Abstract
BACKGROUND AND PURPOSE: [11C]Methionine (MET) PET imaging is a sensitive technique for visualizing primary brain tumors and recurrence/progression after therapy. The aim of this study was to evaluate the relationship between the uptake of MET and histopathologic grading and to investigate the prognostic value of the tracer, in both settings.
METHODS: Cerebral uptake of MET was determined in 52 patients: in 26 patients for primary staging (group A) and 26 patients with suspected brain tumor recurrence/progression after therapy (group B). Semiquantitative methionine uptake indices (UI) defined by the tumor (maximum)-to-background ratio was correlated with tumor grade and final outcome.
RESULTS: Overall median survival was 34.9 months. MET showed pathologically increased uptake in 41 of 52 scans. Although a weak linear correlation between MET uptake and grading was observed (R = 0.38, P = .028), analysis of variance showed no significant differences in MET UI between tumor grades for either group A or B. Benign and grade I lesions showed significant difference in MET uptake in comparison with higher grade lesions (P = .006). Using Kaplan-Meier survival analysis, no thresholds could be found at which MET was predictive for survival. Proportional hazard regression showed that only WHO grading class (low versus high) was predictive of survival (P = .015).
CONCLUSION: Interindividual MET uptake variability does not allow noninvasive grading on an individual patient basis. Moreover, there is no significant prognostic value in studying maximal methionine UI in brain tumors. The clinical use of MET should therefore be primarily focused on questions such as detection of recurrence, biopsy guidance, and radiation therapy target volume delineation.
In the management of brain tumors, accurate pathologic grading of the primary lesion is essential, because histologic features are a major independent prognostic factor next to patient age and performance status.1-4 Because tissue sampling is often obtained by stereotactic biopsy and therefore represents only a small part of the primary tumor, there is a probability of underestimating true tumor grading. Positron- emission tomography (PET) gives metabolic information on the whole tumor in vivo and can guide stereotactic biopsies toward the most metabolically active part of the tumor.5-7 [18F]fluorodeoxyglucose-PET (FDG) has proved to be of value in the assessment of tumor grading and recognizing malignant degeneration,5,6 in differentiating tumor recurrence from tumor necrosis,5,6,8-11 in predicting survival in glioma patients,12 and in therapy response assessment.13 Because many brain tumors also (over)express a variety of l-amino acid transporters, alternative functional biomarkers such as radiolabeled amino acids (eg, l-[11C]methyl-methionine) have been used to visualize amino acid transport and to some extent protein synthesis.14,15 Several studies have shown that methionine (MET) can be successfully applied in the follow-up of low-grade gliomas,16,17 in differentiating recurrent brain tumor from radiation injury,18,19 in guiding optimal biopsy location20 and surgical resection,21 and in delineating the radiation therapy target volume.17,22 There is, however, still some controversy regarding in vivo grading of brain tumors using amino acid analogues and their application as independent prognostic factors.
The aim of this study was 2-fold: (1) to evaluate the relationship between the semiquantitative uptake of MET and histopathologic grading in both primary and suspected recurrence of glioma and (2) to investigate the prognostic value of the MET regarding survival in both settings.
Materials and Methods
Patients
In this retrospective study, histopathologic grading and clinical follow-up data were available from 52 patients (33 male patients and 19 female patients; mean age, 41.5 years; range, 3–72 years [3 children younger than 16 years]) who were referred to the PET center for [11C]methionine-PET scan between 1998 and 2001: 26 patients on primary staging (group A) and 26 other patients with suspected brain tumor recurrence or progression after primary therapy (group B). All PET studies were approved by the local university hospital ethics committee. Follow-up was conducted until the last clinical contact with normal findings (n = 27; average, 24.2 months after PET; range, 1–52 months) or until death (n = 19; average, 13.5 months after PET; range, 1–42 months). Six patients were lost for further follow-up. Demographic, clinical, and histopathologic data are shown in Table 1.
Radiotracer Synthesis
Batches of [11C]CO2 were produced with the use of an IBA Cyclone 10/5 cyclotron (IBA, Louvain-la-Neuve, Belgium). MET was prepared by alkylation of l-homocysteine thiolactone with [11C]CH3I. The [11C]CH3I was prepared by reduction of [11C]CO2 to [11C]CH4 in an IBA synthesis module and subsequent iodination using a custom-built recirculation synthesis module. Quality control of MET was performed using gas chromatography to determine residual solvents; the radiochemical purity was assessed by high-performance liquid chromatography on an Alltech Partisil SCX column (Alltech, Deerfield, Ill). Radiochemical purity of MET was >95%.
Histologic Data
Histopathologic grading was done routinely by an experienced anatomopathologist and was classified according to the WHO system as given from the patients’ records (Table 1). To evaluate MET uptake index according to grade, 2 different grading classifications were used: (1) a classification according to the WHO system as such (grade I, grade I–II, grade II, grade II–III, grade III, grade III–IV, and grade IV) and (2), in those cases where an intermediate grading was given by the pathologist, grade was also reclassified to the higher number (eg, grade I–II was also reclassified as having grade II characteristics).
PET Data
Acquisition.
All PET data were acquired on an ECAT HR+ camera (Siemens, Knoxville, Tenn). PET was performed after at least 4 hours of fasting. Before emission scanning, a 2D interleaved transmission scan was carried out for attenuation correction. Twenty minutes after IV bolus injection of 220 MBq of MET, images were acquired in a 20-minute static scan in 3D acquisition mode. Only these late static images were used for this study. Reconstructed images were obtained by filtered back projection using the manufacturer’s software and postreconstruction Gaussian 2D filter smoothing (with a full-width-at-half-maximum of 6 mm).
Analysis.
All images were evaluated visually for the presence of a lesion. Radiologic (MR imaging/CT) images and protocols were available, but no formal digital coregistration to radiologic data was carried out.
For the semiquantitative analysis, the target-to-background ratio technique was used by a region of interest (ROI) approach. ROIs were manually defined with knowledge of the full clinical and histologic data and of the protocol results of the structural imaging modalities (CT and/or MR imaging). Because the region with the highest tracer uptake reflects the more metabolically active area of the tumor, a tumor ROI (T) was drawn around the lesion on the brain section containing the hottest pixels of the lesion.23 The peak pixel value was taken to define the (maximum) tumor uptake index (UI). Selection of these pixels was facilitated using a 10-step proportional color scale (Bronson 2-scale). If no focus of pathologic increased tracer uptake was found, ROI (T) was defined from visual determination using the structural images. Mean values of background/reference ROIs were obtained by drawing an ROI in the gray matter of the hemispherical half not affected by tumor or—when the tumor crossed the midline—the anterior or posterior quarter of that brain section. For infratentorial lesions, background ROIs were defined on the contralateral cortical hemisphere at a level above the basal ganglia.
Statistical Analysis
All data were analyzed using Statistica v.6.0 (StatSoft, Tulsa, Okla). A general linear model was used to compare MET uptake in both settings (initial staging and recurrence) and pathology grades. Survival intervals were measured from the time of the PET investigation until the date of last follow-up. Survival curves were calculated using the Kaplan-Meier technique. A proportional hazard regression model (Cox model) was used to investigate the predictive value of MET uptake. Correlation analysis was used to investigate a possible relationship between MET uptake index and grading. Discriminant analysis was used to investigate classification accuracy and a possible optimal threshold value for discrimination between histopathologic grades. Significance was defined at the P < .05 level.
Results
Patient Characteristics
The primary tumor type was pilocytic astrocytoma (WHO grade I) in 3, ganglioglioma (WHO grade I–II) in 1, low-grade astrocytoma (WHO grade II) in 7, anaplastic astrocytoma (WHO grade III) in 8, glioblastoma multiforme (WHO grade IV) in 4, 6 mixed oligoastrocytoma (WHO grade I in 1, grade II in 1, grade III in 1, and grade III–IV in 3), and 15 oligodendroglioma (WHO grade I–II in 3, grade II in 5, grade III in 4, grade III–IV in 1, and grade IV in 2). In 8 patients, no evidence of malignancy was found on histology.
Grading versus MET Uptake Index
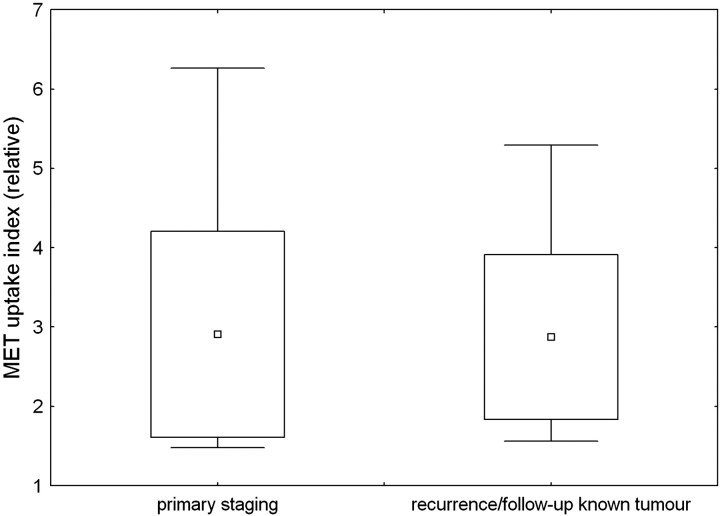
In 41 of 52 scans (79%), visual assessment of MET showed increased uptake compared with background levels. The UI was set to 1.0 for those scans where no abnormal uptake could be visualized. In abnormal scans, the UI ranged from 1.6 to 6.6 (mean, 2.9 ± 1.3) for group A and from 1.6 to 5.6 (mean, 2.9 ± 1.3) for group B. There was no significant difference in uptake between the groups (Fig 1).
Fig 1.
Tumor uptake index for the group of patients on primary staging and for the patients with suspected brain tumor recurrence or progression after primary therapy. Boxes show 1 SD from the mean value; whiskers show the 95% confidence interval limits. There was no significant difference in uptake between both groups.
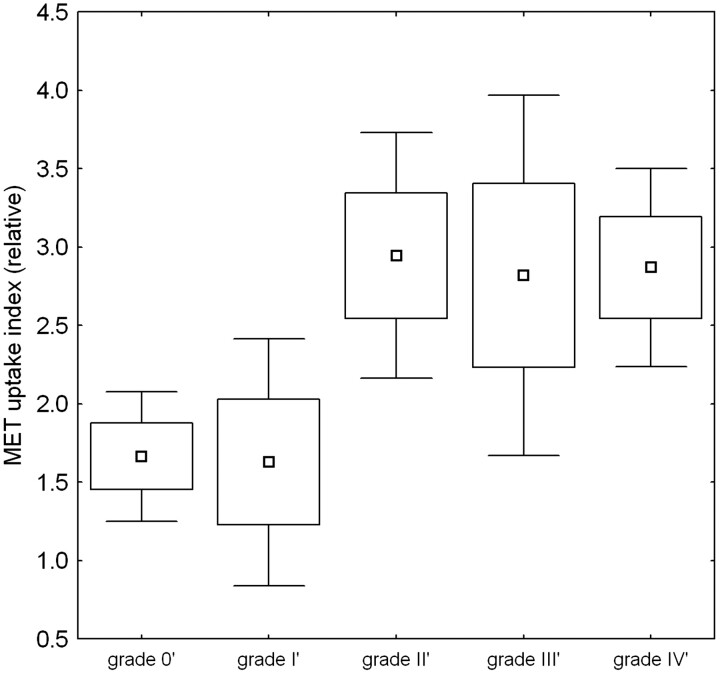
Because there is a tendency in gliomas to progress to more malignant histologic types over time,24 for studying the relationship between UI and grading, only those data in which the time between grading and PET scan did not exceed 12 months were considered, resulting in 43 retained cases. Figure 2 shows the variation in UI with grading. Although a linear correlation between MET uptake and grading was observed (R = 0.34, P = .027) with a lower uptake in benign and grade I lesions, analysis of variance revealed no significant overall MET UI differences between tumor grades (P = .11). When regrouping benign and grade I lesions together, there was a significant difference with grade II–IV lesions: UI = 1.65 versus 2.88, P = .006. In this classification, discriminant analysis showed a total discriminative power of 70% for the whole group (under equal a priori classification probability).
Fig 2.
[11C]Methionine maximum uptake index as a function of grading. Grading values are regrouped as indicated in the text. Grade 0′ (benign) and grade I′ (WHO grade I and intermediate grade I–II) show significantly reduced uptake compared with grade II′–IV′ lesions. Box plots show the mean value, and whiskers show the 95% confidence interval limits. Boxes show 1 SE; whiskers show the 95% confidence interval limits.
Because there is a possible confounding effect of high MET uptake in oligodendroglioma and pilocytic astrocytoma, known for high uptake also in low grades,25 the subgroup of astrocytoma tumors was also analyzed separately. Without oligodendroglioma and pilocytic astrocytoma, the correlation was similar (R = 0.38, P = .028), and analysis of variance revealed no significant differences between individual grading groups.
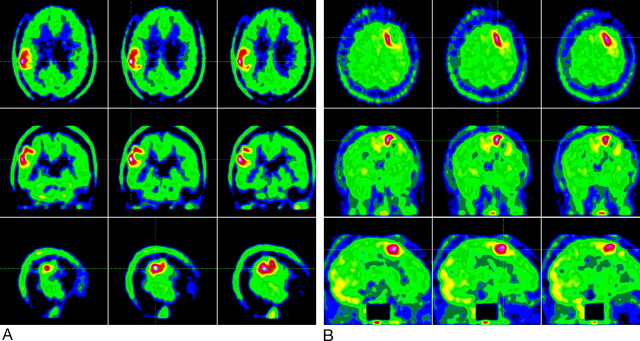
Figure 3 shows example images of MET PET in a patient with grade I–II and another patient with grade IV astrocytoma but comparable MET uptake.
Fig 3.
Example of [11C]methionine PET of a patient with a grade I–II tumor (woman, 31 years old, oligodendroglioma in follow-up) (A) and a patient with a grade IV tumor (woman, 33 years old, glioblastoma multiforme in follow-up), showing comparable tracer uptake (B).
Survival Analysis
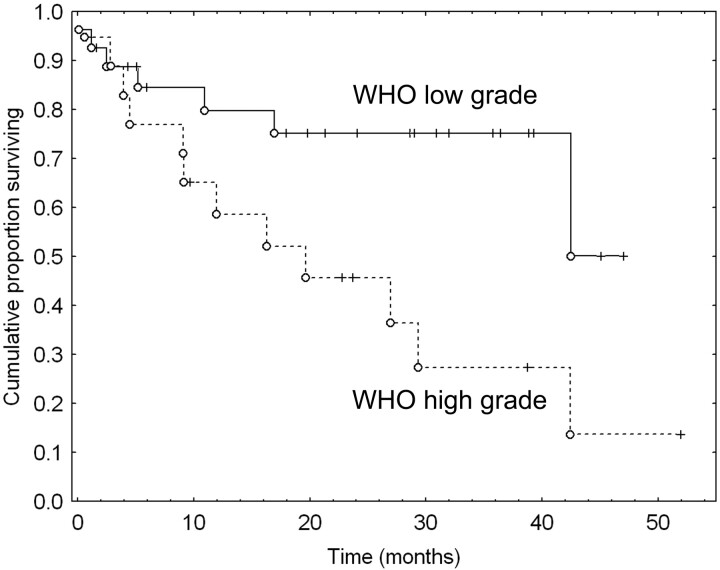
Overall median survival was 34.9 months. In a proportional hazard Cox regression model with age, WHO grading, and MET UI, only WHO grading was significantly predictive of survival (P = .015), whereas age (P = .7) and UI (P = .5) were not significantly predictive. Classification schemes using Kaplan-Meier statistics were calculated for a number of empirically determined cutoff values between 1.6 and 3.0. No thresholds could be found at which MET could be considered predictive of survival. Differences in survival were found only for WHO low-grade versus high-grade (III-IV) tumors (P = .017) (Fig 4). The same results were obtained in the subpopulation without pilocytic astrocytoma and oligodendroglioma tumors.
Fig 4.
Kaplan-Meier (cumulative proportion surviving) curves for WHO low-grade versus high-grade tumors. ○, death; “+” values (patients lost to follow-up).
Discussion
In general, treatment of brain tumors consists of surgical resection, followed by radiation therapy and/or chemotherapy. To determine appropriate individual treatment, accurate pathologic grading is essential. Histopathologic examination is still considered the “gold standard” for determination of tumor grading but is often obtained by stereotactic biopsy and may represent only a small part of the primary tumor. Because many brain tumors, especially gliomas, are heterogeneous and contain microscopic areas of necrosis, there is a probability of underestimating true tumor grading, so in vivo imaging measures of grading would offer advantageous opportunities. In addition, tumor grading is reported as one of the most robust predictors of survival throughout the literature. The aim of this retrospective analysis was to evaluate the value of noninvasive histopathologic grading based on the semiquantitative uptake of MET and to investigate if additional prognostic value could be attributed to semiquantitative tracer uptake, both in a primary and a post-therapeutic setting.
Although a weak positive correlation between MET uptake and grading was found on a group basis, no significant differences between individual grades was found. Only between grade I and higher-graded tumors could a significant difference be found. However, even though only those data where the time between grading and PET scan did not exceed 12 months were considered, it should be emphasized that there is a tendency in gliomas to progress to more malignant histologic types over time,24 which might have had an influence on our results.
Our findings confirm previous reports in the setting of grading of primary brain tumors.26-29 In most of these studies, a weak relationship between MET uptake and the histologic WHO grade of gliomas was noted. Early reports by Derlon et al27 in 22 patients showed increasing uptake with grade II–IV primary glioma, and Ogawa et al28 found, in 50 patients with primary tumors, that a significant difference between low- and high-grade glioma was present (P < .001) but noted high variability and poor in vivo classification accuracy for individual subjects based on MET alone. Kaschten et al26 evaluated 41 primary glioma patients and found no differentiation possible between grade II and III tumors, which is in line with our findings (Fig 3). Utriainen et al29 specifically studied 27 childhood primary brain tumors and also found an increased uptake of MET but again with a considerable overlap. In the setting of recurrence, where there is an increased chance of anaplastic alterations of the primary tumors associated with higher MET uptake, no systematic study on in vivo grading of recurrent brain tumors has been conducted to our knowledge. Our results in this group are in agreement with the findings in primary tumor setting.
The absence of a clear relationship with grading is most likely the consequence of the mechanism of uptake of amino acid analogues. At the normal blood-brain barrier, the main mechanism of amino acid uptake into the cytoplasm of the tumor occurs via sodium-dependent transport systems in the cell membrane,30 one of them being the A (alanine-preferring) system of short-chain, neutral amino acid transport. A variety of factors can influence the activity of this system, including pH, hormones, growth factors, amino acid availability, and cellular proliferation rate. Although an acidic extracellular milieu decreases the activity, the system is overexpressed in neoplastic cells and this seems to be correlated to tumor cell growth rate.31 Apart from overexpression of the amino acid transporter, which parallels the degree of anaplasia,32 other factors may be responsible for variable uptake, such as microvessel attenuation. Studies have shown a strong correlation between MET uptake and microvessel attenuation, indicating a strong relation between increased amino acid uptake and angiogenesis in evolving gliomas.25 A study by Kiessling et al33 using perfusion MR imaging showed a correlation between perfusion and microvessel attenuation, whereas permeability correlated moderately with maximal constrast enhancement but not with perfusion and microvessel attenuation. Tumor growth depends on sufficient oxygen and nutrients supplied by the development of angiogenesis. However, the resulting vascular system is irregular and heterogeneous, resulting in areas of rich and areas of poor vascular attenuation. Bogin et al34 evaluated the distribution of permeability and flow and showed a high capillary permeability, especially in new microcapillaries, and a progressive decrease in permeability as the microvessels mature during tumor growth.
Tumor cells in vitro can also up-regulate amino acid transporters in their cell membranes when growth conditions become adverse and less than optimal. This phenomenon may also occur in vivo and could be the basis for selective imaging of brain tumors by radiolabeled amino acids such as methionine.35 Because the bloodstream provides an available pool of amino acids that can be taken up by all cells of the body, disruption of the blood-brain barrier can be responsible for increased availability and transport of methionine, and it is known that such rupture can also be present in (radio)necrosis without active tumor tissue.36 Aspecific inflammatory changes resulting in an increased FDG-uptake, as seen in radiation injury and normal healing processes, up to 3 months in the immediate postsurgical period, may also cause increased MET uptake.15 However, whether this initial increase of MET uptake immediately after therapy represents an acute reaction to therapy or a lack of treatment efficiency has not yet been determined.37 Considering our own patient population, 26 patients received therapy and 2 underwent stereotactic biopsy before PET scan. Of these interventions, 6 took place within the critical interval of 3 months before MET-PET (range, 2–7 weeks before PET). In only 1 patient with a stereotactic biopsy 2 weeks earlier than MET-PET was a clearly higher tracer uptake seen compared with the other patients within the same grading group (MET UI 6.59 versus 2.25–3.4 in grading group III). Of all patients (group A and B), 8 patients were concurrently being treated with steroids at the time of PET scan. Although amino acids can diffuse into cells, the accumulation of methionine is due largely to carrier-mediated transport,30 which is not substantially reduced by dexamethasone.38 Therefore, a possible confounding effect of steroid treatment is unlikely to be important.
As for assessing the prognosis of primary brain tumors using MET, De Witte et al39 found that high MET uptake represents a prognostic factor for WHO grade II and III tumors considered separately; high quantitative uptake is associated with poor survival. Our series was not large enough to be able to separate all grading classes separately, so these results could not be compared with sufficient statistical power. Interestingly, Weckesser et al found that in primary tumors, the extent but not maximum uptake of iodo-α-methyltyrosine uptake on single-photon emission CT (SPECT) was an independent prognostic factor.40 As in our study, where we were not able to digitally coregister anatomic information, the viable tumor extent was not used as parameter. It does seem plausible, however, that total active tumor burden (in combination with location) might be a more appropriate prognostic factor where amino acid imaging can play an important role. Schmidt et al also used the same tracer and could detect no relationship between cerebral primary astrocytoma uptake intensity and survival time.41
In a previous study comparing FDG to MET PET in recurrence detection and prognosis, we have shown that MET maximum uptake with cutoff values between 2.0 and 2.4 just reached significance in predicting survival, a finding that was restricted to the setting of recurrence in a group of primary astrocytoma42 and a finding that was in line with that of a study of Kim et al,43 suggesting that MET PET is a useful biologic prognostic marker in glioma patients. Extension of this subgroup and further follow-up in our current study did not allow us to reach the same conclusions however. A combination of the information given by MET and FDG provides a more detailed metabolic picture of the regional situation of the tumor, as well as a better prediction of histologic grade of astrocytoma,44 suggesting a complementary role of these tracers. In most clinical situations, however, a choice has to be made between these radioligands. The use of amino acid tracers could be advocated for several reasons. MET shows a higher sensitivity for detection and delineation of a tumor. Even brain lesions that show hypometabolism or isometabolism on FDG PET can be detected and differentiated with high sensitivity by using methionine.45
In a study by Weckesser et al,46 the differential uptake of O-(2-[18F]fluorethyl)-l-tyrosine (FET) was evaluated in suspected primary brain tumors, showing different uptake kinetics of FET in high- and low-grade brain tumors. Other PET imaging ligands that reflect cellular turnover, such as [18F]fluorothymidine,47 might be better suited for grading purposes and might have more predictive power with respect to tumor progression and survival,48 but further studies are needed to confirm these results.
Conclusion
We found that although MET uptake in brain tumors is positively correlated with malignancy grade, interindividual tumor uptake variability of [11C]methionine did not allow noninvasive grading on an individual patient basis in either clinical setting (primary tumor versus recurrence detection).
Moreover, we found no significant prognostic value in studying the maximal methionine uptake index in the tumor. Despite some remaining controversy in the literature on grading and prognosis, the optimal clinical use of MET PET as an in vivo biomarker for brain tumors should be primarily focused on detection of recurrence, guidance for biopsies, and delineation of radiation therapy target volume.
Demographic, clinical, and histopathologic data of patients included in this study
| Gender | |
| Male | 33 |
| Female | 19 |
| Age (y) | |
| Average | 41.5 |
| Range | 3–72 |
| WHO classification | |
| Pilocytic astrocytoma | |
| Grade I | 3 |
| Ganglioglioma | |
| Grades I–II | 1 |
| Low-grade astrocytoma | |
| Grade II | 7 |
| Anaplastic astrocytoma | |
| Grade III | 8 |
| Glioblastoma multiforme | |
| Grade IV | 4 |
| Mixed oligoastrocytoma | 6 |
| Grade I | 1 |
| Grade II | 1 |
| Grade III | 1 |
| Grades III–IV | 3 |
| Oligodendroglioma | 15 |
| Grades I–II | 3 |
| Grade II | 5 |
| Grade III | 4 |
| Grades III–IV | 1 |
| Grade IV | 2 |
| No evidence of malignancy | 8 |
| Therapy prior to PET scan | |
| Surgery | 5 |
| Surgery and radiotherapy | 10 |
| Radiotherapy | 7 |
| Chemotherapy | 1 |
| Radiotherapy with chemotherapy | 3 |
| Surgery, radiotherapy, and chemotherapy | 2 |
| Therapy after PET scan | |
| Surgery | 8 |
| Surgery and radiotherapy | 3 |
| Radiotherapy | 4 |
| Chemotherapy | 4 |
| Surgery and chemotherapy | 2 |
| Radiotherapy and chemotherapy | 3 |
| Surgery, radiotherapy, and chemotherapy | 2 |
Acknowledgments
The technical assistance of Marva Bex and the PET radiopharmacy group is greatly appreciated.
Footnotes
S.C. and K.V.L. contributed equally to this work.
References
- 1.DeAngelis LM. Brain tumors. N Engl J Med 2001;344:114–23 [DOI] [PubMed] [Google Scholar]
- 2.Berg G, Blomquist E, Cavallin-Stahl E. A systematic overview of radiation therapy effects in brain tumours. Acta Oncol 2003;42:582–88 [DOI] [PubMed] [Google Scholar]
- 3.Barker FG, Chang SM, Gutin PH, et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 1998;42:709–20 [DOI] [PubMed] [Google Scholar]
- 4.Kondziolka D, Flickinger JC, Bissonette DJ, et al. Survival benefit of stereotactic radiosurgery for patients with malignant glial neoplasms. Neurosurgery 1997;41:776–83 [DOI] [PubMed] [Google Scholar]
- 5.Mirzaei S, Knoll P, Kohn H. Diagnosis of recurrent astrocytoma with fludeoxyglucose F18 PET scanning. N Engl J Med 2001;344:2030–31 [DOI] [PubMed] [Google Scholar]
- 6.Goldman S, Levivier M, Pirotte B, et al. Regional glucose metabolism and histopathology of gliomas. A study based on positron emission tomography-guided stereotactic biopsy. Cancer 1996;78:1098–106 [DOI] [PubMed] [Google Scholar]
- 7.Pirotte B, Goldman S, Massager N, et al. Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J Nucl Med 2004;45:1293–98 [PubMed] [Google Scholar]
- 8.Langleben DD, Segall GM. PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med 2000;41:1861–67 [PubMed] [Google Scholar]
- 9.Barker FG, Chang SM, Valk PE, et al. 18-Fluorodeoxyglucose uptake and survival of patients with suspected recurrent malignant glioma. Cancer 1997;79:115–26 [PubMed] [Google Scholar]
- 10.Chao ST, Suh JH, Raja S, et al. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer 2001;96:191–97 [DOI] [PubMed] [Google Scholar]
- 11.Di CG, Oldfield E, Wright DC, et al. Cerebral necrosis after radiotherapy and/or intraarterial chemotherapy for brain tumors: PET and neuropathologic studies. AJR Am J Roentgenol 1988;150:189–97 [DOI] [PubMed] [Google Scholar]
- 12.Ribom D, Eriksson A, Hartman M, et al. Positron emission tomography 11C-methionine and survival in patients with low-grade gliomas. Cancer 2001;92:1541–49 [DOI] [PubMed] [Google Scholar]
- 13.Brock CS, Young H, O’Reilly SM, et al. Early evaluation of tumour metabolic response using [18F]fluorodeoxyglucose and positron emission tomography: a pilot study following the phase II chemotherapy schedule for temozolomide in recurrent high-grade gliomas. Br J Cancer 2000;82:608–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishiwata K, Kubota K, Murakami M, et al. Re-evaluation of amino acid PET studies: can the protein synthesis rates in brain and tumor tissues be measured in vivo? J Nucl Med 1993;34:1936–43 [PubMed] [Google Scholar]
- 15.Jager PL, Vaalburg W, Pruim J, et al. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med 2001;42:432–45 [PubMed] [Google Scholar]
- 16.Ribom D, Engler H, Blomquist E, et al. Potential significance of (11)C-methionine PET as a marker for the radiosensitivity of low-grade gliomas. Eur J Nucl Med Mol Imaging 2002;29:632–40 [DOI] [PubMed] [Google Scholar]
- 17.Nuutinen J, Sonninen P, Lehikoinen P, et al. Radiotherapy treatment planning and long-term follow-up with [(11)C]methionine PET in patients with low-grade astrocytoma. Int J Radiat Oncol Biol Phys 2000;48:43–52 [DOI] [PubMed] [Google Scholar]
- 18.Sonoda Y, Kumabe T, Takahashi T, et al. Clinical usefulness of 11C-MET PET and 201T1 SPECT for differentiation of recurrent glioma from radiation necrosis. Neurol Med Chir (Tokyo) 1998;38:342–47 [DOI] [PubMed] [Google Scholar]
- 19.Ogawa T, Kanno I, Shishido F, et al. Clinical value of PET with 18F-fluorodeoxyglucose and l-methyl-11C-methionine for diagnosis of recurrent brain tumor and radiation injury. Acta Radiol 1991;32:197–202 [PubMed] [Google Scholar]
- 20.Pirotte B, Goldman S, Salzberg S, et al. Combined positron emission tomography and magnetic resonance imaging for the planning of stereotactic brain biopsies in children: experience in 9 cases. Pediatr Neurosurg 2003;38:146–55 [DOI] [PubMed] [Google Scholar]
- 21.Pirotte B, Goldman S, Van BP, et al. Integration of [11C]methionine-positron emission tomographic and magnetic resonance imaging for image-guided surgical resection of infiltrative low-grade brain tumors in children. Neurosurgery 2005;57:128–39 [DOI] [PubMed] [Google Scholar]
- 22.Levivier M, Wikler D, Jr., Massager N, et al. The integration of metabolic imaging in stereotactic procedures including radiosurgery: a review. J Neurosurg 2002;97:542–50 [DOI] [PubMed] [Google Scholar]
- 23.Meyer PT, Schreckenberger M, Spetzger U, et al. Comparison of visual and ROI-based brain tumour grading using 18F-FDG PET: ROC analyses. Eur J Nucl Med 2001;28:165–74 [DOI] [PubMed] [Google Scholar]
- 24.Dropcho EJ, Soong SJ. The prognostic impact of prior low grade histology in patients with anaplastic gliomas: a case-control study. Neurology 1996;47:684–90 [DOI] [PubMed] [Google Scholar]
- 25.Kracht LW, Friese M, Herholz K, et al. Methyl-[11C]-l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging 2003;30:868–73 [DOI] [PubMed] [Google Scholar]
- 26.Kaschten B, Stevenaert A, Sadzot B, et al. Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med 1998;39:778–85 [PubMed] [Google Scholar]
- 27.Derlon JM, Bourdet C, Bustany P, et al. [11C]l-methionine uptake in gliomas. Neurosurgery 1989;25:720–28 [DOI] [PubMed] [Google Scholar]
- 28.Ogawa T, Shishido F, Kanno I, et al. Cerebral glioma: evaluation with methionine PET. Radiology 1993;186:45–53 [DOI] [PubMed] [Google Scholar]
- 29.Utriainen M, Metsahonkala L, Salmi TT, et al. Metabolic characterization of childhood brain tumors: comparison of 18F-fluorodeoxyglucose and 11C-methionine positron emission tomography. Cancer 2002;95:1376–86 [DOI] [PubMed] [Google Scholar]
- 30.Souba WW, Pacitti AJ. How amino acids get into cells: mechanisms, models, menus, and mediators. JPEN J Parenter Enteral Nutr 1992;16:569–78 [DOI] [PubMed] [Google Scholar]
- 31.Bading JR, Kan-Mitchell J, Conti PS. System A amino acid transport in cultured human tumor cells: implications for tumor imaging with PET. Nucl Med Biol 1996;23:779–86 [DOI] [PubMed] [Google Scholar]
- 32.Goldman S, Levivier M, Pirotte B, et al. Regional methionine and glucose uptake in high-grade gliomas: a comparative study on PET-guided stereotactic biopsy. J Nucl Med 1997;38:1459–62 [PubMed] [Google Scholar]
- 33.Kiessling F, Krix M, Heilmann M, et al. Comparing dynamic parameters of tumor vascularization in nude mice revealed by magnetic resonance imaging and contrast-enhanced intermittent power Doppler sonography. Invest Radiol 2003;38:516–24 [DOI] [PubMed] [Google Scholar]
- 34.Bogin L, Margalit R, Mispelter J, et al. Parametric imaging of tumor perfusion using flow- and permeability-limited tracers. J Magn Reson Imaging 2002;16:289–99 [DOI] [PubMed] [Google Scholar]
- 35.Sasajima T, Miyagawa T, Oku T, et al. Proliferation-dependent changes in amino acid transport and glucose metabolism in glioma cell lines. Eur J Nucl Med Mol Imaging 2004;31:1244–56 [DOI] [PubMed] [Google Scholar]
- 36.Jacobs A. Amino acid uptake in ischemically compromised brain tissue. Stroke 1995;26:1859–66 [DOI] [PubMed] [Google Scholar]
- 37.Herholz K, Kracht LW, Heiss WD. Monitoring the effect of chemotherapy in a mixed glioma by C-11-methionine PET. J Neuroimaging 2003;13:269–71 [PubMed] [Google Scholar]
- 38.Herholz K, Holzer T, Bauer B, et al. 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology 1998;50:1316–22 [DOI] [PubMed] [Google Scholar]
- 39.De Witte O, Goldberg I, Wikler D, et al. Positron emission tomography with injection of methionine as a prognostic factor in glioma. J Neurosurg 2001;95:746–50 [DOI] [PubMed] [Google Scholar]
- 40.Weckesser M, Matheja P, Schwarzrock A, et al. Prognostic significance of amino acid transport imaging in patients with brain tumors. Neurosurgery 2002;50:958–64 [DOI] [PubMed] [Google Scholar]
- 41.Schmidt D, Gottwald U, Langen KJ, et al. 3-[123I]Iodo-alpha-methyl-L-tyrosine uptake in cerebral gliomas: relationship to histological grading and prognosis. Eur J Nucl Med 2001;28:855–61 [DOI] [PubMed] [Google Scholar]
- 42.Van LK, Ceyssens S, Van CF, et al. Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, inter-observer variability and prognostic value. Eur J Nucl Med Mol Imaging 2005;32:39–51 [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Chung JK, Im SH, et al. 11C-methionine PET as a prognostic marker in patients with glioma: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging 2005;32:52–59 [DOI] [PubMed] [Google Scholar]
- 44.Giammarile F, Cinotti LE, Jouvet A, et al. High and low grade oligodendrogliomas (ODG): correlation of amino-acid and glucose uptakes using PET and histological classifications. J Neurooncol 2004;68:263–74 [DOI] [PubMed] [Google Scholar]
- 45.Chung JK, Kim YK, Kim SK, et al. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging 2002;29:176–82 [DOI] [PubMed] [Google Scholar]
- 46.Weckesser M, Langen KJ, Rickert CH, et al. O-(2-[18F]Fluorethyl)-l-tyrosine PET in the clinical evaluation of primary brain tumours. Eur J Nucl Med Mol Imaging 2005;32:422–29 [DOI] [PubMed] [Google Scholar]
- 47.Choi SJ, Kim JS, Kim JH, et al. [18F]3′-deoxy-3′-fluorothymidine PET for the diagnosis and grading of brain tumors. Eur J Nucl Med Mol Imaging 2005 [DOI] [PubMed]
- 48.Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med 2005;46:945–52 [PubMed] [Google Scholar]