1. Introduction
The effects of neurodegeneration on the experience of pain remain poorly understood, despite the risk of suffering from both pain and neurodegenerative diseases rising concurrently with age.63,75 Given the anticipated increase in magnitude and median age of the global population,76,152 the interaction of these 2 clinically unmet needs will become an increasingly pressing challenge. In particular, a significant proportion of patients with Alzheimer disease (AD) and Parkinson disease (PD), the 2 most prevalent neurodegenerative diseases, suffer chronic pain of variable origin (Box 1). As such, they have been the most extensively studied and, for brevity, will be the focus of this review. Persistent pain in AD and PD is partially attributable to various concomitant disease manifestations and comorbidities (Fig. 1).43,117 In addition, disease-specific neurodegenerative changes may affect a multitude of regions implicated in the perceptual and cognitive processes underlying pain. Despite this, the precise perceptual sequelae of neurodegenerative pathophysiology in these 2 diseases remain equivocal, and whether this may result in differential responses to analgesic treatment remains largely unexplored.
Box 1. Definitions.
Neurodegenerative disease: a heterogeneous group of disorders that are characterized by the progressive degeneration of the structure and function of the central nervous system or peripheral nervous system.
Dementia: a syndrome that involves severe loss of cognitive abilities as a result of disease or injury. Dementia caused by traumatic brain injury is often static, whereas dementia caused by neurodegenerative disorders, such as AD, is usually progressive and can eventually be fatal.
Alzheimer disease: a progressive neurodegenerative disease that impairs memory and cognitive judgment and is often accompanied by mood swings, disorientation, and eventually delirium. The most common cause of dementia.
Parkinson disease: a progressive neurodegenerative disorder, characterized by motor symptoms, such as tremor, rigidity, slowness of movement, and problems with gait. Motor symptoms are often accompanied with fatigue, depression, pain, and cognitive problems.
Figure 1.
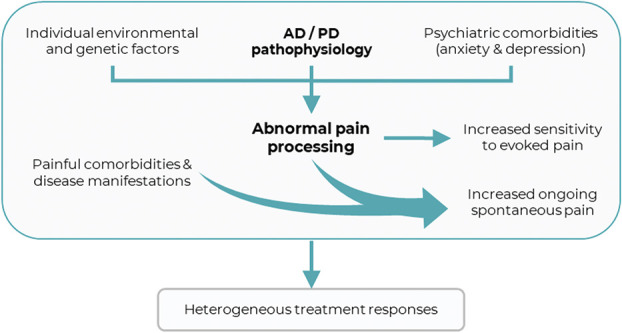
Conceptual framework relating the respective neurodegenerative pathophysiology within AD and PD to pain processing and treatment. AD, Alzheimer disease; PD, Parkinson disease.
Three key principles lay conceptual foundations for the investigation of the effects of neurodegenerative pathophysiology on treatment mechanisms: (1) a given intensity of stimulus produces heterogeneous levels of reported pain and unpleasantness,30,60,110,109,111,154 (2) genetic and environmental factors predispose some to chronic pain,1,47,48,87 and (3) diversity of pain physiology and pathophysiology results in heterogeneous responses to pharmacotherapy46,100,134,153. Collectively, these support the notion that heterogeneous physiology and pathophysiology can give rise to divergent treatment responses. Within this framework, neurodegeneration and its effects on the central nervous system can be considered as one such external factor contributing to heterogeneity, resulting in putative perturbation of pain processing (1 and 2) and responses to analgesic treatments (3) (Fig. 1).
Chronic pain in AD and PD not only impacts patients' quality of life but also presents a formidable healthcare and socioeconomic challenge. Drugs available for treatment of chronic pain are associated with high numbers needed to treat and may have serious side effects.145 Moreover, poorly managed pain is associated with depression,33 anxiety,139 and functional loss.38 Given the high prevalence of pain and frailty in these patient groups, clear scientific rationale is imperative to ensure safe and effective clinical management (Fig. 2). In this article, we discuss pain processing and perception in AD and PD as well as its emerging relevance to pharmacological treatment.
Figure 2.
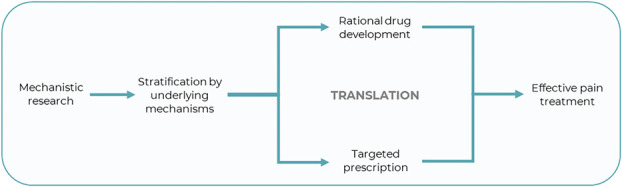
Importance of mechanistic research for evidence-based pain medicine.
2. Alzheimer disease
Alzheimer disease is the most common form of dementia affecting more than 45 million people worldwide119 and is clinically characterised by progressive cognitive deterioration.25,43,77 The prevalence of chronic pain in dementia is between 30% and 80%.43 However, patients with AD do not report pain as often and are prescribed analgesics less frequently, compared with healthy age-matched individuals.34,129 Pain is a key trigger for behavioural and psychological symptoms of dementia such as agitation and mood disorders, which are a major treatment challenge and can result in overprescribing of harmful antipsychotic medications.10,52,123 Pathologically, the basal forebrain and medial temporal lobe are amongst the first regions affected before progression to neocortical regions.18,108 Notably, the sensory cortices remain relatively unaffected until terminal stages. The significance of this is multifaceted: (1) the regions affected partially overlap with regions implicated in the processing of pain, (2) the regions affected are believed to be involved more in emotional-affective rather than sensory-discriminative dimensions, and (3) the cognitive deficits within memory, attention, and communication render self-report of pain increasingly unreliable with disease severity. Specifically, a reduced capacity to comprehend and complete standardised pain assessments as well as an overall reduction in reporting of pain.2,78,84,113 Therefore, altered pain processing (1 and 2) is challenging to disentangle from a diminished capacity to accurately provide self-report (3), highlighting the need for investigation at a mechanistic level.
2.1. Pain processing is altered in Alzheimer disease
Many psychophysical studies investigating noxious stimuli have demonstrated altered pain processing in AD compared with healthy controls. However, the directionality of these changes remain equivocal. Thresholds have been reported to be increased15,35,66,106 or similar to cognitively intact controls.15,81,79,82,93 Similarly, pain tolerance has been reported to be reduced,11,35,79,82 equal,35,66,81,89,88 and increased.122 In addition, behavioural responses to pain have been shown to be augmented in AD,72,89,88 with enhanced facial responses throughout the spectrum of disease severity.12 Patients with AD have also shown a reduced threshold in the nociceptive flexion reflex (NFR), possibly indicating differences in pain processing further down the neuroaxis.89 Overall, disparities are likely due to differences in pathophysiological mechanisms, disease progression, modalities of evoked pain used, and, crucially, outcome measures used. Collectively, these findings allude to patients with AD potentially suffering more despite reporting pain less.
Neuroimaging studies have suggested that neural activity in patients with AD may be augmented in response to noxious stimulation, despite relative preservation of sensory-discriminative facets of pain. Patients show greater amplitude and duration of blood oxygenation level dependent (BOLD) signals (an indirect index of brain activity relating to neurovascular coupling) during noxious pressure stimulation within sensory, affective, and cognitive regions, including the dorsolateral prefrontal cortex (dlPFC).35 Consistent with altered cognition being functionally related to pain processing, patients also show enhanced functional connectivity between the dlPFC and anterior midcingulate, periaqueductal grey (PAG), thalamus, and hypothalamus.36 Indeed, the dlPFC plays a central role in both general cognitive function70 as well as pain modulation.95,130,149 Furthermore, diffusion tensor imaging has evidenced anatomical connectivity between the right dlPFC, hypothalamus, and PAG,71 in which activity has been associated with pain-related escape responses in rodents.86,98 This may reflect a failure to adequately contextualise and appraise painful experiences resulting in uncertainty and a higher threat value ascribed to noxious stimulation. Furthermore, a lack of contextualising features within scanning environments may compound this.36 Delineation of the impact of context and setting warrants further investigation. Collectively, neuroimaging studies indicate greater emotional reactivity and pain processing, despite equal or mildly diminished thresholds.
The implication of regions including the dlPFC, PAG, and hypothalamus overlaps with the neural substrates of placebo analgesia through which context and expectation can profoundly alter treatment responses.36,118,150 Patients with AD with reduced frontal lobe function exhibited diminished placebo responses in an open-hidden paradigm, requiring escalation of analgesic dose.16 Furthermore, executive function is the domain of cognition that best predicts variance in facial responsiveness to noxious electrical stimulation and the NFR.90 Thus, patients with milder disease severity may benefit more from analgesics because of relative preservation of placebo mechanisms. The placebo response is engaged in the administration of all pharmacotherapy to some extent and accounts for a large portion of the reduction in pain produced, over and above pharmacological efficacy.14,17,37,148 Therefore, patients with attenuated placebo responses should require larger doses to produce the same level of analgesia as controls. Worryingly, as AD and age progress, patients become increasingly frail, hence dose escalation may be a major concern given that age is a significant predictor of opioid-related harm.28,57,85 Placebo analgesia and opioid analgesia partially share neuroanatomical substrates; covariation has been observed between the activity in the rostral anterior cingulate cortex (ACC) and the brainstem during both placebo and opioid analgesia, but not during pain alone.114,135 Postmortem AD brains also show reduced μ-/δ-opioid receptor binding.104 Patients with AD may thus present alterations in centrally mediated opioid analgesia. Further application of open-hidden paradigms alongside pharmacoimaging may offer insights into how the combined magnitude of pharmacological and placebo analgesia can be maximised clinically.
2.2. Pharmacotherapy of pain in Alzheimer disease
Overall, patients with AD seem to be prescribed fewer analgesics than healthy individuals.10,73,128 Conversely, recent studies from Scandinavia have reported an opposite trend.80,96,126 Paracetamol/acetaminophen remain the principal treatment for mild-to-moderate pain in AD with additional use of nonsteroidal anti-inflammatory drugs and opioids.3 However, studies providing mechanistic insight remain scarce.3,53 For example, of the 3 randomised control trials (RCTs) investigating opioids, 2 were underpowered and in one investigating the buprenorphine transdermal system, 23 of the 44 patients withdrew treatment because of adverse events.52,97,101 No trials have investigated antidepressants and antiepileptics.3,77 Further RCTs will be necessary to not only produce evidence-based treatment guidelines but also to provide insights into the putative perturbation of neurotransmitter systems.
3. Parkinson disease
Pain is a prevalent nonmotor symptom in people with PD (PwP), acknowledged by James Parkinson in 1817,112 affecting 68% to 85% of patients.13,23,103,116,127 Despite this, it remains underdiagnosed and undertreated.6,31,41,58,83,156 Pain in PwP is multifaceted and may result from comorbidities, be caused or amplified by motor symptoms, and is subject to abnormal nociceptive processing, as PD-specific neurodegeneration affects peripheral, spinal, and cerebral pain pathways.42,125 Attempts have been made to synthesize a clear picture of heterogeneous pain in PD (Table 1)6,58,151; however, to date, our basic understanding of the relationship between PD pathophysiology and pain remains underdeveloped. Identifying well-defined subtypes, and elucidating their concomitant underlying mechanisms, should facilitate the development of personalised treatment of pain in PwP.24,143
Table 1.
Overview of the classification systems to date for pain in people with Parkinson disease.
| Quinn et al.121 | A) Pain preceding diagnosis of Parkinson disease B) Off-period pain (without dystonia) in patients with a fluctuating response to levodopa 1. Morning pain 2. Wearing-off pain 3. Beginning-of-dose pain 4. End-of-dose pain C) Painful dystonic spasms 1. Early morning dystonia 2. Off-period dystonia 3. Beginning-of-dose dystonia 4. End-of-dose dystonia D) Peak-dose pain |
| Ford61 | 1. Musculoskeletal (aching, cramping, arthralgic, and myalgic sensations in joints and muscles) 2. Radicular/neuropathic (pain in a root or nerve territory) 3. Dystonia (associated with sustained twisting movements and postures) 4. Central or primary pain (burning, tingling, formication, and “neuropathic” sensations, often relentless and bizarre in quality) 5. Akathisia (subjective sense of restlessness, often accompanied by an urge to move) |
| Wasner and deuschl151 | A) Pain related to Parkinson disease: 1. Nociceptive: Musculoskeletal (joint pain, pain linked to motor fluctuations—dystonic or nondystonic, back pain, and pain linked to autonomic failure), visceral (abdominal pain, gastrointestinal discomfort, constipation, and involuntary dystonic contraction of anal sphincter), and cutaneous (pressure sores) 2. Neuropathic: Peripheral (radicular) or central Parkinson pain 3. Miscellaneous: pain preceding Parkinson disease, pain linked to restless leg syndrome and akathisia, and pain linked to depression. B) Pain unrelated to Parkinson disease —different pain syndromes. |
| Chaudhuri et al.32 | 1. Musculoskeletal pain (pain around joints) 2. Chronic pain (a generalised constant, dull, aching pain or pain related to an internal organ) 3. Fluctuation-related pain (dyskinetic pain, “off”-period dystonia, and generalised “off”-period pain) 4. Nocturnal pain (pain related to periodic limb movement and restless leg syndrome or pain related to difficulties turning around in bed) 5. Oro-facial pain (pain when chewing, pain due to grinding the teeth, and burning mouth syndrome) 6. Discolouration/oedema and swelling (burning pain in limbs and generalised lower abdominal pain) 7. Radicular pain (a shooting pain/pins and needles down the limbs) |
| Mylius et al.107 | A) Non–Parkinson disease-related pain B) Parkinson disease-related pain: 1. Musculoskeletal pain 2. Psychomotor restlessness pain 3. Neuropathic pain |
3.1. Pain processing is altered in Parkinson disease
Studies have largely reported reduced pain thresholds (greater sensitivity to pain) and lower pain tolerance in PwP (for meta-analysis, see Ref. 141). Interestingly, no relationship between pain sensitivity and disease duration was reported across 26 studies.141 Moreover, significant heterogeneity is seen within and across studies suggesting considerable interindividual differences with multiple contributory factors. Surveys have found intensity and frequency of pain to be higher in patients with more advanced PD; however, this likely reflects an increased incidence of musculoskeletal pain.141 A study using quantitative sensory testing failed to find a difference between drug-naive pain-free patients and controls suggesting that abnormalities may arise later in the disease duration, relate to dopaminergic therapy, or be associated with the development of chronic pain.62 In the absence of longitudinal investigation, the effects of disease progression are impossible to delineate but the power advantages of meta-analysis add credence to the possibility that enhanced pain sensitivity is engaged at a certain point during pathogenesis with a strong ceiling effect. Early pathophysiology within the midbrain and brainstem regions may therefore be important for elevated psychophysical pain sensitivity and reduced pain thresholds. Conversely, conditioned pain modulation paradigms, which assess the functionality of descending modulatory mechanisms, have been found to be comparable in controls and patients with PD in both ON and OFF states.68,69 However, trend significant differences were seen between PD subtypes (akinetic rigid, tremor dominant, and mixed). Given the low power of the study, this supports the heterogeneity of pain processing in PwP and emphasises the need for large studies that allow for adequately powered substratification.
Functional magnetic resonance imaging has revealed maladaptation of pain networks present even at early disease stages in pain-free PwP compared with healthy controls. Increased pain-related BOLD activation was observed in the somatosensory cortex, cerebellum, and caudal pons.138 Furthermore, activity in descending pain modulatory regions, such as the dlPFC, dorsal ACC, and subgenual ACC, is lower in PwP than in healthy individuals, and connectivity between dorsal ACC and dlPFC during anticipation of pain is reduced.138 The bilateral activation of the nucleus accumbens (NA) in PwP is also lower than that in healthy controls, suggesting altered processing of cognitive and evaluative facets of pain.120,140 A network-based analysis has shown dysfunction in reward pathways in PwP suffering from persistent pain, but not those without, with disconnection of the right NA and left hippocampus.118 The NA has been implicated in the transition from acute to chronic pain across a variety of human and animal studies.8,29,51,56,155 The direction of causality remains unclear, but dysfunction of reward and modulatory networks may predispose PwP to develop chronic pain and offer therapeutic targets.
3.2. Pharmacotherapy of pain in Parkinson disease
Pain in PwP remains neglected and poorly understood, with only a minority of patients receiving adequate treatment.13 People with PD are more likely to be prescribed analgesics, such as opiates, acetaminophen, antiepileptics, and antidepressants, as well as receive chronic prescriptions, risking polypharmacy or burdensome side effects.22 Dopaminergic replacement therapy might lead to pain relief in some PwP.92,142 For example, a 2-fold improvement in the King's Parkinson Disease Pain Scale domain “fluctuation-related pain” was observed with rotigotine vs placebo.124 l-Dopa administration reversed the reduction of pain threshold seen in PwP during the off-state64 and normalised abnormally increased pain-related activation within sensory-discriminative (insula) and cognitive-affective (prefrontal cortex and ACC) regions in a positron emission tomography study.21 Interestingly, pain reduction from l-dopa administration or deep brain stimulation [for review, see; Refs. 39,45,91] does not correlate with motor improvement suggesting it may act directly on pain circuitry.40,92,102,142 l-Dopa is not only converted exclusively into dopamine but also into noradrenaline and may act as a false neurotransmitter within serotonergic terminals.50 As both monoamines play a role in descending pain modulation and are affected by PD-specific neurodegenerative changes at prodromal stages, the pain modifying effect of l-dopa may be partially mediated through nondopaminergic systems.9,19,20,44,74 Accordingly, duloxetine led to some degree of pain relief in an open-label study.49 Cannabis has shown an ability to markedly reduce both sensory and affective facets of pain in PwP.132 Interestingly, an oxycodone RCT failed to reach significance for the primary end point of reducing 24 hour pain scores.144 There was a trend reduction in pain, and the dosage may have been inadequate. However, opioidergic circuitry is known to be perturbed by PD pathophysiology, and this may affect the efficacy of opioid analgesia.54,115,136,140 Safinamide, with actions on dopamine through monoamine oxidase-B inhibition as well as modulating abnormal glutamate release, has also shown a benefit in PwP.26,27,65 Rotigotine, a purely dopaminergic agonist, produces limited benefit for overall pain in PwP suggesting that safinamide may well impart a benefit through glutamatergic actions and this warrants future investigation.124 However, there remains a paucity of robust studies with the Movement Disorder Society non-motor symptoms treatment recommendation identifying only 2 as sufficiently high quality to include.131 The multiplicity of neurotransmitter systems through which these drugs act eludes to the complexity of pain in PD. Future research should use refined populations, or those large enough for substratification, to further elucidate how these interventions differentially interact with PD subtypes.
3.3. Utility of animal models
Animal models offer a unique opportunity to probe mechanisms of pain and pharmacotherapy. This has been well reviewed for PD,55,147 but remains understudied in AD. Mirroring clinical populations most studies report altered pain thresholds compared with controls.7,59,67,94,99,105,133,137 A chemically induced model of osteoarthritis through an intra-articular injection of monosodium iodoacetate within transgenic TASTPM AD mice has provided insights into interactions between clinically relevant pain, neurodegenerative pathophysiology, and opioid analgesia.4,5 TASTPM mice demonstrate an age-dependent reduction in thermal nociception that coincides with amyloid pathology in pain-related brain regions.4 Naloxone, an opioid antagonist, restored thermal nociceptive thresholds to that of wild-type controls. Mice modelling with combined AD and osteoarthritis exhibited impaired mechanical hypersensitivity and a lack of weight asymmetry. Subsequent administration of morphine not only produced an antinociceptive effect but also increased the noxious threshold significantly greater than that seen in wild-type animals.5 Conversely, gabapentin showed no efficacy. Thus, altered processing within opioidergic circuitry may partially mediate altered pain processing as well as influence both efficacy and centrally mediated side effects of opioidergic pharmacotherapy. Additional preclinical investigation may yield similar avenues for translational investigation.
4. Conclusion
Pain processing is altered in both AD and PD, but research to date has been focussed on evoked pain. During chronic pain, structural and functional reorganisation that takes place can be conceptualised as normal pain processing by the nervous system interacting with a given aetiology to produce a novel chronic pain brain state.146 These perturbed states further interact with neurodegenerative pathophysiology in a manner yet to be investigated; whether this produces differential responses to analgesic pharmacotherapy to those seen in the general population remains unclear. However, the theoretical basis outlined here is compelling and mechanistic-level investigation will be crucial to translate our emerging understanding of dysfunctional pain processing to inform safe and effective clinical management. Although our focus here has been on AD and PD, these constructs likely extend to other neurodegenerative diseases that require similar mechanism-based investigation to facilitate therapeutic development.
Conflict of interest statement
C. Ballard reports grants and personal fees from Acadia pharmaceutical company, grants and personal fees from Lundbeck, personal fees from Roche, personal fees from Otsuka, personal fees from Biogen, personal fees from Eli Lilly, personal fees from Novo Nordisk, personal fees from AARP, grants and personal fees from Synexus, and personal fees from Exciva, all outside the submitted work. The remaining authors have no conflicts of interest to declare.
Acknowledgements
T. Lawn is in receipt of a PhD studentship funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The authors acknowledge funds from the European Union's Horizon 2020 research and innovation programme “TOBeATPAIN” under the Marie Skłodowska-Curie grant agreement No 764860. The views expressed are those of the author and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Timothy Lawn, Email: timothy.lawn@kcl.ac.uk.
Yahyah Aman, Email: yahyah.aman@medisin.uio.no.
Katarina Rukavina, Email: katarina.rukavina@nhs.net.
George Sideris-Lampretsas, Email: george.sideris@kcl.ac.uk.
Matthew Howard, Email: matthew.howard@kcl.ac.uk.
Clive Ballard, Email: c.ballard@exeter.ac.uk.
Kallol Ray Chaudhuri, Email: ray.chaudhuri@kcl.ac.uk.
References
- [1].Abbott CA, Malik RA, van Ross ERE, Kulkarni J, Boulton AJM. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011;34:2220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Achterberg W, Pieper MJC, van Dalen-Kok AH, de Waal MWM, Husebo BS, Lautenbacher S, Kunz M, Scherder EJA, Corbett A. Pain management in patients with dementia. Clin Interv Aging 2013;8:1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Achterberg W, Lautenbacher S, Husebo B, Erdal A, Herr K. Pain in dementia. Pain reports 2020;5:e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aman Y, Pitcher T, Simeoli R, Ballard C, Malcangio M. Reduced thermal sensitivity and increased opioidergic tone in the TASTPM mouse model of Alzheimerʼs disease. Pain 2016;157:2285–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aman Y, Pitcher T, Ballard C, Malcangio M. Impaired chronic pain‐like behaviour and altered opioidergic system in the TASTPM mouse model of Alzheimer’s disease. Eur J Pain 2019;23:91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Antonini A, Tinazzi M, Abbruzzese G, Berardelli A, Chaudhuri KR, Defazio G, Ferreira J, Martinez-Martin P, Trenkwalder C, Rascol O. Pain in Parkinson’s disease: facts and uncertainties. Eur J Neurol 2018;25:917–e69. [DOI] [PubMed] [Google Scholar]
- [7].Baeta-Corral R, Defrin R, Pick CG, Giménez-Llort L. Tail-flick test response in 3×Tg-AD mice at early and advanced stages of disease. Neurosci Lett 2015;600:158–163. [DOI] [PubMed] [Google Scholar]
- [8].Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 2010;66:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bannister K, Dickenson AH. What do monoamines do in pain modulation?. Curr Opin Support Palliat Care 2016;10:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bauer U, Pitzer S, Schreier MM, Osterbrink J, Alzner R, Iglseder B. Pain treatment for nursing home residents differs according to cognitive state – a cross-sectional study. BMC Geriatr 2016;16:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Beach PA, Huck JT, Miranda MM, Bozoki AC. Autonomic, Behavioral, and Subjective Pain Responses in Alzheimer’s Disease. Pain Med 2015;16:1930–1942. [DOI] [PubMed] [Google Scholar]
- [12].Beach PA, Huck JT, Miranda MM, Foley KT, Bozoki AC. Effects of Alzheimer Disease on the Facial Expression of Pain. Clin J Pain 2016;32:478–487. [DOI] [PubMed] [Google Scholar]
- [13].Beiske AGG, Loge JHH, Rønningen A, Svensson E. Pain in Parkinson’s disease: Prevalence and characteristics. PAIN 2009;141:173–177. [DOI] [PubMed] [Google Scholar]
- [14].Benedetti F, Amanzio M, Maggi G. Potentiation of placebo analgesia by proglumide. Lancet (London, England) 1995;346:1231. [DOI] [PubMed] [Google Scholar]
- [15].Benedetti F, Vighetti S, Ricco C, Lagna E, Bergamasco B, Pinessi L, Rainero I. Pain threshold and tolerance in Alzheimer’s disease. Pain 1999;80:377–82. [DOI] [PubMed] [Google Scholar]
- [16].Benedetti F, Arduino C, Costa S, Vighetti S, Tarenzi L, Rainero I, Asteggiano G. Loss of expectation-related mechanisms in Alzheimer’s disease makes analgesic therapies less effective. Pain 2006;121:133–144. [DOI] [PubMed] [Google Scholar]
- [17].Bingel U, Wanigasekera V, Wiech K, Mhuircheartaigh RN, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: Imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med 2011;3. [DOI] [PubMed] [Google Scholar]
- [18].Braak E, Griffing K, Arai K, Bohl J, Bratzke H, Braak H. Neuropathology of Alzheimer’s disease: what is new since A. Alzheimer?. Eur Arch Psychiatry Clin Neurosci 1999;249(Suppl 3):14–22. [DOI] [PubMed] [Google Scholar]
- [19].Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 2004;318:121–134. [DOI] [PubMed] [Google Scholar]
- [20].Braak H, Sastre M, Bohl JRE, de Vos RAI, Del Tredici K. Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol 2007;113:421–429. [DOI] [PubMed] [Google Scholar]
- [21].Brefel-Courbon C, Payoux P, Thalamas C, Ory F, Quelven I, Chollet F, Montastruc JL, Rascol O. Effect of levodopa on pain threshold in Parkinson’s disease: A clinical and positron emission tomography study. Mov Disord 2005;20:1557–1563. [DOI] [PubMed] [Google Scholar]
- [22].Brefel-Courbon C, Grolleau S, Thalamas C, Bourrel R, Allaria-Lapierre V, Loï R, Micallef-Roll J, Lapeyre-Mestre M. Comparison of chronic analgesic drugs prevalence in Parkinson’s disease, other chronic diseases and the general population. Pain 2009;141:14–18. [DOI] [PubMed] [Google Scholar]
- [23].Broen MPG, Braaksma MM, Patijn J, Weber WEJ. Prevalence of pain in Parkinson’s disease: A systematic review using the modified QUADAS tool. Mov Disord 2012;27:480–484. [DOI] [PubMed] [Google Scholar]
- [24].Buhidma Y, Rukavina K, Chaudhuri KR, Duty S. Potential of animal models for advancing the understanding and treatment of pain in Parkinson’s disease. npj Park Dis 2020;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Canter RG, Penney J, Tsai LH. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016;539:187–196. [DOI] [PubMed] [Google Scholar]
- [26].Cattaneo C, Barone P, Bonizzoni E, Sardina M. Effects of Safinamide on Pain in Fluctuating Parkinson’s Disease Patients: A Post-Hoc Analysis. J Parkinsons Dis 2017;7:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cattaneo C, Kulisevsky J, Tubazio V, Castellani P. Long-term Efficacy of Safinamide on Parkinson’s Disease Chronic Pain. Adv Ther 2018;35:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cepeda M. Side effects of opioids during short-term administration: effect of age, gender, and race. Clin Pharmacol Ther 2003;74:102–112. [DOI] [PubMed] [Google Scholar]
- [29].Chang P-C, Pollema-Mays SL, Centeno MV, Procissi D, Contini M, Baria AT, Martina M, Apkarian AV. Role of nucleus accumbens in neuropathic pain: Linked multi-scale evidence in the rat transitioning to neuropathic pain. Pain 2014;155:1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chapman WP, Jones CM. Variations in cutaneous and visceral pain sensitivity in normal subjects. J Clin Invest 1944;23:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P. Parkinson’s disease: The non-motor issues. Parkinsonism Relat Disord 2011;17:717–723. [DOI] [PubMed] [Google Scholar]
- [32].Chaudhuri KR, Rizos A, Trenkwalder C, Rascol O, Pal S, Martino D, Carroll C, Paviour D, Falup-Pecurariu C, Kessel B, Silverdale M, Todorova A, Sauerbier A, Odin P, Antonini A, Martinez-Martin P. King’s Parkinson’s disease pain scale, the first scale for pain in PD: An international validation. Mov Disord 2015;30:1623–1631. [DOI] [PubMed] [Google Scholar]
- [33].Cohen-Mansfield J, Marx MS. Pain and depression in the nursing home: corroborating results. J Gerontol 1993;48:P96–7. [DOI] [PubMed] [Google Scholar]
- [34].Cohen-Mansfield J.. Agitated behavior in persons with dementia: The relationship between type of behavior, its frequency, and its disruptiveness. J Psychiatr Res 2008;43:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cole LJ, Farrell MJ, Duff EP, Barber JB, Egan GF, Gibson SJ. Pain sensitivity and fMRI pain-related brain activity in Alzheimer’s disease. Brain 2006;129:2957–2965. [DOI] [PubMed] [Google Scholar]
- [36].Cole LJ, Gavrilescul M, Johnstonl LA, Gibsonl SJ, Farrelll MJ, Eganl GF. The impact of Alzheimer’s disease on the functional connectivity between brain regions underlying pain perception. Eur J Pain 2011;15:568.e1–568.e11. [DOI] [PubMed] [Google Scholar]
- [37].Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol 2004;3:679–684. [DOI] [PubMed] [Google Scholar]
- [38].Creamer P, Lethbridge‐Cejku M, Hochberg MC. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology 2000;39:490–496. [DOI] [PubMed] [Google Scholar]
- [39].Cury RG, Galhardoni R, Fonoff ET, Dos Santos Ghilardi MG, Fonoff F, Arnaut D, Myczkowski ML, Marcolin MA, Bor-Seng-Shu E, Barbosa ER, Teixeira MJ, De Andrade DC. Effects of deep brain stimulation on pain and other nonmotor symptoms in Parkinson disease. Neurology 2014;83:1403–1409. [DOI] [PubMed] [Google Scholar]
- [40].Cury RG, Galhardoni R, Fonoff ET, Perez Lloret S, dos Santos Ghilardi MG, Barbosa ER, Teixeira MJ, Ciampi de Andrade D. Sensory abnormalities and pain in Parkinson disease and its modulation by treatment of motor symptoms. Eur J Pain 2016;20:151–165. [DOI] [PubMed] [Google Scholar]
- [41].Defazio G, Berardelli A, Fabbrini G, Martino D, Fincati E, Fiaschi A, Moretto G, Abbruzzese G, Marchese R, Bonuccelli U, Del Dotto P, Barone P, De Vivo E, Albanese A, Antonini A, Canesi M, Lopiano L, Zibetti M, Nappi G, Martignoni E, Lamberti P, Tinazzi M. Pain as a Nonmotor Symptom of Parkinson Disease. Arch Neurol 2008;65:1191–4. [DOI] [PubMed] [Google Scholar]
- [42].Defazio G, Tinazzi M, Berardelli A. How pain arises in Parkinson’s disease?. Eur J Neurol 2013;20:1517–1523. [DOI] [PubMed] [Google Scholar]
- [43].Defrin R, Amanzio M, de Tommaso M, Dimova V, Filipovic S, Finn DP, Gimenez-Llort L, Invitto S, Jensen-Dahm C, Lautenbacher S, Oosterman JM, Petrini L, Pick CG, Pickering G, Vase L, Kunz M. Experimental pain processing in individuals with cognitive impairment. Pain 2015;156:1396–1408. [DOI] [PubMed] [Google Scholar]
- [44].Dellapina E, Gerdelat-Mas A, Ory-Magne F, Pourcel L, Galitzky M, Calvas F, Simonetta-Moreau M, Thalamas C, Payoux P, Brefel-Courbon C. Apomorphine effect on pain threshold in Parkinson’s disease: A clinical and positron emission tomography study. Mov Disord 2011;26:153–157. [DOI] [PubMed] [Google Scholar]
- [45].Dellapina E, Ory-Magne F, Regragui W, Thalamas C, Lazorthes Y, Rascol O, Payoux P, Brefel-Courbon C. Effect of subthalamic deep brain stimulation on pain in Parkinson’s disease. PAIN 2012;153:2267–2273. [DOI] [PubMed] [Google Scholar]
- [46].Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: A randomised, double-blind, placebo-controlled phenotype-stratified study. PAIN 2014;155:2263–2273. [DOI] [PubMed] [Google Scholar]
- [47].Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci 2014;17:192–200. [DOI] [PubMed] [Google Scholar]
- [48].Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet 2005;365:965–973. [DOI] [PubMed] [Google Scholar]
- [49].Djaldetti R, Yust-Katz S, Kolianov V, Melamed E, Dabby R. The effect of duloxetine on primary pain symptoms in Parkinson disease. Clin Neuropharmacol 2007;30:201–205. [DOI] [PubMed] [Google Scholar]
- [50].Dolphin A, Jenner P, Marsden CD. Noradrenaline synthesis from L-DOPA in rodents and its relationship to motor activity. Pharmacol Biochem Behav 1976;5:431–439. [DOI] [PubMed] [Google Scholar]
- [51].DosSantos MF, Moura B de S, DaSilva AF. Reward Circuitry Plasticity in Pain Perception and Modulation. Front Pharmacol 2017;8:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Erdal A, Flo E, Aarsland D, Ballard C, Slettebo DD, Husebo BS. Efficacy and Safety of Analgesic Treatment for Depression in People with Advanced Dementia: Randomised, Multicentre, Double-Blind, Placebo-Controlled Trial (DEP.PAIN.DEM). Drugs Aging 2018;35:545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Erdal A, Ballard C, Vahia IV, Husebo BS. Analgesic treatments in people with dementia - how safe are they? A systematic review. Expert Opin Drug Saf 2019;18:511–522. [DOI] [PubMed] [Google Scholar]
- [54].Erga AH, Dalen I, Ushakova A, Chung J, Tzoulis C, Tysnes OB, Alves G, Pedersen KF, Maple-Grødem J. Dopaminergic and Opioid Pathways Associated with Impulse Control Disorders in Parkinson’s Disease. Front Neurol 2018;9:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Faivre F, Joshi A, Bezard E, Barrot M. The hidden side of Parkinson’s disease: Studying pain, anxiety and depression in animal models. Neurosci Biobehav Rev 2019;96:335–352. [DOI] [PubMed] [Google Scholar]
- [56].Farmer MA, Baliki MN, Apkarian AV. A dynamic network perspective of chronic pain. Neurosci Lett 2012;520:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ferrell BA. Pain Evaluation and Management in the Nursing Home. Ann Intern Med 1995;123:681. [DOI] [PubMed] [Google Scholar]
- [58].Fil A, Cano-de-la-Cuerda R, Muñoz-Hellín E, Vela L, Ramiro-González M, Fernández-de-las-Peñas C. Pain in Parkinson disease: A review of the literature. Parkinsonism Relat Disord 2013;19:285–294. [DOI] [PubMed] [Google Scholar]
- [59].Filali M, Lalonde R, Theriault P, Julien C, Calon F, Planel E. Cognitive and non-cognitive behaviors in the triple transgenic mouse model of Alzheimer’s disease expressing mutated APP, PS1, and Mapt (3xTg-AD). Behav Brain Res 2012;234:334–342. [DOI] [PubMed] [Google Scholar]
- [60].Fillingim RB. Individual differences in pain responses. Curr Rheumatol Rep 2005;7:342–347. [DOI] [PubMed] [Google Scholar]
- [61].Ford B. Pain in Parkinson’s disease. Clin Neurosci 1998;5:63–72. [PubMed] [Google Scholar]
- [62].Fründt O, Grashorn W, Buhmann C, Forkmann K, Mainka T, Bingel U, Schmidt K. Quantitative Sensory Testing (QST) in Drug-Naïve Patients with Parkinson’s Disease. J Parkinsons Dis 2019;9:369–378. [DOI] [PubMed] [Google Scholar]
- [63].Gagliese L. Pain and Aging: The Emergence of a New Subfield of Pain Research. J Pain 2009;10:343–353. [DOI] [PubMed] [Google Scholar]
- [64].Gerdelat-Mas A, Simonetta-Moreau M, Thalamas C, Ory-Magne F, Slaoui T, Rascol O, Brefel-Courbon C. Levodopa raises objective pain threshold in Parkinson’s disease: a RIII reflex study. J Neurol Neurosurg Psychiatry 2007;78:1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Geroin C, Di Vico IA, Squintani G, Segatti A, Bovi T, Tinazzi M. Effects of safinamide on pain in Parkinson’s disease with motor fluctuations: an exploratory study. J Neural Transm 2020;127:1143–1152. [DOI] [PubMed] [Google Scholar]
- [66].Gibson SJ, Voukelatos X, Ames D, Flicker L, Helme RD. An Examination of Pain Perception and Cerebral Event-Related Potentials following Carbon Dioxide Laser Stimulation in Patients with Alzheimer’s Disease and Age-Matched Control Volunteers. Pain Res Manag 2001;6:126–132. [DOI] [PubMed] [Google Scholar]
- [67].Gong WY, Wang R, Liu Y, Jin H, Zhao ZW, Wang YL, Li HY, Zhang X, Ni JX. Chronic monoarthritis pain accelerates the processes of cognitive impairment and increases the NMDAR subunits NR2B in CA3 of hippocampus from 5-month-old transgenic APP/PS1 mice. Front Aging Neurosci 2017;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Granovsky Y, Schlesinger I, Fadel S, Erikh I, Sprecher E, Yarnitsky D. Asymmetric pain processing in Parkinson’s disease. Eur J Neurol 2013;20:1375–1382. [DOI] [PubMed] [Google Scholar]
- [69].Grashorn W, Schunke O, Buhmann C, Forkmann K, Diedrich S, Wesemann K, Bingel U. Influence of Dopaminergic Medication on Conditioned Pain Modulation in Parkinson’s Disease Patients. PLoS One 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, Gee J. What’s in a name: Voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain 2004;127:628–649. [DOI] [PubMed] [Google Scholar]
- [71].Hadjipavlou G, Dunckley P, Behrens TE, Tracey I. Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: A diffusion tensor imaging study in healthy controls. PAIN 2006;123:169–178. [DOI] [PubMed] [Google Scholar]
- [72].Hadjistavropoulos T, LaChapelle DL, MacLeod FK, Snider B, Craig KD. Measuring Movement-Exacerbated Pain in Cognitively Impaired Frail Elders. Clin J Pain 2000;16:54–63. [DOI] [PubMed] [Google Scholar]
- [73].Horgas AL, Tsai P-F. Analgesic Drug Prescription and Use in Cognitively Impaired Nursing Home Residents. Nurs Res 1998;47:235–242. [DOI] [PubMed] [Google Scholar]
- [74].Hornykiewicz O. Biochemical aspects of Parkinson’s disease. Neurology 1998;51:S2–S9. [DOI] [PubMed] [Google Scholar]
- [75].Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 2019;15:565–581. [DOI] [PubMed] [Google Scholar]
- [76].Howdon D, Rice N. Health care expenditures, age, proximity to death and morbidity: Implications for an ageing population. J Health Econ 2018;57:60–74. [DOI] [PubMed] [Google Scholar]
- [77].Husebo BS, Achterberg W, Flo E. Identifying and Managing Pain in People with Alzheimer’s Disease and Other Types of Dementia: A Systematic Review. CNS Drugs 2016;30:481–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jensen-Dahm C, Vogel A, Waldorff FB, Waldemar G. Discrepancy Between Self- and Proxy-Rated Pain in Alzheimer’s Disease: Results from the Danish Alzheimer Intervention Study. J Am Geriatr Soc 2012;60:1274–1278. [DOI] [PubMed] [Google Scholar]
- [79].Jensen-Dahm C, Werner MU, Dahl JB, Jensen TS, Ballegaard M, Hejl A-M, Waldemar G. Quantitative sensory testing and pain tolerance in patients with mild to moderate Alzheimer disease compared to healthy control subjects. PAIN 2014;155:1439–1445. [DOI] [PubMed] [Google Scholar]
- [80].Jensen-Dahm C, Gasse C, Astrup A, Mortensen PB, Waldemar G. Frequent use of opioids in patients with dementia and nursing home residents: A study of the entire elderly population of Denmark. Alzheimer’s Dement 2015;11:691–699. [DOI] [PubMed] [Google Scholar]
- [81].Jensen-Dahm C, Madsen CS, Waldemar G, Ballegaard M, Hejl A-M, Johnsen B, Jensen TS. Contact Heat Evoked Potentials (CHEPs) in Patients with Mild-Moderate Alzheimer’s Disease and Matched Control—A Pilot Study. Pain Med 2015;17:pnv012. [DOI] [PubMed] [Google Scholar]
- [82].Jensen-Dahm C, Werner MU, Jensen TS, Ballegaard M, Andersen BB, Høgh P, Waldemar G. Discrepancy between stimulus response and tolerance of pain in Alzheimer disease. Neurology 2015;84:1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Jost WH, Buhmann C. The challenge of pain in the pharmacological management of Parkinson’s disease. Expert Opin Pharmacother 2019;20:1847–1854. [DOI] [PubMed] [Google Scholar]
- [84].Kaasalainen S, Crook J. A comparison of pain-assessment tools for use with elderly long-term-care residents. Can J Nurs Res 2003;35:58–71. [PubMed] [Google Scholar]
- [85].Kamal-Bahl SJ, Stuart BC, Beers MH. Propoxyphene use and risk for hip fractures in older adults. Am J Geriatr Pharmacother 2006;4:219–226. [DOI] [PubMed] [Google Scholar]
- [86].Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev 2001;25:669–678. [DOI] [PubMed] [Google Scholar]
- [87].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–1625. [DOI] [PubMed] [Google Scholar]
- [88].Kunz M, Scharmann S, Hemmeter U, Schepelmann K, Lautenbacher S. The facial expression of pain in patients with dementia. Pain 2007;133:221–228. [DOI] [PubMed] [Google Scholar]
- [89].Kunz M, Mylius V, Scharmann S, Schepelman K, Lautenbacher S. Influence of dementia on multiple components of pain. Eur J Pain 2009;13:317–325. [DOI] [PubMed] [Google Scholar]
- [90].Kunz M, Mylius V, Schepelmann K, Lautenbacher S. Loss in Executive Functioning Best Explains Changes in Pain Responsiveness in Patients with Dementia-Related Cognitive Decline. Behav Neurol 2015;2015:878157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kurtis MM, Rajah T, Delgado LF, Dafsari HS. The effect of deep brain stimulation on the non-motor symptoms of Parkinson’s disease: A critical review of the current evidence. npj Park Dis 2017;3:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lim SY, Farrell MJ, Gibson SJ, Helme RD, Lang AE, Evans AH. Do dyskinesia and pain share common pathophysiological mechanisms in Parkinson’s disease?. Mov Disord 2008;23:1689–1695. [DOI] [PubMed] [Google Scholar]
- [93].Limongi F, Radaelli S, Noale M, Maggi S, Crepaldi G. Somatosensory Evoked Potentials and pain assessment in Alzheimer’s disease. Eur Geriatr Med 2013;4:384–388. [Google Scholar]
- [94].López-González I, Aso E, Carmona M, Armand-Ugon M, Blanco R, Naudí A, Cabré R, Portero-Otin M, Pamplona R, Ferrer I. Neuroinflammatory Gene Regulation, Mitochondrial Function, Oxidative Stress, and Brain Lipid Modifications With Disease Progression in Tau P301S Transgenic Mice as a Model of Frontotemporal Lobar Degeneration-Tau. J Neuropathol Exp Neurol 2015;74:975–999. [DOI] [PubMed] [Google Scholar]
- [95].Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003;126:1079–1091. [DOI] [PubMed] [Google Scholar]
- [96].Lövheim H, Karlsson S, Gustafson Y. The use of central nervous system drugs and analgesics among very old people with and without dementia. Pharmacoepidemiol Drug Saf 2008;17:912–918. [DOI] [PubMed] [Google Scholar]
- [97].Lukas A, Hagg-Grün U, Mayer B, Fischer T, Schuler M. Pain assessment in advanced dementia. Validity of the German PAINAD - A prospective double-blind randomised placebo-controlled trial. Pain 2019;160:742–753. [DOI] [PubMed] [Google Scholar]
- [98].Lumb BM. Inescapable and escapable pain is represented in distinct hypothalamic-midbrain circuits: Specific roles for Aδ- and C-nociceptors. Exp Physiol 2002;87:281–286. [DOI] [PubMed] [Google Scholar]
- [99].Ma Y, Wang S, Tian Y, Chen L, Li G, Mao J. Disruption of persistent nociceptive behavior in rats with learning impairment. PLoS One 2013;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Mainka T, Malewicz NM, Baron R, Enax-Krumova EK, Treede R-D, Maier C. Presence of hyperalgesia predicts analgesic efficacy of topically applied capsaicin 8% in patients with peripheral neuropathic pain. Eur J Pain 2016;20:116–129. [DOI] [PubMed] [Google Scholar]
- [101].Manfredi PL, Breuer B, Wallenstein S, Stegmann M, Bottomley G, Libow L. Opioid Treatment for Agitation in Patients With Advanced Dementia. Int J Geriatr Psychiatry 2003;18. [DOI] [PubMed] [Google Scholar]
- [102].Marques A, Chassin O, Morand D, Pereira B, Debilly B, Derost P, Ulla M, Lemaire JJ, Durif F. Central pain modulation after subthalamic nucleus stimulation: A crossover randomized trial. Neurology 2013;81:633–640. [DOI] [PubMed] [Google Scholar]
- [103].Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR; NMSS Validation Group. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord 2011;26:399–406. [DOI] [PubMed] [Google Scholar]
- [104].Mathieu-Kia A-M, Fan L-Q, Kreek MJ, Simon EJ, Hiller JM. μ-, δ- and κ-opioid receptor populations are differentially altered in distinct areas of postmortem brains of Alzheimer’s disease patients. Brain Res 2001;893:121–134. [DOI] [PubMed] [Google Scholar]
- [105].Mellone M, Kestoras D, Andrews MR, Dassie E, Anthony Crowther R, Stokin GB, Tinsley J, Horne G, Goedert M, Tolkovsky AM, Spillantini MG. Tau pathology is present in vivo and develops in vitro in sensory neurons from human P301S tau transgenic mice: A system for screening drugs against tauopathies. J Neurosci 2013;33:18175–18189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Monroe TB, Beach PA, Bruehl SP, Dietrich MS, Rogers BP, Gore JC, Atalla SW, Cowan RL. The Impact of Alzheimer’s Disease on the Resting State Functional Connectivity of Brain Regions Modulating Pain: A Cross Sectional Study. J Alzheimer’s Dis 2017;57:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mylius V, Ciampi de Andrade D, Cury RG, Teepker M, Ehrt U, Eggert KM, Beer S, Kesselring J, Stamelou M, Oertel WH, Möller JC, Lefaucheur J-P. Pain in Parkinson’s Disease: Current Concepts and a New Diagnostic Algorithm. Mov Disord Clin Pract 2015;2:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Nelson PT, Braak H, Markesbery WR. Neuropathology and Cognitive Impairment in Alzheimer Disease: A Complex but Coherent Relationship. J Neuropathol Exp Neurol 2009;68:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Nielsen CS, Stubhaug A, Price DD, Vassend O, Czajkowski N, Harris JR. Individual differences in pain sensitivity: Genetic and environmental contributions. Pain 2008;136:21–29. [DOI] [PubMed] [Google Scholar]
- [110].Nielsen CS, Staud R, Price DD. Individual Differences in Pain Sensitivity: Measurement, Causation, and Consequences. J Pain 2009;10:231–237. [DOI] [PubMed] [Google Scholar]
- [111].Norbury TA, MacGregor AJ, Urwin J, Spector TD, McMahon SB. Heritability of responses to painful stimuli in women: a classical twin study. Brain 2007;130:3041–3049. [DOI] [PubMed] [Google Scholar]
- [112].Parkinson J. An essay on the shaking palsy. J Neuropsychiatry Clin Neurosci 2002:14. [DOI] [PubMed] [Google Scholar]
- [113].Pautex S, Michon A, Guedira M, Emond H, Lous P, Samaras D, Michel J-P, Herrmann F, Giannakopoulos P, Gold G. Pain in Severe Dementia: Self-Assessment or Observational Scales?. J Am Geriatr Soc 2006;54:1040–1045. [DOI] [PubMed] [Google Scholar]
- [114].Petrovic P. Opioid and placebo analgesia share the same network. Semin Pain Med 2005;3:31–36. [Google Scholar]
- [115].Piccini P, Weeks RA, Brooks DJ. Alterations in opioid receptor binding in Parkinson’s disease patients with levodopa-induced dyskinesias. Ann Neurol 1997;42:720–726. [DOI] [PubMed] [Google Scholar]
- [116].Politis M, Wu K, Molloy S, Bain P G., Chaudhuri KR, Piccini P. Parkinson’s disease symptoms: The patient’s perspective. Mov Disord 2010;25:1646–1651. [DOI] [PubMed] [Google Scholar]
- [117].Polli A, Weis L, Biundo R, Thacker M, Turolla A, Koutsikos K, Chaudhuri KR, Antonini A. Anatomical and functional correlates of persistent pain in Parkinson’s disease. Mov Disord 2016;31:1854–1864. [DOI] [PubMed] [Google Scholar]
- [118].Price DD, Finniss DG, Benedetti F. A Comprehensive Review of the Placebo Effect: Recent Advances and Current Thought. Annu Rev Psychol 2008;59:565–590. [DOI] [PubMed] [Google Scholar]
- [119].Prince M, Ali G-C, Guerchet M, Prina AM, Albanese E, Wu Y-T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 2016;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Qiu Y-H, Wu X-Y, Xu H, Sackett D. Neuroimaging study of placebo analgesia in humans. Neurosci Bull 2009;25:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Quinn NP, Husain FA. Parkinson’s disease. Br Med J (Clin Res Ed) 1986;293:379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Rainero I, Vighetti S, Bergamasco B, Pinessi L, Benedetti F. Autonomic responses and pain perception in Alzheimer’s disease. Eur J Pain 2000;4:267–274. [DOI] [PubMed] [Google Scholar]
- [123].Rajkumar AP, Ballard C, Fossey J, Orrell M, Moniz-Cook E, Woods RT, Murray J, Whitaker R, Stafford J, Knapp M, Romeo R, Woodward-Carlton B, Khan Z, Testad I, Corbett A. Epidemiology of Pain in People With Dementia Living in Care Homes: Longitudinal Course, Prevalence, and Treatment Implications. J Am Med Dir Assoc 2017;18:453.e1–453.e6. [DOI] [PubMed] [Google Scholar]
- [124].Rascol O, Zesiewicz T, Chaudhuri KR, Asgharnejad M, Surmann E, Dohin E, Nilius S, Bauer L. A Randomized Controlled Exploratory Pilot Study to Evaluate the Effect of Rotigotine Transdermal Patch on Parkinson’s Disease-Associated Chronic Pain. J Clin Pharmacol 2016;56:852–61. [DOI] [PubMed] [Google Scholar]
- [125].Rukavina K, Leta V, Sportelli C, Buhidma Y, Duty S, Malcangio M, Ray Chaudhuri K. Pain in Parkinsonʼs disease. Curr Opin Neurol 2019;32:579–588. [DOI] [PubMed] [Google Scholar]
- [126].Sandvik R, Selbaek G, Kirkevold O, Aarsland D, Sandgathe Husebo B. Corrigendum to ‘Analgesic prescribing patterns in Norwegian nursing homes from 2000 to 2011: trend analyses of four data samples. Age Ageing 2016;45:323. [DOI] [PubMed] [Google Scholar]
- [127].Schapira AH V., Chaudhuri KR, Jenner P.. Erratum: Non-motor features of Parkinson disease. Nat Rev Neurosci 2017;18:509. [DOI] [PubMed] [Google Scholar]
- [128].Scherder E, Oosterman J, Swaab D, Herr K, Ooms M, Ribbe M, Sergeant J, Pickering G, Benedetti F. Recent developments in pain in dementia. BMJ 2005;330:461–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Scherder EJA. Low Use of Analgesics in Alzheimer’s Disease: Possible Mechanisms. Psychiatry 2000;63:1–12. [DOI] [PubMed] [Google Scholar]
- [130].Seminowicz DA, Moayedi M. The Dorsolateral Prefrontal Cortex in Acute and Chronic Pain. J Pain 2017;18:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Seppi K, Ray Chaudhuri K, Coelho M, Fox SH, Katzenschlager R, Perez Lloret S, Weintraub D, Sampaio C. the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee and the collaborators of the PDU on NSSG on behalf of the MDSEM. Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord 2019;34:180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Shohet A, Khlebtovsky A, Roizen N, Roditi Y, Djaldetti R. Effect of medical cannabis on thermal quantitative measurements of pain in patients with Parkinson’s disease. Eur J Pain 2017;21:486–493. [DOI] [PubMed] [Google Scholar]
- [133].Shukla M, Quirion R, Ma W. Reduced expression of pain mediators and pain sensitivity in amyloid precursor protein over-expressing CRND8 transgenic mice. Neuroscience 2013;250:92–101. [DOI] [PubMed] [Google Scholar]
- [134].Simpson DM, Schifitto G, Clifford DB, Murphy TK, Durso-De Cruz E, Glue P, Whalen E, Emir B, Scott GN, Freeman R. 1066 HIV Neuropathy Study Group. Pregabalin for painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial. Neurology 2010;74:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Spanagel R, Herz A, Shippenberg TS, Fields H. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci 1992;89:2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Stefano GB, Mantione KJ, Králíčková M, Ptacek R, Kuzelova H, Esch T, Kream RM. Parkinson’s disease, L-DOPA, and endogenous morphine: a revisit. Med Sci Monit 2012;18:RA133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Takeuchi H, Iba M, Inoue H, Higuchi M, Takao K, Tsukita K, Karatsu Y, Iwamoto Y, Miyakawa T, Suhara T, Trojanowski JQ, Lee VMY, Takahashi R. P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS One 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Tessitore A, Russo A, De Micco R, Fratello M, Caiazzo G, Giordano A, Cirillo M, Tedeschi G, Esposito F. Central pain processing in “drug‐naïve” pain‐free patients with Parkinson’s disease. Hum Brain Mapp 2018;39:932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Thibodeau MA, Welch PG, Katz J, Asmundson GJG. Pain-related anxiety influences pain perception differently in men and women: A quantitative sensory test across thermal pain modalities. Pain 2013;154:419–426. [DOI] [PubMed] [Google Scholar]
- [140].Thobois S, Brefel-Courbon C, Le Bars D, Sgambato-Faure V. Molecular Imaging of Opioid System in Idiopathic Parkinson’s Disease. Int Rev Neurobiol 2018;141:275–303. [DOI] [PubMed] [Google Scholar]
- [141].Thompson T, Gallop K, Correll CU, Carvalho AF, Veronese N, Wright E, Stubbs B. Pain perception in Parkinson’s disease: A systematic review and meta-analysis of experimental studies. Ageing Res Rev 2017;35:74–86. [DOI] [PubMed] [Google Scholar]
- [142].Tinazzi M, Del Vesco C, Defazio G, Fincati E, Smania N, Moretto G, Fiaschi A, Le Pera D, Valeriani M. Abnormal processing of the nociceptive input in Parkinson’s disease: A study with CO2 laser evoked potentials. PAIN 2008;136:117–124. [DOI] [PubMed] [Google Scholar]
- [143].Titova N, Chaudhuri KR. Personalized Medicine and Nonmotor Symptoms in Parkinson’s Disease. Int Rev Neurobiol 2017;134:1257–1281. [DOI] [PubMed] [Google Scholar]
- [144].Trenkwalder C, Chaudhuri KR, Martinez-Martin P, Rascol O, Ehret R, Vališ M, Sátori M, Krygowska-Wajs A, Marti MJ, Reimer K, Oksche A, Lomax M, DeCesare J, Hopp M. Prolonged-release oxycodone–naloxone for treatment of severe pain in patients with Parkinson’s disease (PANDA): a double-blind, randomised, placebo-controlled trial. Lancet Neurol 2015;14:1161–1170. [DOI] [PubMed] [Google Scholar]
- [145].Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet 2011;377:2226–2235. [DOI] [PubMed] [Google Scholar]
- [146].Vachon-Presseau E, Centeno MV, Ren W, Berger SE, Tétreault P, Ghantous M, Baria A, Farmer M, Baliki MN, Schnitzer TJ, Apkarian AV. The Emotional Brain as a Predictor and Amplifier of Chronic Pain. J Dent Res 2016;95:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Valek L, Auburger G, Tegeder I. Sensory neuropathy and nociception in rodent models of Parkinson’s disease. Dis Model Mech 2019;12:dmm039396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci 2015;16:403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-Induced Changes in fMRI in the Anticipation and Experience of Pain. Science 2004;303:1162–1167. [DOI] [PubMed] [Google Scholar]
- [150].Wagner IC, Rütgen M, Hummer A, Windischberger C, Lamm C. Placebo-induced pain reduction is associated with inverse network coupling at rest. bioRxiv 2019:735563. [DOI] [PubMed] [Google Scholar]
- [151].Wasner G, Deuschl G. Pains in Parkinson disease-many syndromes under one umbrella. Nat Rev Neurol 2012;8:284–294. [DOI] [PubMed] [Google Scholar]
- [152].United Nations, Department of Economic and Social Affairs PD. World Population Prospects 2019: Highlights. 2019. [Google Scholar]
- [153].Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012;153:1193–1198. [DOI] [PubMed] [Google Scholar]
- [154].Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: Between pro- and antinociception. Pain 2014;155:663–665. [DOI] [PubMed] [Google Scholar]
- [155].Zhou H, Martinez E, Lin HH, Yang R, Dale JA, Liu K, Huang D, Wang J. Inhibition of the Prefrontal Projection to the Nucleus Accumbens Enhances Pain Sensitivity and Affect. Front Cell Neurosci 2018;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Zis P, Martinez-Martin P, Sauerbier A, Rizos A, Sharma JC, Worth PF, Sophia R, Silverdale M, Chaudhuri KR. Non-motor symptoms burden in treated and untreated early Parkinson's disease patients: argument for non-motor subtypes. Eur J Neurol 2015;22:1145–1150. [DOI] [PubMed] [Google Scholar]


