Abstract
SUMMARY: This report presents the imaging findings of an unusual case of Epstein-Barr virus (EBV) encephalitis. A young man presented with a short-lasting history of febrile infection, neuropsychologic deficits, ataxia, and seizures. MR imaging revealed fully reversible signal intensities (T2, diffusion-weighted imaging with a decreased apparent diffusion coefficient) in the splenium of the corpus callosum and both posterior hemispheres. EBV infection must be added to the list of differential diagnoses of (reversible) splenial lesions.
Epstein-Barr virus (EBV) is the underlying pathogen of infectious mononucleosis, which is usually a benign, self-limiting disease. Neurologic symptoms have been described and comprise seizures, polyradiculomyelitis, transverse myelitis, encephalitis, and cranial nerve palsies.1,2 The overall incidence of neurologic complications has been reported to be <7% and central nervous system (CNS) symptoms can be the sole manifestation of EBV infection. Therefore, EBV has to be considered in a variety of acute neurologic illnesses. We describe a young man with EBV encephalitis with multiple reversible MR lesions, including the splenium of the corpus callosum (SCC) and excellent clinical recovery. Similar lesions of the splenium can occur in a variety of (treatable) pathologic conditions and present with nonspecific neuropsychologic and neurologic signs.3, 4 Until now, different MR pictures of EBV-encephalitis have been described, and to our knowledge this is the first report of a reversible splenial lesion in EBV infection.1, 5,6
Case Report
A 21-year-old male carpenter was admitted to our unit from a nearby hospital with a few days’ history of febrile infection, sickness, dizziness, ataxia, nystagmus, and confusion. Gastritis had been suspected, and the patient was treated with metoclopramide. This treatment was stopped once the nystagmus and the ataxia occurred. He had no other history of disease and was immunocompetent. On his way to our hospital, he experienced several generalized tonic clonic seizures and was treated with diazepam. On arrival, he did not show any focal neurologic signs but was disorientated. An initial cranial CT scan was normal. He became drowsy and had to be intubated. Anticonvulsive treatment with valproate was initiated. Lab results showed a marginal leukocytosis (16% monocytes) but no other abnormality. A lumbar puncture revealed 18 cells/μL with 64% lymphocytes and a protein content of 1500 mg/L. Antiviral and antibiotic treatment was started with acyclovir, ceftriaxon, and ampicillin. A cranial MR imaging scan was obtained, which showed multiple diffuse T2, fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted imaging (DWI) signal intensity hyperintensities in different, especially parieto-occipital, cortical areas, and, most marked, in the SCC, with a reduction of the apparent diffusion coefficient (ADC) values in these regions (Fig 1). There was no contrast enhancement. Serologic response to EBV was positive for heterophilic antibodies as well as for specific antibodies to viral capsid antigen (VCA-IgM) and early antigen. EBV DNA, detected by polymerase chain reaction (PCR), was positive in peripheral mononuclear cells. In CSF, EBV DNA PCR was also positive, and VCA-IgM was weakly positive on one occasion with a normal EBV-IgG antibody index of 1.1.
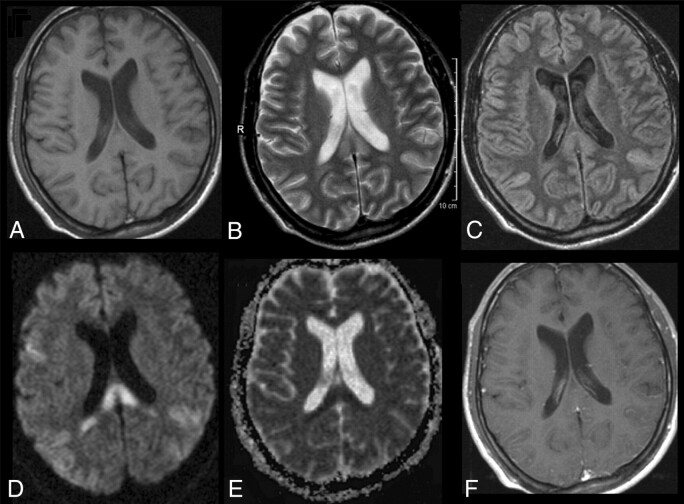
Fig 1.
Initial MR imaging of the patient. T1-weighted image does not reveal any abnormality (A). T2-weighted image depicts high signal intensity in the SCC (B). Turbo spin-echo FLAIR sequence shows additional high signals in both posterior hemispheres (C). All lesions had clearly elevated diffusion coefficients with high signal intensity on DWI (D), whereas ADC maps in the splenium were decreased (E). The T1-weighted image with gadolinium shows no contrast enhancement.
Serologic testing for tick-borne encephalitis, Lyme borreliosis, listeriosis, cytomegalovirus, herpes virus and varicella-zoster virus, and HIV and PCR for herpes simplex and varizella zoster virus were all negative. This confirmed the diagnosis of EBV infection with CNS manifestation. High-dose steroids were administered for 5 days and tapered off. Extubation twice failed as another generalized seizure developed and a tracheotomy was necessary. Levetiracetam was added to the anticonvulsive treatment. CSF 8 days after admission showed 1 cell/μL and a protein content of 626 mg/L. After 14 days, the patient breathed spontaneously and subsequently made a gradual clinical recovery with no focal neurologic signs but confusion, verbal influency, and amnesic deficits on neuropsychologic testing. Follow-up MR imaging on day 21 was completely normal (Fig 2). Four months later he had almost fully recovered: he reported rare episodes with decreased cognitive velocity, and a final neuropsychologic evaluation revealed high levels of cognitive function with rare verbal memory deficits. He experienced several provoked seizures and has continued to receive levetiracetam monotherapy.
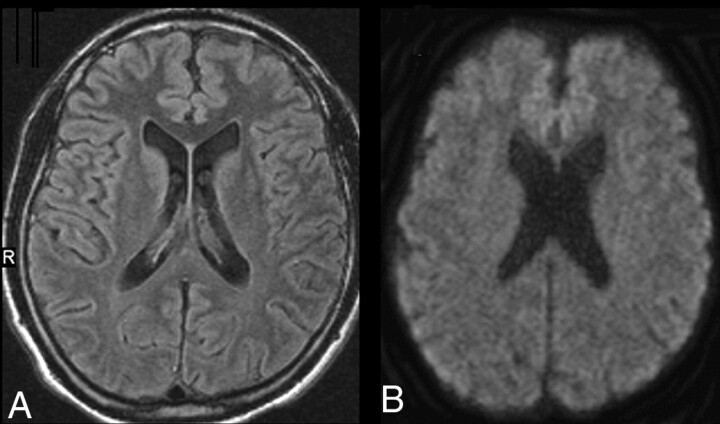
Fig 2.
MR imaging oft the patient on follow-up 3 weeks later. All signal intensities are normalized (A, FLAIR; B, DWI; ADC maps, not shown).
Discussion
In this case, the diagnosis of EBV infection is ascertained by the detection of EBV PCR and a weakly positive VCA-IgM titer in the CSF and serum.2 The clinical, as well as the neuroimaging, pictures, however, are less unequivocal. He had seizures and temporary focal neurologic deficits pointing toward involvement of cerebellum and brain stem but predominantly neuropsychologic signs and confusion. This is compatible with EBV infection, but hardly specific. Clinically, EBV can present with meningitis, encephalitis, acute demyelinating encephalomyelitis (ADEM), cranial nerve palsies, cerebellitis, myelitis, or seizures. EBV can also present with a wide spectrum of imaging abnormalities reaching from normal to diffuse signal intensity changes either in gray or white matter, sometimes resembling ADEM.1,5,6
The distribution of the signal intensities in our patient is confined to the SCC and both parieto-occipital cortical areas. This is interesting, because the latter shows gray matter involvement, whereas the lesion in the SCC is a white matter lesion. (Reversible) lesions of the SCC have been described in a variety of conditions without any specific clinical syndrome and often without a disconnection syndrome.3 Confusion was the most frequent symptom followed by ataxia and seizures, which is in good agreement with our patient’s illness syndrome. Other described etiologies of reversible splenial lesions include other viral CNS infections, Legionnaire disease, hypoglycemia, alcohol, malnutrition, ADEM, electrolyte imbalances, anticonvulsant drug withdrawal, and seizures.3,4,7, 8 The underlying mechanisms are not yet clear: an impairment of cellular fluid regulation has been proposed, which could be induced by direct toxic effects on cell membranes or by transient energy depletion with shifts of electrolytes into the cells, during either seizure activity, hypoglycemia, or transient incomplete ischemia.3, 7,8 Most of the conditions mentioned above were excluded in our patient.
We cannot, however, rule out that the lesions in our patient were not caused primarily by the EBV but by the series of seizures or as an epiphenomenon of an EBV-induced ADEM.1,3 In all cases with epileptic seizure-induced reversible SCC lesions, a high number of seizures was reported or patients developed series of seizures after withdrawal of anticonvulsant medication, making it impossible to pinpoint the reason for the lesion.8,9 The patient presented here did not receive anticonvulsants beforehand and did experience only a small number of generalized seizures, which makes it unlikely that seizures were the underlying etiology.
No study of EBV CNS infections has used DWI and calculation of ADC maps. This technique is sensitive to diffusion of water in the tissue and is assumed to allow discrimination of intracellular and extracellular water. Reduced ADC values may represent cytotoxic edema with bound water within the cell and are believed to represent severe tissue damage progressing to necrosis. On the other hand, reversible ADC decrements have also been reported (eg, in transient ischemia, after seizures, and in hypoglycemia), which also indicates that functional disturbances can go along with an ADC decline.7,10–12 It is noteworthy that many of the published cases with lesions of the SCC, either inflammatory or of other origin, reported diffusion changes, also with reversible ADC reduction. This suggests that this region has an unexplained increased propensity for cytotoxic edema. Because there are basically only axonal and glial elements but no neurons in the corpus callosum, it is conceivable that glial water uptake is the underlying mechanism of the described signal intensity change. In case of axonal swelling, more severe and long-lasting, if not persistent, deficits had to be expected.
Acyclovir and corticosteroids have been recommended for treatment of EBV encephalitis, but their effectiveness is uncertain.2,13 Nevertheless, the course of the illness is most often benign, though fatal cases have been reported.14,15 This report demonstrates that EBV can present with a peculiar distribution of reversible diffusion changes on MR imaging, including a lesion of the SCC, which has not been reported before. Therefore, EBV infection should be considered in otherwise unexplained cases with a splenial lesion.3
Acknowledgments
We thank C. Fitzek, M. Wohlfahrt, and O. W. Witte for helpful comments and critical reading of the manuscript.
References
- 1.Fujimoto H, Asaoka K, Imaizumi T, et al. Epstein-Barr virus infections of the central nervous system. Intern Med 2003;42:33–40 [DOI] [PubMed] [Google Scholar]
- 2.Volpi A. Epstein-Barr virus and human herpesvirus type 8 infections of the central nervous system. Herpes 2004;11 (suppl 2):120A–27A [PubMed] [Google Scholar]
- 3.Doherty MJ, Jayadev S, Watson NF, et al. Clinical implications of splenium magnetic resonance imaging signal changes. Arch Neurol 2005;62:433–37 [DOI] [PubMed] [Google Scholar]
- 4.Tada H, Takanashi J, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 2004;63:1854–58 [DOI] [PubMed] [Google Scholar]
- 5.Shian WJ, Chi CS. Epstein-Barr virus encephalitis and encephalomyelitis: MR findings. Pediatr Radiol 1996;26:690–93 [DOI] [PubMed] [Google Scholar]
- 6.Ono J, Shimizu K, Harada K, et al. Characteristic MR features of encephalitis caused by Epstein-Barr virus: a case report. Pediatr Radiol 1998;28:569–70 [DOI] [PubMed] [Google Scholar]
- 7.Bottcher J, Kunze A, Kurrat C, et al. Localized reversible reduction of apparent diffusion coefficient in transient hypoglycemia-induced hemiparesis. Stroke 2005;36:e20–22 [DOI] [PubMed] [Google Scholar]
- 8.Oster J, Doherty C, Grant PE, et al. Diffusion-weighted imaging abnormalities in the splenium after seizures. Epilepsia 2003;44:852–54 [DOI] [PubMed] [Google Scholar]
- 9.Mirsattari SM, Lee DH, Jones MW, et al. Transient lesion in the splenium of the corpus callosum in an epileptic patient. Neurology 2003;60:1838–41 [DOI] [PubMed] [Google Scholar]
- 10.Hufnagel A, Weber J, Marks S, et al. Brain diffusion after single seizures. Epilepsia 2003;44:54–63 [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa Y, Formato JE, Latour LL, et al. Severe transient hypoglycemia causes reversible change in the apparent diffusion coefficient of water. Stroke 1996;27:1648–55; discussion 1655–56 [DOI] [PubMed] [Google Scholar]
- 12.Fiehler J, Knudsen K, Kucinski T, et al. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke 2004;35:514–19 [DOI] [PubMed] [Google Scholar]
- 13.Hausler M, Ramaekers VT, Doenges M, et al. Neurological complications of acute and persistent Epstein-Barr virus infection in paediatric patients. J Med Virol 2002;68:253–63 [DOI] [PubMed] [Google Scholar]
- 14.Shian WJ, Chi CS. Fatal brainstem encephalitis caused by Epstein-Barr virus. Pediatr Radiol 1994;24:596–97 [DOI] [PubMed] [Google Scholar]
- 15.Francisci D, Sensini A, Fratini D, et al. Acute fatal necrotizing hemorrhagic encephalitis caused by Epstein-Barr virus in a young adult immunocompetent man. J Neurovirol 2004;10:414–17 [DOI] [PubMed] [Google Scholar]