Abstract
PURPOSE: This study sought to determine whether the angiographic demonstration of slow antegrade contrast opacification of an occluded cerebral artery distal to the thrombus (clot outline sign) on cerebral arteriograms performed immediately before thrombolytic treatment is associated with higher recanalization rates relative to patients without antegrade contrast opacification distal to the occlusion site.
METHODS: The angiographic images of 100 consecutive arteriograms performed before thrombolysis in patients eligible for intra-arterial thrombolysis from May 1995 to February 2005 were reviewed. A modified Thrombolysis in Myocardial Infarction flow grade (mTIMI) was adapted to grade recanalization after cerebral thrombolysis. Clot outline sign was defined as slow antegrade contrast opacification distal to the thrombus on the delayed images of the presenting arteriogram. Logistic regression analysis for mTIMI grade included the following potential predictors: presence of outline sign, age, time to treatment, sex, site of occlusion, presenting National Institutes of Health Stroke Scale (NIHSS) score, presenting platelets, presenting systolic blood pressure, presence of pial collaterals, and admitting glucose value.
RESULTS: Eighty-seven arteriograms were reviewed. Of these, 19 (22%) displayed the clot outline sign. Thirteen (69%) of 19 had clot outline sign, and 16 of 68 (29%) were not completely recanalized (mTIMI = 3); 95% with clot outline sign and 54% without were associated with either mTIMI 2 or 3 (P = .0055, Pearson correlation). Logistic regression analysis for recanalization relative to other predictors indicates that only the clot outline sign could act as a statistically significant predictor for recanalization (P = .0007).
CONCLUSION: Prethrombolysis cerebral arteriograms demonstrating delayed antegrade contrast opacification distal to the occlusion site are associated with higher recanalization rates.
Recanalization of occluded intracranial vessels after intra-arterial thrombolytic treatment delivered within 6 hours after symptom onset in acute ischemic stroke has been shown to improve outcomes and reduce infarct volumes.1–3 Complete recanalization rates, however, vary between studies involving patients undergoing intra-arterial thrombolysis for acute ischemic stroke.1–5 Few factors associated with successful recanalization have been reported. Site of occlusion and angiographic appearance have been shown to be associated with differing recanalization rates.5–7 This investigation sought to determine whether the objective angiographic finding of slow antegrade contrast opacification of an occluded cerebral artery distal to thrombus (clot outline sign) on cerebral arteriograms immediately before intra-arterial thrombolytic treatment is associated with higher recanalization rates.
The clot outline sign is similar to what is defined as Thrombolysis in Myocardial Infarction (TIMI) grade 1 (penetration of contrast distal to the occlusion site). Morphologic descriptions of the occlusion site as “tram track appearance” or “tapered” have been associated with higher recanalization rates.7 This type of description, however, is somewhat subjective. Identification of the contrast distal to the occlusion site depends on a single finding and would be expected to be more objective and more reproducible than a morphologic description as “tram track” or “tapered,” which introduces an element of subjectivity.
Materials and Methods
This study retrospectively reviewed the records and available images from 100 consecutive arteriograms performed before thrombolysis in patients eligible for intra-arterial thrombolysis. Patients were excluded if the arteriogram was not available for review, if the operator purposefully occluded the parent vessel (ie, treated parent vessel perforation), or the microcatheter was not able to reach the occlusion site. Patients in whom balloon angioplasty was used to assist recanalization were also excluded. Patients underwent angiography and thrombolytic treatment with the use of local anesthesia, aseptic technique, and digital fluoroscopic control in a biplane angiographic suite. Sedatives were avoided where possible. Methods of thrombolytic therapy for patients included continuous infusion or pulse spray. Microcatheter positioning relative to the thrombus was determined by the operator but was typically within the thrombus. Mechanical methods for clot disruption, such as balloon angioplasty or clot retrieval, were not included. Thrombolytic agents infused in this study were either intra-arterial tissue plasminogen activator (up to 100 mg), urokinase (up to 1 million units), or pro-urokinase (up to 1 million units).
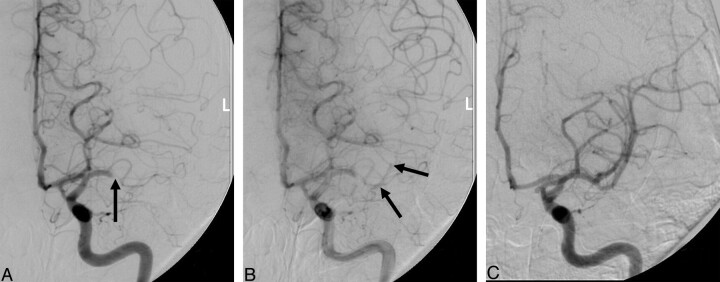
Arteriograms performed before thrombolytic treatment were reviewed separately from arteriograms performed after thrombolytic treatment. Review of arteriograms before thrombolysis included identification of clot outline sign, site of occlusion, and extent of pial collaterals. Clot outline sign was defined as slow antegrade contrast opacification distal to the thrombus on the delayed images of the presenting arteriogram (Fig. 1). Presence of “clot outline sign” was made by evaluating the sequence of arteriographic images obtained during the pretreatment evaluation of the occlusion site. It implies that the vessel in question is almost completely occluded and has a minute blood flow past the clot. As is often the case, the vascular bed distal to the occlusion site is also perfused by pial collateral vessels. Site of occlusion was defined as either proximal or distal. Proximal occlusion included occlusions at the internal carotid artery, basilar artery, A1 segment, M1 segment, or P1 segment. All other occlusions were considered to be distal. Cerebral arterial segments are defined elsewhere.8 Post-thrombolysis arteriograms were reviewed to identify recanalization on the basis of a modified version of the TIMI flow grading scheme.9 No recanalization was given a grade of 0, minor recanalization (<33% of the territory recanalized) was a grade of 1, partial recanalization with reperfusion of more than 33% of the involved territory was a grade of 2, and complete recanalization with no visible vessel occlusion at the end of the procedure was a grade of 3. The presence of a clot outline sign was then correlated to modified TIMI (mTIMI) flow grade using contingency analysis, and Pearson correlation value was identified for significance. Logistic regression analysis for the mTIMI grade was performed that included presence of outline sign, age, site of occlusion, time to treatment, sex, presenting National Institutes of Health Stroke Scale (NIHSS) score, systolic blood pressure, platelet count on admission, presence of pial collaterals, and blood glucose value on admission. To facilitate regression analysis, NIHSS was treated as a continuous variable. The regression analyses were repeated using backward selection, and then variables with estimates having a probability of proving the null hypothesis greater than 0.2, based on the Wald test, were rejected.
Fig 1.
Prethrombolysis midarterial phase (A) and late arterial phase (B) angiographic images and post-thrombolysis angiographic image (C) from a patient who underwent intra-arterial thrombolysis with complete recanalization (C). This patient presented with a left m1 segment occlusion (A, arrow). Note the delayed opacification of the distal m1 and proximal m2 segments (arrows).
Results
A total of 87 of the 100 consecutive patients identified were studied. Seven patients were excluded because arteriograms were not available for review. In 3 patients, the microcatheter was not able to reach the occlusion site. In 1 patient, the occluded vessel was coiled as a result of intraprocedural perforation, and 2 patients were excluded because angioplasty was used to assist recanalization. Only 1 of the 6 patients in whom films were available but were excluded had an outline sign present. In that patient, the parent vessel was occluded with coils to treat an iatrogenic perforation. The patients studied included 40 women and 47 men with a mean age of 65.1 (σ = 14.4). The mean time to treatment was 264.5 minutes (σ = 84.0), median presenting NIHSS was 16 (quartiles 11–20), mean platelet count on admission value was 224 K/μL (σ = 65.5), mean blood glucose on admission value was 132 mg/dL (σ = 49.8), and mean presenting systolic blood pressure was 145.1 (σ = 25.0). Occlusion sites distributed as follows: middle cerebral artery (MCA), −71%; internal carotid artery (ICA), 16%; anterior cerebral artery, 4% basilar artery, 1%; and posterior inferior cerebellar artery, 1%. Seventy-five percent of occlusions were proximal, and the remaining 25% were distal. Nineteen of 87 patients with arteriograms available for review displayed the clot outline sign. Thirteen of these 19 (68.4%) went on to completely recanalize (Table 1). Only 20 (29.4%) of the 68 patients without demonstration of the clot outline sign went on to complete recanalization. Eighteen of 19 patients (94.7%) displaying the clot outline sign were associated with a mTIMI grade of 2 or 3, whereas only 37 (54.4%) of those without the clot outline sign were associated with mTIMI grades of 2 or greater (P = .0055). Logistic regression analysis Table 2 for mTIMI grades relative to other predictors indicates that only the clot outline sign is significantly associated with recanalization (P = .0007).
Table 1:
Post-thrombolysis mTIMI grades in patients with angiographic demonstration of a clot outline sign versus those who did not demonstrate a clot outline sign
| mTIMI Grade (%) |
||||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Outline sign (n = 19) | 1 (5.3) | 0 (0) | 5 (26.3) | 13 (68.4) |
| No outline sign (n = 68) | 21 (30.1) | 10 (14.7) | 17 (25) | 20 (29.4) |
| Total (n = 87) | 22 (25.3) | 10 (11.5) | 22 (25.3) | 33 (37.9) |
Note:—mTIMI indicates modified Thrombolysis in Myocardial Infarction flow grade.
Pearson correlation (P = .0055).
Table 2:
Multivariate logistic regression analysis for mTIMI grade after rejecting variables with P > .2
| Parameter | Estimate | P > χ2 |
|---|---|---|
| Outline sign | 0.300 | .0007 |
| Age | −0.025 | .1183 |
| Time to treatment (min) | 0.004 | .1305 |
| Systolic blood pressure (SBP) | −0.0137 | .1401 |
| Glucose | 0.009 | .0561 |
Discussion
Recanalization is a strong predictor for clinical outcome and infarct volume after thrombolytic treatment.1,3,5,10 Methods used to recanalize patients have included intravenous and intra-arterial thrombolysis, angioplasty, and clot retrieval. Recanalization rates and clinical outcomes after intra-arterial thrombolysis vary between studies depending on the type of thrombolytic agent, technique, clot volume, and occlusion site.11 Therefore, it is important to identify predictors for improving recanalization rates to determine treatment efficacy. Thus far, few investigations have been able to identify predictors for recanalization. Although MCA and ICA trunk occlusions have been shown to achieve successful recanalization (mTIMI 2–3) more frequently than those with distal occlusions by other investigators,5–7 this was not confirmed in this study. Initial angiographic morphologic features have been shown to correlate with recanalization. In a study performed by Pillai et al,7 all 3 patients with “tram track” appearance of the clot and 6 of 7 patients with “tapered” appearance of clot had complete recanalization. Based on those descriptions, it is evident that the “tram track” appearance would qualify as the “outline sign” described in the current work. It is also possible that some of the patients with “tapered” appearance of the clot would also qualify as an “outline sign.” The current study evaluates contrast opacification distal to the occlusion site rather than the angiographic morphology. Unlike the descriptions of the “tram track” appearance and the “tapered” appearance, the outline sign takes into account the appearance of contrast distal to the occlusion site even on delayed images rather than a single arteriographic picture. It is significant that the observation in the current study of “outline sign” was made after data were compiled, indicating that this observation was made independently of other factors. The number of patients included in the current study provides statistically significant support for this finding. The cohort of patients in this study included occlusions from all sites of the brain, though most had MCA (71%) and ICA (16%) occlusions. In this study, good recanalization (mTIMI 2–3) was evident in 94.7% of patients with a clot outline sign, whereas only 54.4% of patients without the outline sign had mTIMI grades of 2 and above Table 1.
In the present study, predictors of recanalization tested included: presence of outline sign, age, site of occlusion, time to treatment (minutes), sex, presenting systolic blood pressure, presenting NIHSS score, admitting platelets, presence of pial collaterals, and admitting glucose value. Clinical studies have demonstrated that all of these variables are predictive of either clinical outcome or hemorrhage in acute stroke.1,3,12–24 As a result, they were included in our analysis. The only predictor that proved to have significance for recanalization among the variables tested in this study was clot outline sign Table 2. Support that contrast opacification distal to the occlusion site may be associated with higher recanalization rates is also found in coronary artery occlusions. TIMI grade 3 reperfusion is significantly higher in morphologic descriptions in which there is contrast-opacifying vasculature distal to the occlusion site.25
Because only endovascular pharmacologic treatment was used for thrombolysis in this study, it is not known whether the presence of an “outline sign” would be associated with higher recanalization rates with mechanical thrombolysis. If a difference did exist, presence of “outline sign” may influence the selection of treatment method and help the operator make a decision on endovascular treatment method. Although the prognostic value for the “clot outline sign” was not investigated in this study, it is suspected that its association with higher recanalization rates would account for any potential clinical changes. It is assumed that the miniscule blood flow around the blood clot in patients with this sign would not account for any independent differences in clinical outcome.
In the current study, there are several limitations. The dataset was compiled retrospectively and therefore is prone to misclassification bias. To reduce this bias, prethrombolysis arteriograms were reviewed separately from postthrombolysis arteriograms, thereby blinding the reviewer from outcome. A total of 13 patients were excluded; however, these patients had baseline characteristics similar to those of the study population, and therefore no bias was introduced in the study selection. The study population is heterogeneous in that the patients received 1 of 3 intra-arterial thrombolytic agents; however, most were given tissue plasminogen activator. In addition, patients had occlusions in both proximal and distal sites. These differences were taken into account when performing analysis, and therefore allowed the comparison of the clot outline sign with recanalization rates. Other factors that may affect recanalization have not been studied, such as source or makeup of clot and operator performing the procedure. Because of the overwhelming difference in recanalization rates when the clot outline sign was present, it is not likely that such factors would substantially affect our conclusions.
Conclusion
Prethrombolysis arteriography demonstrating delayed antegrade contrast opacification distal to the occlusion site is associated with higher recanalization rates after local intra-arterial administration of thrombolytic agents. This angiographic finding can provide an objective means to assess potential for recanalization before thrombolytic treatment.
Acknowledgments
We thank Peggy Notestine, Hoda Jradi, Donald Chakeres, Eric Bourekas, and Wayne Slone.
Footnotes
This work has been presented previously at the 2005 meeting of the American Society of Interventional and Therapeutic Neuroradiology; May 20–22, 2005; Toronto, Canada, and at the 42nd Annual Meeting of the American Society of Neuroradiology; June 5–11, 2004.
References
- 1.Del Zoppo G, Higashida R, Furlan A, et al. PROACT: A Phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke 1998;29:4–11 [DOI] [PubMed] [Google Scholar]
- 2.Furlan A, Higashida R, Weschler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study. JAMA 1999;282:2003–11 [DOI] [PubMed] [Google Scholar]
- 3.Christoforidis GA, Mohammad Y, Kehagias D, et al. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 2005;26:1789–97 [PMC free article] [PubMed] [Google Scholar]
- 4.Zeumer H, Freitag HJ, Zanella F, et al. Local intra-arterial fibrinolytic therapy in patients with stroke: urokinase versus recombinant tissue plasminogen activator (r-TPA). Neuroradiology 1993;35:159–62 [DOI] [PubMed] [Google Scholar]
- 5.Suarez J, Sunshine J, Tarr R, et al. Predictors of clinical improvement, angiographic recanalization, and intracranial hemorrhage after intra-arterial thrombolysis for acute ischemic stroke. Stroke 1999;30:2094–100 [DOI] [PubMed] [Google Scholar]
- 6.Kucinski T, Koch C, Eckert B, et al. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology 2003;45:11–18 [DOI] [PubMed] [Google Scholar]
- 7.Pillai JJ, Lanzier CF, Trinidad SB, et al. Initial angiographic appearance of intracranial vascular occlusions in acute stroke asa predictor of outcome of thrombolysis: initial experience. Radiology 2001;218:733–38 [DOI] [PubMed] [Google Scholar]
- 8.Morris P. Practical neuroangiography. Baltimore: Williams and Wilkins;1997. :87–278
- 9.The Thrombolysis in Myocardial Infarction (TIMI) trial: Phase I findings: TIMI Study Group. N Engl J Med 1985;312:932–36 [DOI] [PubMed] [Google Scholar]
- 10.Von Kummer R, Holle R, Rosin L, et al. Does arterial recanalization improve outcome in carotid territory stroke? Stroke 1995;26:581–87 [DOI] [PubMed] [Google Scholar]
- 11.Sorimachi T, Fujii Y, Tsuchiya N, et al. Recanalization by mechanical embolus disruption during intra-arterial thrombolysis in the carotid territory. AJNR Am J Neuroradiol 2004;25:1391–402 [PMC free article] [PubMed] [Google Scholar]
- 12.The National Institute of Neurological Disorders and Stroke (NINDS) trial. N Engl J Med 1995;33:1581–87 [Google Scholar]
- 13.Clark WM, Wissman S, Albers GW, et al. Recombinant tissue-type plasminogen activator(alpteplase)for ischemic stroke 3 to 5 hours after symptom onset: the ATLANTIS study. JAMA 1999;282:2019–26 [DOI] [PubMed] [Google Scholar]
- 14.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the european cooperative acute stroke study (ECASS). JAMA 1995;274:1017–25 [PubMed] [Google Scholar]
- 15.Del Zoppo G, Poeck K, Pessin M, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992;32:78–86 [DOI] [PubMed] [Google Scholar]
- 16.Von Kummer R, Hacke W. Safety and efficacy of intravenous tissue plasminogen activator and heparin in acute middle cerebral artery stroke. Stroke 1992;23:646–52 [DOI] [PubMed] [Google Scholar]
- 17.Levy D, Brott T, Haley C, et al. Factors related to intracranial hematoma formation in patients receiving tissue-type plasminogen activator for acute ischemic stroke. Stroke 1994;25:291–97 [DOI] [PubMed] [Google Scholar]
- 18.Jaillard A, Cornu C, Durieux A. Hemorrhagic transformation in acute ischemic stroke: the MAST-E study. Stroke 1999;30:1326–32 [DOI] [PubMed] [Google Scholar]
- 19.Larrue V, von Kummer R, del Zoppo G, et al. Hemorrhagic transformation in acute ischemic stroke potential contributing factors in the european cooperative acute stroke study. Stroke 1997;28:957–60 [DOI] [PubMed] [Google Scholar]
- 20.Demchuk A, Morgenstern L, Krieger D, et al. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke 1999;30:34–39 [DOI] [PubMed] [Google Scholar]
- 21.Kase C, Furlan AJ, Weschler L, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke:The PROACT II Trial. Neurology 2001;57. [DOI] [PubMed]
- 22.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). The Lancet 1998;353:1245–51 [DOI] [PubMed] [Google Scholar]
- 23.Weir C, Murray G, Dyker A, et al. Is hyperglycemia an independent predictor of poor outcome after acute stroke:Results of a long term follow up study. BMJ 1997;314:1303–06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidwell CS, Saver JL, Carnado J, et al. Predictors of hemorrhagic transformation in patients receiving intra-arterial thrombolysis. Stroke 2002;33:717. [DOI] [PubMed] [Google Scholar]
- 25.Yip HK, Chen MC, Chang HW, et al. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and no-reflow phenomenon. Chest 2002;122:1322–32 [DOI] [PubMed] [Google Scholar]