Abstract
BACKGROUND AND PURPOSE: Dentatorubral-pallidoluysian atrophy (DRPLA) is an autosomal dominant spinocerebellar ataxia. Techniques for the quantitative assessment of neurodegenerative lesions remain to be established in this disease. We attempted to quantify global and region-specific neurodegeneration in DRPLA using analysis of apparent diffusion coefficient (ADC) maps.
METHODS: Diffusion-weighted images (b = 1000 s/mm2) by echo-planar sequences were obtained with the use of a 1.5T clinical scanner. Whole-brain histogram and region of interest (ROI) analyses of ADC values as well as conventional MR imaging studies were performed in 6 patients with genetically confirmed DRPLA.
RESULTS: Histograms demonstrated significantly higher mean ADC values in the patients than in age- and sex-matched control subjects (P < .01). ROI analysis revealed that the patients had significantly higher ADC values in the cerebellum and globus pallidus, preferentially affected regions (P < .05), but not in the thalamus, the region relatively spared in this disease. ADC values in the white matter were higher only in patients with adult-onset disease. Histogram analyses could more sensitively identify abnormalities than ROI analyses, because the former avoided errors associated with setting ROIs and thus had smaller P values on statistical analysis than the latter.
CONCLUSIONS: Histogram ADC analyses were more sensitive for the detection of neurodegeneration in DRPLA than ROI analyses, whereas ROI analyses revealed regional alterations reflecting the distribution of pathologic changes. Thus, histogram and ROI analyses complement each other and may permit the sensitive, quantitative evaluation of neurodegeneration in DRPLA, especially that involving the globus pallidus showing normal T2 signals.
Dentatorubral-pallidoluysian atrophy (DRPLA) is an autosomal dominant spinocerebellar ataxia caused by CAG repeat expansions in the DRPLA gene.1,2 Clinical manifestations depend on the age at onset of disease and the size of the repeat expansions and include ataxia, choreoathetosis, and dementia in adult-onset disease and progressive myoclonus epilepsy in juvenile-onset disease.3 Despite the distinct phenotypes of these types of DRPLA, neurodegeneration commonly occurs in the basal ganglia (especially the globus pallidus), cerebral white matter, brain stem, and cerebellum.4 Neurodegeneration can be qualitatively evaluated by conventional MR imaging in most of these regions, excluding the basal ganglia5; however, techniques for quantitative assessment remain to be established. To this end, analysis of apparent diffusion coefficient (ADC) maps has been found to be a reproducible, sensitive, and quantitative technique for detecting increased water diffusion in neurodegenerative lesions.6 In particular, histogram ADC analysis of the whole brain has been shown to more sensitively detect global abnormalities than conventional region of interest (ROI) measurements because of smaller interstudy variations.7 We report ADC findings obtained by histogram analyses of the whole brain combined with ROI analyses in 6 patients with genetically confirmed DRPLA.
Methods
Subjects
Six patients with genetically confirmed DRPLA (juvenile-onset in 3 and adult-onset in 3; 3 men and 3 women; age range, 26–56 years; mean age, 40.8 years), including 2 parents (patients 1 and 2) and their offspring (patients 4 and 5, respectively) pairs, and 6 age- and sex-matched healthy control subjects were studied. All 12 subjects were Japanese and resided in a similar geographic location. No patient had a history of any other neurologic diseases. Informed consent was obtained from all patients or their guardians as well as from all control subjects before genetic analysis or MR imaging studies. The clinical features of the patients are summarized in the Table.
MR Imaging
All MR images were obtained with a 1.5T whole-body MR system (Magnetom Sonata, Siemens, Erlangen, Germany) using turbo spin-echo sequences for T1- and T2-weighted imaging (repetition time [TR]/echo time [TE] = 450/9 for T1-weighted imaging; TR/TE = 4000/123; echo-train length [ETL] = 11 for T2-weighted imaging) and a multisection, single-shot, echo-planar sequence (TR/TE = 180/96, b = 1000 s/mm2; matrix, 128 × 128; field of view, 230 mm) for diffusion-weighted (DW) imaging. Three experienced neurologists independently evaluated atrophy of the cerebrum and cerebellum on T1-weighted imaging and signal intensity changes in the globus pallidus, thalamus, and white matter on T2-weighted imaging according to a 4-grade scale (severity, − to +++). If the scores did not agree, that agreed on by 2 of the neurologists was adopted. Nineteen contiguous diffusion-weighted sections were then acquired on axial planes perpendicular to the brain stem with a single-shot echo-planar sequence. Diffusion-sensitizing gradients were applied along the 6 axes, using b values of 0 and 1000 s/mm2. ADC maps were created by signals obtained from images with the 2 b values (b = 0 and 1000). For each DW section, a mean diffusivity (D) map, representing the diffusivity derived from the 6 measurements in orthogonal directions, was computed on a PC workstation with the use of image processing software (Dr. View/LINVX R1.0; Asahikasei Information System, Tokyo, Japan). Whole-brain parenchyma ADC histograms were then computed. To exclude CSF spaces from the ADC map histograms, CSF segmentation was performed on a preselected intracranial volume using a threshold value corresponding to the mean ADC of CSF minus 2 SD for each patient. This procedure should enable exclusion of 97.5% of the CSF-containing pixels, assuming a Gaussian distribution of the CSF ADC values.8 To correct for intersubject differences in brain volume, each histogram was normalized by dividing the height of each histogram bin by the total number of pixels contributing to the histogram. For each histogram, the following measures were then derived: relative peak height, peak location ADC value, and the means of these values. The regional ADC was evaluated from circular ROIs, ∼5 mm in diameter, placed in the right external globus pallidus, thalamus, temporal white matter, and cerebellum (inset of Fig 2). The ROIs were carefully defined to avoid partial volume effects of CSF.
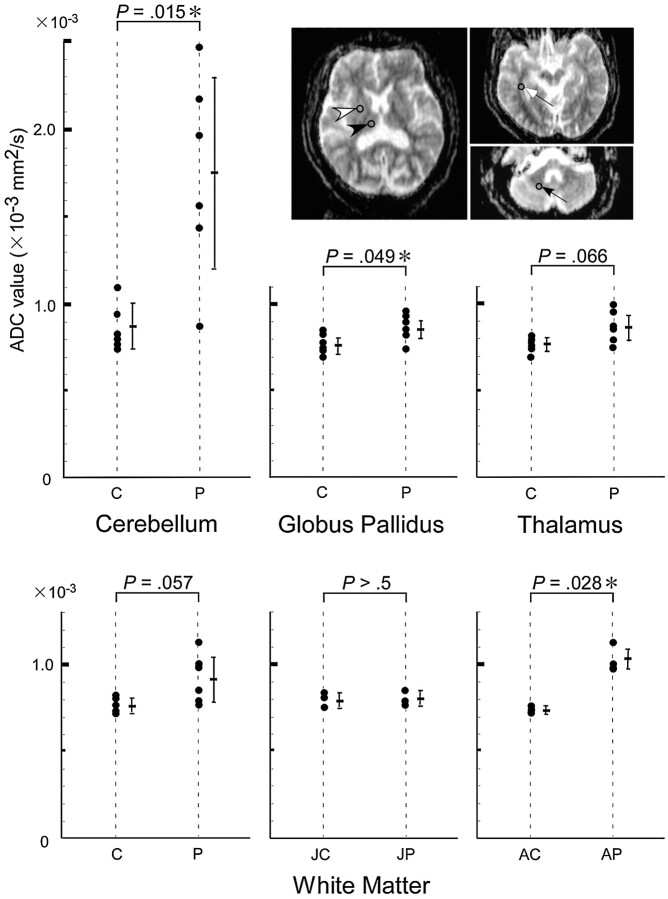
Fig 2.
Region of interest (ROI) analyses. ROIs were set in the globus pallidus (white arrowhead), thalamus (black arrowhead), temporal white matter (white arrow), and cerebellum (black arrow). The ADC values in the globus pallidus and cerebellum of patients with DRPLA (P) were significantly higher than those of age- and sex-matched control subjects (C). In contrast, the ADC values did not differ in the thalamus. ADC values in the white matter were higher in patients with adult-onset disease (AP) than in age-matched control subjects (AC). ADC values in the white matter did not differ significantly between patients with juvenile-onset disease (JP) and age- and sex-matched control subjects (JC).
Statistical Analyses
ADC values in patients were compared with those in age- and sex-matched control subjects. We also compared ADC values in adult-onset patients with those in juvenile-onset patients, the values in adult-onset patients with those in corresponding control subjects, or the values in juvenile-onset patients with those in corresponding control subjects. The statistical significance of differences between groups was analyzed with the use of either the Welch t test or Student t test. P values of less than 0.05 were considered to indicate statistical significance. Correlations of the number of CAG repeats with mean ADC values, peak height of ADC histograms, and peak locations of ADC histograms were assessed by linear regression analysis.
Results
Histogram Analyses of the Whole Brain
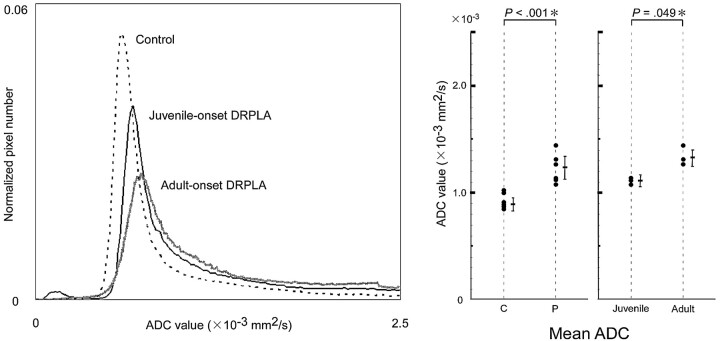
The mean whole-brain ADC value in the patients with DRPLA (1.249 ± 0.120 × 10−3 mm2/s, mean ± SD) was significantly higher (P < .001; t test) than that in the age- and sex-matched control subjects (0.924 ± 0.068 × 10−3 mm2/s) (Fig 1). Peak location (0.766 ± 0.047 × 10−3 mm2/s) was significantly higher (P < .05; t test) and peak height (0.035 ± 0.008, normalized pixel number) was significantly lower (P < .001; t test) in patients than in control subjects (0.688 ± 0.041 × 10−3 mm2/s, 0.057 ± 0.002). The mean whole-brain ADC value in adult-onset DRPLA (1.339 × 10−3± 0.081 × 10−3 mm2/s) was significantly higher (P < .05; t test) and peak height (0.029 ± 0.004) was significantly lower (P < .05; t test) than the respective values in juvenile-onset DRPLA (1.125 ± 0.028 × 10−3 mm2/s, 0.041 ± 0.006). Linear regression analysis showed no significant correlation of the mean ADC value, peak height, or peak location of the DRPLA patients with either the duration of disease or the number of CAG repeats. In this study, the age- and-sex matched control subjects showed no significant physiologic increase in the mean ADC value (r = 0.619; P = .190),9,10 perhaps because the number of subjects was smaller than that in previous studies.
Fig 1.
Histogram analyses. Representative measurements of a control subject, a patient with juvenile-onset DRPLA, and a patient with adult-onset DRPLA are plotted (left). The peak heights were lower and peak locations higher in patients with DRPLA than in controls, with greater changes in adult-onset disease. Mean ADC values were significantly higher in patients with DRPLA (P) than in age- and sex-matched control subjects (C) (center). Mean ADC values were higher in adult-onset disease than in juvenile-onset disease (right).
ROI-Based Analyses
ADC values at the globus pallidus (0.823 ± 0.051 × 10−3 mm2/s) and cerebellum (1.748 ± 0.575 × 10−3 mm2/s) in the patients with DRPLA were significantly higher (P < .05; t test, P < .05; t test) than those in the age- and sex-matched control subjects (0.760 ± 0.047 × 10−3 mm2/s, 0.872 ± 0.126 × 10−3 mm2/s) (Fig 2). The ADC value at the thalamus did not differ significantly between the patients (0.833 ± 0.084 × 10−3 mm2/s) and control subjects (0.753 ± 0.033 × 10−3 mm2/s). The ADC value at the white matter in the patients with adult-onset disease (1.030 ± 0.087 × 10−3 mm2/s) was significantly higher (P < .05; t test) than that in control subjects (0.730 ± 0.017 × 10−3 mm2/s). The ADC values at the white matter did not differ significantly between the patients with juvenile-onset disease (0.790 ± 0.017 × 10−3 mm2/s) and the control subjects (0.803 ± 0.031 × 10−3 mm2/s).
Discussion
Our study demonstrated, for the first time to our knowledge, that mean ADC values on whole-brain histogram analysis were higher in patients with DRPLA than in age- and sex-matched control subjects. Although the number of subjects was small, the histogram shapes obtained for the patients were considerably altered, with significantly lower peak height and higher peak location compared with the control histograms. These findings suggest that ADC analyses are useful to assess neurodegeneration in DRPLA. This notion is supported by the fact that DRPLA involves both gray and white matter, and involvement of either of these regions has been extensively documented by ADC analyses in gray matter diseases (eg, Alzheimer disease) as well as in white matter diseases (eg, multiple sclerosis).11,12
It is noteworthy that patients with adult-onset DRPLA showed a more abnormal histogram shape and thus higher mean ADC values than did patients with juvenile-onset disease, despite similar disease durations in both types of DRPLA. This finding may not be attributed solely to the physiologic age-dependent increase in ADC values, because the increment (19.1%) was much larger than values expected (1.5–6.8%, corresponding to the mean age difference of 26.3 years) based on calculations with the formulas used in previous studies.9,10 Thus, more severe neurodegeneration in patients with adult-onset disease was suggested but was inconsistent with the fact that they had shorter mean CAG repeat expansions, reflecting milder neurotoxicity of the resulting polyglutamine protein.13 ADC findings, however, were in accord with our results of conventional MR imaging in adult-onset patients, indicating increased T2-signal intensity in the white matter and more severe atrophy in the cerebrum and cerebellum. Our ADC findings also agree with the previously reported pathologic characteristics of white matter changes between adult- and juvenile-onset patients.14 Possible explanations for the inconsistency between MR imaging or pathologic findings and the smaller number of CAG repeats include a longer subclinical period before recognizable clinical onset in adult-onset patients; ie, clinical onset of disease is more obscure in adult-onset DRPLA with chronic dementia or ataxia than in juvenile-onset DRPLA with epilepsy. Although the presence of such a subclinical period has yet to be established in this disease, it is partly supported by the findings of previous MR imaging studies of another triplet repeat disease, adult-onset Huntington disease, which has a subclinical period of approximately 6 years.15
To examine the relation between the high mean ADC values on histogram analysis and regional alterations, we performed ROI analyses. Our results showed significantly higher ADC values in the globus pallidus and cerebellum, regions preferentially involved in DRPLA. The high ADC value in the globus pallidus was especially important clinically, because conventional MR imaging in the present on previous studies detected no or mild abnormalities in this region.5 Previous studies have demonstrated pathologic alterations in the globus pallidus, even with a normal T2 signal intensity.16 Thus, ADC findings might more sensitively reflect microscopic neurodegeneration than T2 signal intensity changes. White matter showed significantly increased ADC values only in adult-onset disease associated with high T2 signals, not in juvenile-onset disease with normal T2 signals. This finding is consistent with the results of a previous study of juvenile-onset patients whose ages and CAG repeats were similar to those of our patients, showing no or only mild pathologic alterations in the white matter.14 In contrast, no significant changes in either type of disease were found in the thalamus, the region relatively spared in DRPLA.17 All of these findings indicate that the high mean ADC values in the whole brain resulted from increased ADC values in preferentially affected regions, the cerebellum and globus pallidus, suggesting that our data closely reflected the pathologic effects of this disease. However, confirmation should await further studies because of the small number of the patients evaluated.
In summary, histogram and ROI analyses in this study demonstrated significantly higher ADC values in patients with DRPLA than in well-matched control subjects. Histogram analyses were more sensitive than ROI analyses for the detection of neurodegeneration in DRPLA, because the former method avoided errors associated with setting ROIs and thus had smaller P values on statistical analysis than the latter method. Despite lower sensitivity, ROI analyses revealed regional alterations reflecting the reported distribution of pathologic changes. Thus, histogram and ROI analyses complement each other and may permit the sensitive and quantitative evaluation of neurodegeneration in DRPLA, especially that involving the globus pallidus showing normal T2 signals. Automated quantitative methods such as whole-brain ADC analysis are likely to play an important role in future therapeutic clinical trials, which may include studies currently being conducted for other triplet repeat diseases sharing a common polyglutamine-mediated mechanism of pathogenesis18,19 (eg, studies of phenylbutyrate, a histone deacetylase inhibitor, in patients with Huntington disease).
Clinical, genetic, and neuroradiologic information of patients with DRPLA
| Juvenile-Onset Cases |
Adult-Onset Cases |
||||||
|---|---|---|---|---|---|---|---|
| Patient No.: | 1 | 2 | 3 | 4 | 5 | 6 | |
| Age (y) | 26 | 28 | 29 | 53 | 56 | 53 | |
| Sex | F | M | F | M | M | F | |
| CAG repeat | 65 | 65 | 60 | 63 | 60 | 59 | |
| Duration (y) | 10 | 15 | 10 | 10 | 15 | 10 | |
| Onset (y) | 16 | 13 | 19 | 43 | 41 | 43 | |
| Epilepsy | +++ | plus;+ | + | − | − | − | |
| Dementia | + | + | + | ++ | ++ | +++ | |
| Ataxia | ++ | + | − | ++ | ++ | ++ | |
| Chorea | + | − | − | + | ++ | + | |
| MR images | |||||||
| Atrophy | |||||||
| Cerebrum | − | − | − | + | + | + | |
| Cerebellum | + | + | + | ++ | + | + | |
| T2 high-signal GP | − | − | − | − | − | − | |
| Thalamus | − | − | − | − | − | − | |
| White matter | − | − | − | + | ++ | ++ | |
Note:—DRPLA indicates dentatorubral-pallidoluysian atrophy; GP, globus pallidus; −, not apparent; +, mild; ++, moderate; +++, severe.
References
- 1.Nagafuchi S, Yanagisawa H, Sato K, et al. Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nat Genet 1994;6:14–18 [DOI] [PubMed] [Google Scholar]
- 2.Knoide R, Ikeuchi T, Onodera O, et al. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet 1994;6:9–13 [DOI] [PubMed] [Google Scholar]
- 3.Kanazawa I. Dentatorubral-pallidoluysian atrophy or Naito-Oyanagi disease. Neurogenetics 1998;2:1–17 [DOI] [PubMed] [Google Scholar]
- 4.Naito H, Oyanagi S. Familial myoclonus epilepsy and choreoathetosis: hereditary dentatorubral-pallidoluysian atrophy. Neurology 1982;32:798–807 [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki M, Hashimoto T, Yoneda Y, et al. Proton magnetic resonance spectroscopy on juvenile-onset dentatorubral-pallidoluysian atrophy (DRPLA). Brain Dev 1996;18:142–46 [DOI] [PubMed] [Google Scholar]
- 6.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Res B 1996;111:209–19 [DOI] [PubMed] [Google Scholar]
- 7.Cercignani M, Bammer R, Sormani MP, et al. Inter-sequence and inter-imaging unit variabilia of diffusion tensor MR imaging histogram-derived metrics of the brain in healthy volunteers. AJNR Am J Neuroradiol 2003;24:638–43 [PMC free article] [PubMed] [Google Scholar]
- 8.Mascalchi M, Tessa C, Moretti M, et al. Whole brain apparent diffusion coefficient histogram: a new tool for evaluation of leukoaraiosis. J Magn Reson Imaging 2002;15:144–48 [DOI] [PubMed] [Google Scholar]
- 9.Chun T, Filippi CG, Zimmerman RD, et al. Diffusion changes in the aging human brain. AJNR Am J Neuroradiol 2000;21:1078–83 [PMC free article] [PubMed] [Google Scholar]
- 10.Nusbaum AO, Tang CY, Buchsbaum MS, et al. Regional and global changes in cerebral diffusion with normal aging. AJNR Am J Neuroradiol 2001;22:136–42 [PMC free article] [PubMed] [Google Scholar]
- 11.Bozzali M, Franceschi M, Falini A, et al. Quantification of tissue damage in AD using diffusion tensor and magnetization transfer MRI. Neurology 2001;57:1135–37 [DOI] [PubMed] [Google Scholar]
- 12.Nusbaum AO, Tang CY, Wei T, et al. Whole-brain diffusion MR histograms differ between MS subtypes. Neurology 2000;54:1421–27 [DOI] [PubMed] [Google Scholar]
- 13.Yamada M, Wood JD, Shimohata T, et al. Widespread occurrence of intranuclear atrophin-1 accumulation in the central nervous system neurons of patients with dentatorubral-pallidoluysian atrophy. Ann Neurol 2001;49:14–23 [DOI] [PubMed] [Google Scholar]
- 14.Yamada M, Sato T, Tsuji S, et al. Oligodendrocytic polyglutamine pathology in dentatorubral-pallidoluysian atrophy. Ann Neurol 2002;52:670–74 [DOI] [PubMed] [Google Scholar]
- 15.Harris GJ, Codori AM, Lewis RF, et al. Reduced basal ganglia blood flow and volume in pre-symptomatic, gene-tested persons at-risk for Huntington’s disease. Brain 1999;122:1667–78 [DOI] [PubMed] [Google Scholar]
- 16.Munoz E, Mila M, Sanchez A, et al. Dentatorubropallidoluysian atrophy in a Spanish family: a clinical, radiological, pathological, and genetic study. J Neurol Neurosurg Psychiatry 1999;67:811–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uyama E, Kondo I, Uchino M, et al. Dentatorubral-pallidoluysian atrophy (DRPLA): clinical, genetic, and neuroradiologic studies in a family. J Neurol Sci 1995;130:146–53 [DOI] [PubMed] [Google Scholar]
- 18.Gardian G, Browne SE, Choi DK, et al. Neuroprotective effects of phenylbutyrate in the N171–82Q transgenic mouse model of Huntington’s disease. J Biol Chem 2005;280:556–63 [DOI] [PubMed] [Google Scholar]
- 19.Kariya S, Hirano M, Uesato S, et al. Cytoprotective effect of novel histone deacetylase inhibitors against polyglutamine toxicity. Neurosci Lett 2005;392:213–15 [DOI] [PubMed] [Google Scholar]