Abstract
BACKGROUND AND PURPOSE: Brain imaging is an integral part of the diagnostic work-up for metabolic disorders, and the bedside availability of cranial ultrasonography (cUS) allows very early brain imaging in symptomatic neonates. Our aim was to investigate the role and range of abnormalities seen on cUS in neonates presenting with metabolic disorders. A secondary aim, when possible, was to address the question of whether brain MR imaging is more informative by comparing cUS to MR imaging findings.
MATERIALS AND METHODS: Neonates with a metabolic disorder who had at least 1 cUS scan were eligible. cUS images were reviewed for anatomic and maturation features, cysts, calcium, and other abnormalities. When an MR imaging scan had been obtained, both sets of images were compared.
RESULTS: Fifty-five infants (35 also had MR imaging) were studied. The most frequent findings were in oxidative phosphorylation disorders (21 cUS and 12 MR imaging): ventricular dilation (11 cUS and 6 MR imaging), germinolytic cysts (GLCs; 7 cUS and 5 MR imaging), and abnormal white matter (7 cUS and 6 MR imaging); in peroxisomal biogenesis disorders (13 cUS and 9 MR imaging): GLCs (10 cUS and 6 MR imaging), ventricular dilation (10 cUS and 5 MR imaging), abnormal cortical folding (8 cUS and 7 MR imaging), and lenticulostriate vasculopathy (8 cUS); in amino acid metabolism and urea cycle disorders (14 cUS and 11 MR imaging): abnormal cortical folding (9 cUS and 4 MR imaging), abnormal white matter (8 cUS and 8 MR imaging), and hypoplasia of the corpus callosum (7 cUS and 6 MR imaging); in organic acid disorders (4 cUS and 2 MR imaging): periventricular white matter echogenicity (2 cUS and 1 MR imaging); and in other disorders (3 cUS and 1 MR imaging): ventricular dilation (2 cUS and 1 MR imaging). cUS findings were consistent with MR imaging findings. cUS was better for visualizing GLCs and calcification. MR imaging was more sensitive for subtle tissue signal intensity changes in the white matter and abnormality in areas difficult to visualize with cUS, though abnormalities of cortical folding suggestive of polymicrogyria were seen on cUS.
CONCLUSION: A wide range of abnormalities is seen using cUS in neonatal metabolic disorders. cUS is a reliable bedside tool for early detection of cysts, calcium, structural brain abnormalities, and white matter echogenicity, all suggestive of metabolic disorders.
Metabolic disorders are individually rare and often a diagnostic challenge. Many present in the neonatal period with encephalopathy and nonspecific symptoms, such as lethargy, floppiness, poor feeding and vomiting, apnea or tachypnea, seizures, metabolic disturbance, or dysmorphic features.1,2 Recent advances in diagnosis and treatment have substantially improved the prognosis for some of these disorders,1,2 making early diagnosis important for the prevention of death or long-term sequelae.
Neuroimaging forms a part of the investigation of neonates with metabolic disorders. MR imaging is generally considered the optimal technique.3,4 However, there is a substantial overlap and a lack of specificity in the pattern of imaging findings in neonatal metabolic disorders,3,4 and MR imaging may not be readily available on admission to a neonatal unit, and infants are often too unstable to be sedated and transported at this time. Cranial ultrasonography (cUS) can be performed bedside, is safe, and can be easily repeated. If performed by an experienced person using a modern scanner with optimal techniques and settings, it is very good for detecting most structural brain abnormalities, destructive lesions, and often more subtle tissue abnormality.5–7
Metabolic disorders can be difficult to diagnose, and recognition of characteristic neuroimaging features is very helpful in the diagnostic process.8,9 Structural brain abnormalities and more acute changes detected on cUS scan in metabolic disorders have been described in individual case reports,10–26 but there is no large study systematically reviewing these imaging findings and their accuracy or comparing them with MR imaging. The aim of this study, therefore, was primarily to investigate the role and range of abnormalities seen on cUS examination in a wide range of metabolic disorders that present during the neonatal period; a secondary aim, when possible, was to address the question of whether brain MR imaging is more informative by comparing cUS to MR imaging findings.
Materials and Methods
Patients
Infants born between September 1992 and May 2005 and seen at either the Hammersmith and Queen Charlotte's Hospitals (London, UK) or the Wilhelmina Children's Hospital/University Medical Center Utrecht (the Netherlands), who were diagnosed with a metabolic disorder presenting in the neonatal period and who had at least 1 cUS scan, were eligible for this study. Most of the infants also had a brain MR imaging examination. MR imaging was performed for different indications, including suspected metabolic disorder or hypoxic-ischemic encephalopathy, and because of the abnormalities detected on cUS. Reasons for not performing MR imaging included clinical instability or early death of the infant; some of the infants seen in the early 1990s did not have MR imaging, because it was not such a routine procedure or so readily available at that time. The diagnosis of a metabolic disorder was based on the history, clinical presentation, and examination and was confirmed by a broad range of appropriate biochemical tests. Clinical data were extracted from the infants' notes. The infants were divided into groups according to the classification of metabolic disorders, that is, disorders of oxidative phosphorylation, peroxisomal biogenesis disorders, disorders of amino acid metabolism and urea cycle defects, organic acid disorders, and others.
The following clinical parameters were documented: gestational age, sex, birth weight, head circumference, Apgar scores, cord pH, neurologic status, and occurrence of seizures. The age (days) at cUS examination and MR imaging and time interval (days) between them was documented.
Neuroimaging
cUS.
cUS examination was in most cases done by neonatal neurologists experienced in performing and interpreting neonatal cUS scans (F.M.C. and L.S.d.V.) using a standard protocol. Scanning was done as part of the clinical work-up of the infants using an ATL Ultramark-4 mechanical sector scanner (Advanced Technology Laboratories), with 5.0- and 7.5-MHz probes (Philips Medical Systems, Best, the Netherlands), an Acuson Antares scanner (Siemens Medical Solutions, Bracknell, UK), or an Aplio XG scanner (Toshiba Medical Systems, Zoetermeer, the Netherlands), both with multifrequency transducers. In both centers, it is a routine standard of care that all infants admitted to our neonatal units have a cUS scan as part of their clinical work-up. All of the scans were retrospectively evaluated (F.M.C., L.M.L., and L.S.d.V.) for gyral configuration, presence, size and shape of corpus callosum, cerebellum, cavum septum pellucidum, size of extracerebral space, interhemispheric fissure and ventricles, presence of hemorrhage, germinolytic cysts (GLCs), and lenticulostriate vasculopathy (LSV), and the appearance of the white matter, deep gray matter, and cortex. We used GLC (often called subependymal cysts, germinal matrix cysts, or subependymal pseudocysts) to describe cysts around the anterior horns of the lateral ventricles, in the caudothalamic groove, and along the ventricular margins but not for cysts in white matter, adjacent to the lateral ventricles. Some of the more anteriorly located cysts are often referred to as the subependymal pseudocysts, and those in the caudothalamic groove are often called caudothalamic cysts, but for the purposes of this study we will refer to them as GLCs. LSV was defined as linear echogenicity in the basal ganglia in a vascular distribution. If several cUS scans were obtained, the initial findings and subsequent different findings were described.
MR Imaging.
MR imaging was done as part of the neuroimaging work-up in infants. Ethical approval was given by the Hammersmith Hospitals Research Ethics Committee and Medical Ethical Committee of the University Medical Center Utrecht for the MR imaging study. Parental consent was always obtained. The infants were scanned with a 1.5T Eclipse scanner, a 3T Intera scanner, or a 1.5T ACS-NT system (Philips Medical Systems). All of the infants were sedated.27–29 Heart rate and oxygen saturation were monitored, and an experienced neonatologist was always present. Standard T1- and T2-weighted spin-echo and often diffusion-weighted images were obtained. All of the MR imaging scans were retrospectively evaluated by M.A.R. and F.G. as for the cUS and in addition for myelination and signal intensity of the white matter and deep gray matter.
Results
Patients
During the study period, 55 infants (38 male and 17 female) were diagnosed with a metabolic disorder. Median gestational age was 40.1 weeks (range, 30.0–42.6 weeks), median birth weight was 2895 g (range, 920-4060 g), and median head circumference was 33.9 cm (range, 25.0–38.5 cm). Median Apgar scores were 6 (range, 1–9), 8 (range, 3–10), and 9 (range, 5–10) at 1, 5, and 10 minutes, respectively; median cord pH was 7.18 (range, 6.78–7.31).
Forty-eight infants presented with poor responsiveness (87%), 44 with hypotonia (80%), and 35 with poor feeding (64%). Thirty-seven infants (67%) had seizures at a median age of 3 days (range, 1–61 days; Table). Nine infants (16%) were suspected of having hypoxic-ischemic encephalopathy.
Clinical and cUS and MRI findings in different groups of metabolic disorders
| Diagnosis | Clinical findings |
Characteristic cUS findings | Findings added by MRI | |||
|---|---|---|---|---|---|---|
| Poor responsiveness | Hypotonia | Early seizures | Poor feeding | |||
| Oxidative phosphorylation disorders (n = 21; MRI, n = 12) | 18 (86%) | 14 (67%) | 10 (48%) | 15 (71%) | VD (n = 11; 52%) | Delayed myelination (n = 3, 25%) |
| GLCs; abnormal WM (n = 7; 33%) | Abnormal brainstem (n = 2, 17%) | |||||
| LSV; widened ECS/IHF (n = 5, 24%) | Loss of grey/WM differentiation; large subarachnoid space; intraventricular haemorrhage; abnormal SI in WM; decreased WM volume; abnormal SI in BGT; abnormal hippocampus; abnormal SI in choroid plexus (n = 1, 8%) | |||||
| Abnormal cortical folding; small cerebellum; abnormal BGT (n = 4, 19%) | ||||||
| Thin corpus callosum (n = 3, 14%) | ||||||
| Peroxisomal biogenesis disorders (n = 13; MRI, n = 9) | 12 (92%) | 12 (92%) | 10 (77%) | 8 (62%) | GLCs; VD (n = 10, 77%) | Abnormal/delayed myelination (n = 6, 67%) |
| Abnormal cortical folding; LSV (n = 8, 62%) | Abnormal SI in WM (n = 3, 33%) | |||||
| Abnormal BGT (n = 4, 31%) | Migrational disorder; decreased WM volume (n = 1, 11%) | |||||
| Absent hin corpus callosum; patent CSP (n = 3, 23%) | ||||||
| Amino acid metabolism and urea cycle disorders (n = 14; MRI, n = 11) | 11 (79%) | 14 (100%) | 13 (93%) | 13 (93%) | Abnormal cortical folding (n = 9, 64%) | Delayed myelination (n = 5, 45%) |
| Abnormal WM (n = 8, 57%) | Cortical highlighting (n = 3, 27%) | |||||
| Abnormal corpus callosum (n = 7, 50%) | Punctate WM haemorrhage; abnormal brainstem (n = 2, 18%) | |||||
| Unusually shaped lateral ventricles (n = 5, 36%) | Abnormal SI in cerebellum; subdural haemorrhage (n = 1, 9%) | |||||
| Abnormal BGT (n = 4, 29%) | ||||||
| Small cerebellum; widened ECS/IHF (n = 2, 14%) | ||||||
| Organic acid disorders (n = 4; MRI, n = 2) | 4 (100%) | 3 (75%) | 1 (25%) | 2 (50%) | Echogenic periventricular WM (n = 2, 50%) | Delayed myelination; abnormal SI in globus pallidus (n = 1, 50%) |
| Other disorders (n = 3; MRI, n = 1) | 3 (100%) | 1 (33%) | 0 (0%) | 0 (0%) | Lateral VD (n = 2, 67%) | None |
Note:—Clinical findings are given in number of infants (percentage in group). n indicates number of examinations; VD, ventricular dilatation; GLC, germinolytic cyst; WM, white matter; LSV, lenticulostriate vasculopathy; ECS, extracerebral space; IHF, interhemispheric fissure; BGT, basal ganglia and thalami; SI, signal intensity; CSP, cavum septum pellucidum.
cUS performed bedside showed ventricular dilation in 44% of the infants, GLCs in 38%, abnormal cortical folding in 36%, abnormal white matter in 35%, LSV in 29%, an absent hin corpus callosum in 24%, widened extracerebral space and interhemispheric fissure in 20%, abnormal basal ganglia and thalami in 18%, and an abnormal appearance of the cerebellum in 16%. No evidence of other causes for GLCs or LSV, for example, toxoplasmosis, other viruses, rubella, cytomegalovirus, herpes simplex viruses or other infection, chromosomal disorders, or neonatal lupus erythematosus, was found.
Twenty-one infants (38%) had a oxidative phosphorylation disorder, 13 (24%) a peroxisomal biogenesis disorder, 11 (20%) a disorder of amino acid metabolism, 4 (7%) an organic acid disorder, and 3 (5%) a urea cycle defect. One infant (2%) each had a congenital disorder of glycosylation (CDG), Hurler syndrome, and Smith-Lemli-Opitz (SLO) syndrome. Thirty-three infants (60%) died; 14 (42%) had disorders of oxidative phosphorylation, 11 (33%) disorders of peroxisomal biogenesis, 4 (12%) disorders of amino acid metabolism, 2 (6%) urea cycle defects, and 2 (6%) other metabolic disorders.
Thirty-five infants (64%) had an MR imaging scan. The median age at first cUS scan was 3 days (range, 1–33 days) and at MR imaging was 15 days (range, 1–273 days). Median time interval between the scans was 4 days (range, 0–272 days). The 1 infant with the long time interval (ie, 272 days) was a preterm infant admitted with respiratory difficulties and mild ventriculomegaly in whom the diagnosis of a metabolic disorder was not suspected at that early time.
Of the 20 infants who did not have neonatal MR imaging, 17 died at a median age of 7 days (range, 1–62 days). The 3 infants who survived but who did not have MR imaging examination during the neonatal period were very sick and too unstable for MR imaging at that time; 1 of these infants had methylmalonic acidemia, 1 had propionic acidemia, and 1 had a complex disorder.
cUS
Oxidative Phosphorylation Disorders (n = 21).
The infant with multiple acyl-coenzyme A (CoA) dehydrogenase deficiency (glutaric aciduria type 2) had a thin corpus callosum, multiple GLCs, lateral ventricular dilation, large third ventricle, widened extracerebral space and interhemispheric fissure, and deep sulci. The cerebellum and basal ganglia appeared small on visual assessment.
The cUS scans of the 2 infants with lactic acidosis were normal. Of the 2 infants with cytochrome c oxidase (COX) deficiency, 1 had echogenic sulci and mild ventricular dilation and the other had multiple small GLCs.
Six of the remaining 16 infants had specifically identified complex disorders (3 complex I, 1 complex II, 1 complex III, and 1 complex I and IV). Three had an abnormal gyral pattern and ventricular dilation. Two also had a thin corpus callosum, widened extracerebral space and interhemispheric fissure, and 1 had patchy echogenic white matter. Five infants had GLCs and mild ventricular dilation, with increased white matter echogenicity and LSV each in 2 and an apparently small cerebellum, widened extracerebral space and interhemispheric fissure, and abnormal putamen each in 1. Two infants had an apparently small cerebellum and LSV, associated with mild dilation of lateral ventricles, extracerebral space, and interhemispheric fissure in 1 and a small patent cavum septum pellucidum, dilated third and fourth ventricles, patchy echogenic white matter, and bilateral focal echogenic areas in the basal ganglia in the other. One infant had LSV and echogenic white matter, 1 infant had bilateral echogenic basal ganglia and thalami and diffusely echogenic white matter, and 1 other infant only had loss of tissue definition in the white matter. In most infants with LSV, the calcifications were very obvious. Four infants had normal scans (Figs 1 and 2).
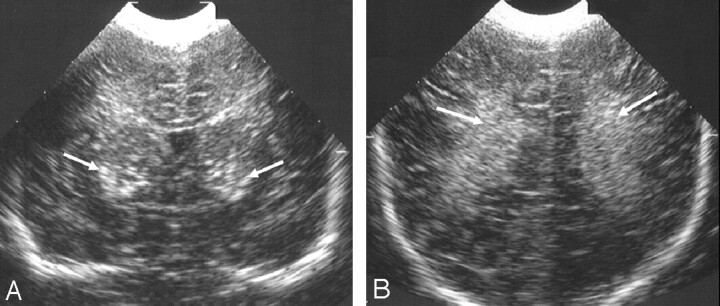
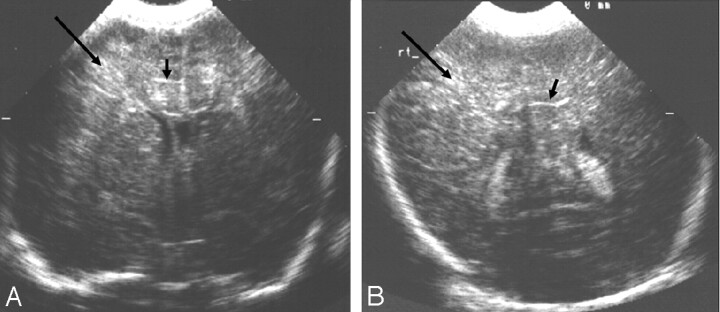
Fig 1.
Ultrasonography images from 1 of 2 siblings with an oxidative phosphorylation disorder.
A and B, Coronal views showing (A) bilateral marked echogenicity in the lower basal ganglia typical of LSV (arrows) and (B) bilateral increased echogenicity in the white matter (arrows). No MR imaging was obtained in this child.
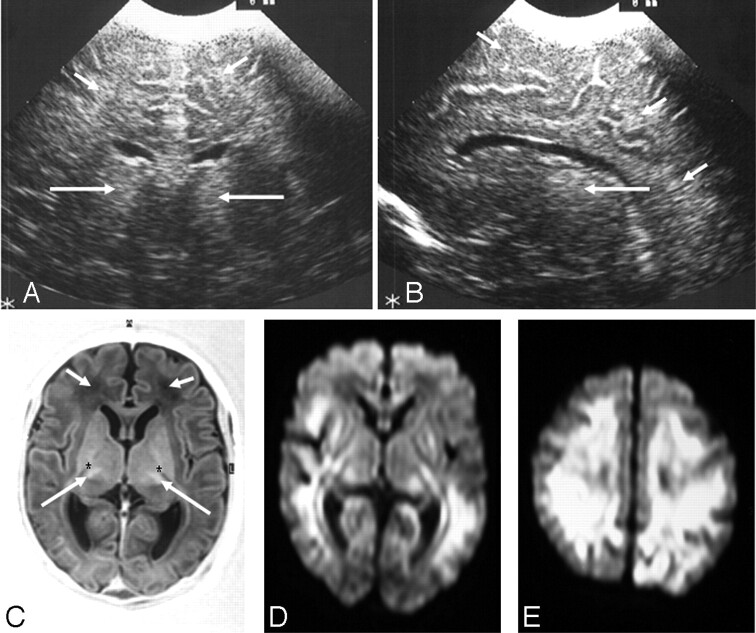
Fig 2.
Ultrasonography (A and B) and MR imaging (C–E) (time interval, 3 days) from an infant with a complex III disorder.
A, Coronal view showing bilateral echogenicity in the thalami (long arrows) and diffusely increased echogenicity in the subcortical and periventricular white matter (short arrows). B, Parasagittal view showing an echogenic thalamus (long arrow) and diffusely increased echogenicity in the periventricular and subcortical white matter (short arrows).
C, Axial inversion recovery MR image showing abnormal signal intensity in the thalami (long arrows), absence of normal signal intensity from the internal capsule (*), and abnormal low signal intensity in the white matter (short arrows).
D and E, Axial diffusion-weighted MR images showing widespread abnormal signal intensity in the periventricular and subcortical white matter and in the lateral basal ganglia and posterior thalami.
Peroxisomal Biogenesis Disorders (n = 13).
The 2 infants with a peroxisomal biogenesis disorder with a neonatal adrenoleukodystrophy phenotype had multiple small GLCs and a patent cavum septum pellucidum. In 1, these findings were associated with an abnormal appearance of the cortex and Sylvian fissures and LSV. The other infant had mild lateral ventricular dilation.
Eight of 10 infants with a peroxisomal biogenesis disorder with a Zellweger phenotype had multiple GLCs. In 7, these were associated with unusually shaped Sylvian fissures and LSV, in 5 with mild lateral ventricular dilation, and in 1 with focal echogenic areas in the basal ganglia and thalami on 1 side. One infant had a partially absent corpus callosum, unusually shaped Sylvian fissures, straight gyri, irregular lateral ventricular dilation, and an abnormal appearance of the cerebellum but no cysts. Two other infants had a thin corpus callosum, associated in 1 with a patent cavum septum pellucidum, mild irregular lateral ventricular dilation, slightly large third and fourth ventricles, widened extracerebral space, a small punctate hemorrhage in the trigonal white matter, and bilateral mildly echogenic basal ganglia and in the other with mild ventricular dilation, widened extracerebral space, and multiple GLCs. The infant with rhizomelic chondrodysplasia punctata (RCDP) had mild ventricular dilation and extensive LSV (Fig 3).
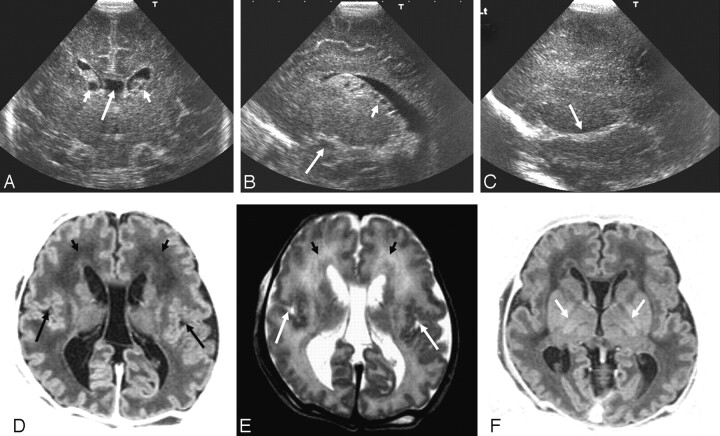
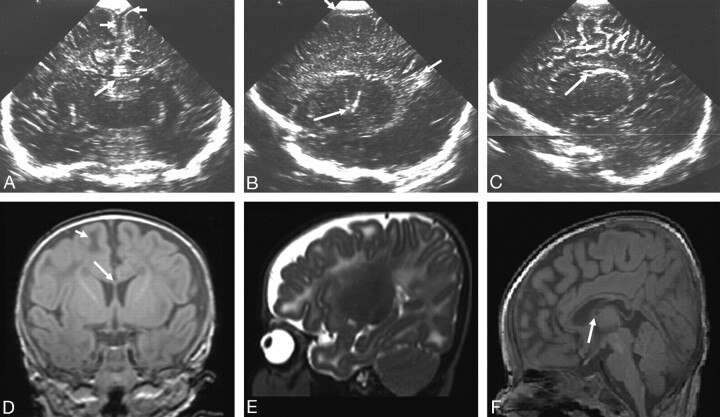
Fig 3.
Ultrasonography (A–C) and MR imaging (D–F) (time interval, 1 day) from an infant with a peroxisomal biogenesis disorder with a Zellweger phenotype.
A, Coronal view showing GLCs (short arrows), a large cavum septum pellucidum (long arrow), and increased echogenicity in the white matter.
B, Parasagittal view showing GLCs, cysts, in the choroid plexus (short arrow) and an abnormal appearance to the insula (long arrow).
C, Extreme parasagittal view showing abnormal development of the Sylvian fissure (arrow) and increased echogenicity in the white matter.
D and E, Axial inversion recovery and T2-weighted MR images showing a large cavum septum pellucidum, abnormal signal intensity in the frontal white matter (short arrows), and polymicrogyria of the Sylvian fissures (long arrows).
F, Axial inversion recovery image showing a lack of myelin in the posterior limb of the internal capsule (arrows).
Amino Acid Metabolism Disorders (n = 11).
Five of 6 infants with nonketotic hyperglycinemia (NKHG) had a thin corpus callosum with small, unusually shaped frontal horns in all; sulci extending very close to the posterior corpus callosum in 4; parallel configuration of gyri in medial parietal lobe in 1; an abnormal cingulate gyrus in 1; and an apparently small cerebellum in 1. Four of these infants also had focal echogenic areas in the white matter. In a sixth infant, the corpus callosum was not visible, the sulci were prominent, the cerebellum appeared small and abnormal on visual assessment, and there was mild ventricular dilation and swollen basal ganglia and thalami.
Of the 3 infants with maple syrup urine disease (MSUD), 1 had focal echogenic areas in the white matter and basal ganglia and thalami, 1 had focal echogenicity in the basal ganglia and thalami alone, and 1 had a mildly increased extracerebral space. The infant with transient hyperhomocysteinemia had unusually appearing gyri, a focal infarct in the white matter, and bilateral echogenic basal ganglia and thalami. The infant with glutathione synthetase deficiency had a GLC (Fig 4).
Fig 4.
Ultrasonography (A–C) and MR imaging (D and E) (time interval, 7 days) from an infant with NKHG.
A–C, Sagittal views showing (A) a hypoplastic corpus callosum (arrow) and increased echogenicity in the white matter, (B) increased echogenicity in the white matter (arrows), and (C) exaggerated contrast between the white matter and the cortex (arrows).
D and E, Axial T1- and T2-weighted MR images showing a lack of myelin in the posterior limb of the internal capsule (arrows) and increased T1 and T2 throughout the white matter.
Urea Cycle Defects (n = 3).
The infant with ornithine transcarbamylase deficiency had straight gyri off the interhemispheric fissure. In addition, there was loss of gray/white matter differentiation and loss of tissue definition in patchy echogenic white matter. The infant with argininosuccinic acid lyase deficiency had a thin corpus callosum, associated with a widened extracerebral space and interhemispheric fissure, a cyst in the choroid plexus, small GLCs, bilateral LSV, and slightly increased echogenicity in the subcortical white matter. The infant with carbamylphosphate synthetase deficiency had mildly increased echogenicity in the periventricular white matter (Figs 5 and 6).
Fig 5.
Ultrasonography images from an infant with ornithine transcarbamylase deficiency.
A and B, Coronal views showing straight gyri off the interhemispheric fissure (short arrow), loss of gray/white matter differentiation, and loss of tissue definition in patchy echogenic white matter (long arrow). This child was too unstable to transfer for MR imaging.
Fig 6.
Ultrasonography (A–C) and MR imaging (D–F) (time interval, 35 days) from an infant with argininosuccinic acid lyase deficiency.
A, Coronal view showing a thin corpus callosum (long arrow) and widened extracerebral space and interhemispheric fissure (short arrows).
B, Sagittal view showing LSV (long arrow), increased echogenicity in the white matter most obvious at the trigone (medium arrow), and widened extracerebral space (short arrow).
C, Sagittal view showing a cyst in the choroid plexus (long arrow) and slightly increased echogenicity in the subcortical white matter (short arrows).
D, Coronal T1-weighted MR image showing a thin corpus callosum (long arrow) and low signal intensity in the peripheral white matter (short arrow).
E, Parasagittal T2-weighted MR image showing widespread abnormal signal intensity in the white matter but not the LSV seen on cUS.
F, Midsagittal T1-weighted MR image showing the small cyst (arrow) also seen on cUS.
Organic Acid Disorders (n = 4).
One of the 2 infants with methylmalonic acidemia had straight sulci coming off the interhemispheric fissure, a widened extracerebral space and interhemispheric fissure, an abnormal appearance to the cerebellum on visual assessment, and bilateral GLCs; the other infant had bilateral LSV. The 2 infants with propionic acidemia had mildly increased periventricular white matter echogenicity (Fig 7).
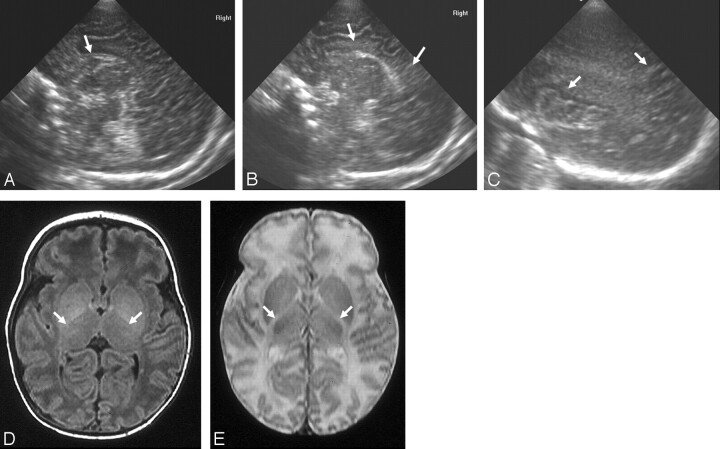
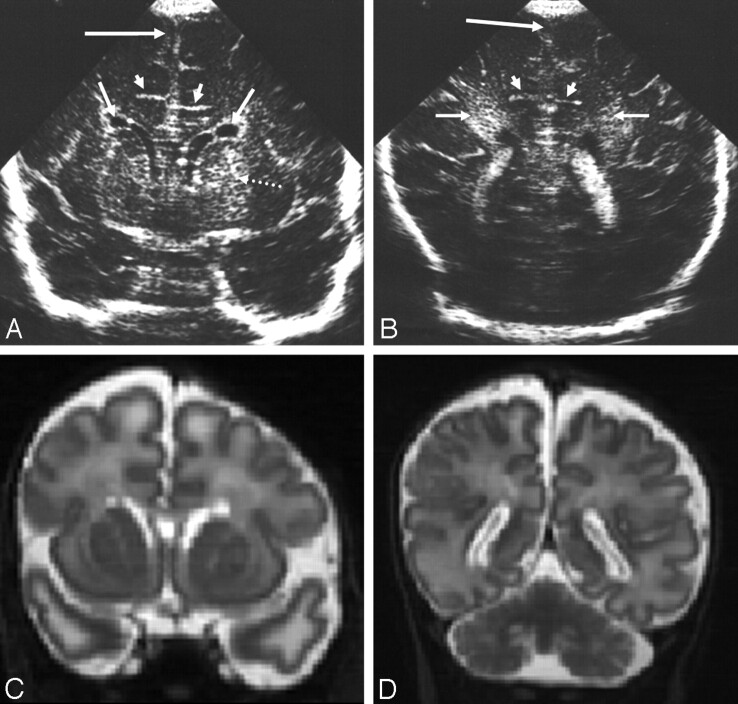
Fig 7.
Ultrasonography (A and B) and MR imaging (C and D) (time interval, 2 days) from an infant with methylmalonic acidaemia.
A, Coronal view showing straight sulci coming off the interhemispheric fissure (short arrows), bilateral GLCs (medium arrows), LSV (dotted arrow), and slightly widened interhemispheric fissure (long arrow).
B, Posterior coronal view showing straight sulci (short arrows), slightly widened interhemispheric fissure and extracerebral space (long arrow), and increased echogenicity in the white matter of the trigone (medium arrows).
C and D, Reconstructed coronal T2-weighted MR images showing features similar to the ultrasonography images except for the LSV (only seen sonographically) and white matter change also seen subcortically on the MR images.
Others (n = 3).
The infant with CDG had an apparently small cerebellum, mild ventricular dilation, and a mild increase in extracerebral space. The infant with Hurler syndrome had initially a mild but then progressive lateral ventricular dilation. The scan of the infant with SLO syndrome was normal (Fig 8).
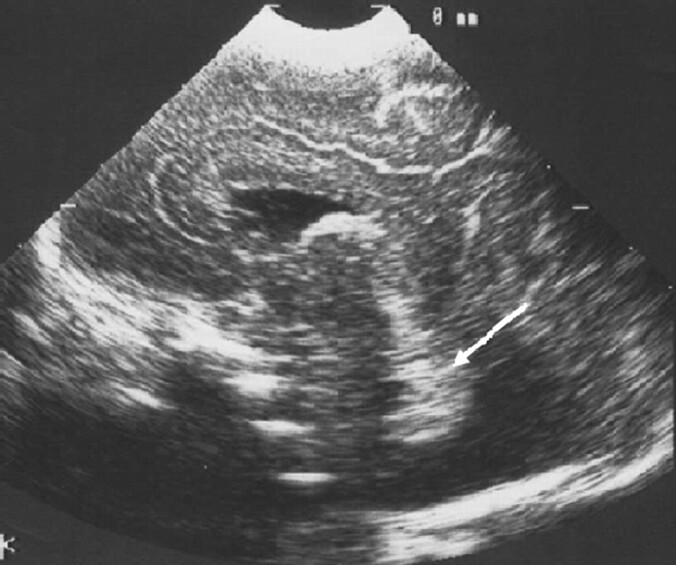
Fig 8.
Ultrasonography image from an infant with CDG. Sagittal view showing an apparently small cerebellum on visual assessment (arrow). No MR imaging was obtained for this child.
Relation between cUS Findings and MR Imaging Findings (n = 35)
See Table.
Oxidative Phosphorylation Disorders (n = 12).
In the infant with multiple acyl-CoA dehydrogenase deficiency, the findings were similar, but the MR imaging also showed a small brain stem, reduced white matter volume with abnormal signal intensity, and delayed myelination.
The cUS scan of an infant with lactic acidosis was thought normal, but the MR imaging showed abnormal signal intensity in the lentiform and caudate nuclei and thin myelin in the internal capsules. The cUS and MR imaging of an infant with COX deficiency identified the same findings.
Nine of 16 infants with a complex disorder had both scans. In 3, the MR imaging was performed postmortem. For most infants, both techniques identified an abnormal gyral pattern, GLCs, an apparently small cerebellum, and ventricular dilation. In 1 infant, cUS showed LSV not identified on MR imaging. In 6 infants, MR imaging showed findings not seen on cUS, that is, loss of gray/white matter differentiation, delayed myelination, a large subarachnoid space, prominent fourth ventricle, intraventricular hemorrhage, and also abnormal signal intensity in the deep gray and white matter. The MR imaging scans were done 2 weeks after the cUS. In 1 infant, both cUS and MR imaging showed findings not seen on the other technique, that is, patchy white matter, dilated lateral, third and fourth ventricles, and LSV on cUS, and a pachygyric cortex and bilateral large GLCs on MR imaging. The cUS was done 2 days after birth, whereas the MR imaging was done postmortem (Fig 2).
Peroxisomal Biogenesis Disorder (n = 9).
In the 2 infants with a neonatal adrenoleukodystrophy phenotype, most findings were detected on both scans. cUS showed LSV not seen on MR imaging, and MR imaging showed subtle changes in signal intensity in the white matter and myelination not seen on cUS.
Seven of 10 infants with a Zellweger phenotype had both scans. In 2, the MR imaging identified polymicrogyric Sylvian fissures and unmigrated cells in the white matter not seen on cUS. Other differences were multiple GLCs, LSV, and echogenic deep gray matter seen on cUS and delayed myelination, abnormal deep gray and white matter, and cerebellar abnormality on MR imaging. In 1 of these infants, the time interval between scans was more than 2 months, and in 4 it was more than 1 week (Fig 3).
Amino Acid Metabolism Disorders (n = 10).
Five infants with NKHG had cUS and MR imaging; both showed a thin corpus callosum, abnormal white matter signal intensity, and swollen basal ganglia and thalami. cUS additionally showed prominent sulci and echolucent caudate nuclei. The differences between cUS and MR imaging are summarized in the Table.
In 2 of the 3 infants with MSUD, MR imaging additionally showed abnormal high signal intensity in myelinated tracts on diffusion-weighted images and abnormal signal intensity in the cerebellum, posterior limb of the internal capsule, and brain stem. In the other infant, cUS showed echogenic basal ganglia and thalami, and MR imaging showed a delay in myelination. The time interval between scans was 7 weeks.
In the infant with transient hyperhomocysteinemia, MR imaging additionally showed abnormal signal intensity of the sulci and a subdural hemorrhage over the tentorium. Both cUS and MR imaging of the infant with glutathione synthetase deficiency showed a single GLC (Fig 4).
Urea Cycle Defects (n = 1).
In the infant with argininosuccinic acid lyase deficiency, most findings were seen on both scans; additionally, cUS showed a choroid plexus cyst, and LSV and MR imaging showed delayed myelination (Fig 6).
Organic Acid Disorders (n = 2).
In the infant with methylmalonic acidemia, most findings were concordant, but immature white matter and delayed myelination were only identified on MR imaging. One infant with propionic acidemia had both scans. cUS showed increased echogenicity in the periventricular white matter, whereas MR imaging a week later showed bilateral abnormal signal intensity in the globus pallidus (Fig 7).
Others.
The infant with Hurler syndrome had bilateral ventricular dilation on both cUS and MR imaging. In this infant, the time interval between scans was long, that is, 272 days (n = 1).
Discussion
Several studies have been performed on brain MR imaging and histologic findings in metabolic disorders presenting during the neonatal period,3,4,21,24,30–49 but cUS findings have only been described in individual case reports.10–26 cUS has the advantage that it is readily available on neonatal units when infants are admitted. We have shown in a large cohort of neonates with diagnosed metabolic disorders that cUS detects many abnormalities that support a clinical diagnosis, detects structural abnormalities that lead to further investigations, or depicts ongoing injurious or metabolic processes. Although some aspects of abnormalities were better detected with MR imaging, most salient features were seen on cUS. Our data show that cUS is a reliable tool for detecting both structural brain abnormalities and more acute changes highly suggestive of metabolic disorders presenting in the neonatal period. Although several of the infants were suspected of having hypoxic-ischemic encephalopathy, the cUS imaging abnormalities seen were rarely suggestive of that diagnosis (the infant illustrated in Fig 2 is an exception). The basal ganglia abnormalities in the metabolic disorders were generally more focal and punctate and did not evolve with time, and the white matter, while often being echogenic, was more uniform in appearance than that encountered in hypoxic-ischemic encephalopathy. The consistency of sequential scans also militated against that diagnosis. Another confounding diagnosis could be congenital infections, but these were excluded in all infants by appropriate serologic testing.
In the oxidative phosphorylation disorders, cUS showed GLCs and ventricular dilation in several infants, though no lesions were seen in infants with lactic acidosis. Most infants with complex disorders had ventricular dilation, frequently associated with abnormal white matter, GLCs, calcification within basal ganglia, widened extracerebral space and interhemispheric fissure, an apparently small cerebellum, and abnormal cortical folding. Our cUS findings in the infants with COX deficiency and complex disorders are consistent with those in the literature.10,13,14,17,26
Most infants with peroxisomal biogenesis disorders had multiple GLCs and ventricular dilation, often with abnormal peri-Sylvian cortical folding and LSV. These findings were apparent on cUS and consistent with case reports,15,19,24 contributing to an early diagnosis. To our knowledge, no cUS findings have been described previously in neonatal adrenoleukodystrophy.
The cUS scans in infants with amino acid metabolism and urea cycle disorders showed abnormal cortical folding, often associated with abnormal white matter, a hypoplastic corpus callosum, unusually shaped lateral ventricles, and echogenic areas in the basal ganglia and thalami. Some of the cUS findings in NKHG, MSUD, and hyperhomocysteinemia have been described,11,16,21–23 but those in glutathione synthetase deficiency only by our own group25 and, to our knowledge, cUS findings in urea cycle defects have not been described before.
The cUS scans in the organic acid disorders showed periventricular white matter echogenicity in the infants with propionic acidemia and mostly structural abnormalities in the infants with methylmalonic acidemia. As far as we know, only MR imaging findings have been described in these disorders, but our data show that cUS detects structural abnormalities and injurious processes, which, in combination with the clinical presentation, are suggestive of organic acid disorders. Although MR imaging findings have been described in CDG, Hurler and SLO syndromes,40,43,47 so far, no cUS findings have been described previously.
In several infants, MR imaging additionally showed findings not identified on cUS, including subtle signal intensity changes, maturation abnormalities, and abnormalities in areas of the brain that are difficult to visualize with cUS. This is partially because of the greater sensitivity of MR imaging for detecting more diffuse, peripheral abnormalities and its ability to show marginal changes in size of structures, neuronal migration disorders, and delayed or abnormal myelination. However, with the substantial improvement in the quality of cUS scanners in recent years and better training in cUS interpretation, the difference in sensitivity between cUS and MR imaging is diminishing.50,51 That MR imaging showed some additional findings is also partially because of the time interval between cUS and MR imaging scans reflecting the natural evolution of cerebral lesions in metabolic disorders.20,43 cUS showed calcification not identified on MR imaging, a useful clue to metabolic diagnoses. GLCs were almost always better seen on cUS, probably caused by partial volume effects from slice thickness.
No major cerebral lesions or structural abnormalities were missed on cUS, and all of the abnormalities thought highly suggestive of metabolic disorders were detected. So, although MR imaging is generally considered the optimal imaging technique in neonatal metabolic disorders, in particular in disorders of oxidative phosphorylation, we found its additional value limited. However, it should be taken into account that this is a retrospective study, and not all infants underwent neonatal MR imaging, which may have influenced our findings. An advantage of MR imaging is that diffusion-weighted images and MR spectroscopy can be performed, both of diagnostic value in metabolic disorders, including MSUD, NKHG, and RCDP.3,4,22,24,42,45,47–49 Additionally, neuronal migration disorders and more diffuse, peripheral white matter changes are more easily identified, and myelination can be evaluated.
We accept that cUS has limitations. It is best used when the fontanelles are open, though with improved scanners, there is increasing experience of scanning through acoustic windows other than the anterior fontanelle, thus extending the use of cUS, especially for posterior fossa and more peripheral structures. The size of most brain structures can be assessed accurately with cUS,52 though assessment of the cortical thickness remains difficult. As yet, myelination in the hemispheres cannot be assessed on cUS, though the posterior myelinated brain stem is of low echogenicity and can reliably be distinguished from the anterior unmyelinated brain stem. MR imaging, though usually abnormal in metabolic disorders presenting neonatally, is often nonspecific, except for MSUD, where it can be diagnostic. Unlike cUS, acquiring the MR images involves inevitable disturbance to the infant and considerable medical expertise at a time when the infants are often unstable and need intensive care.
Conclusion
We described the cUS findings in a wide range of metabolic disorders presenting during the neonatal period. All of the cerebral lesions and major structural brain abnormalities characteristic of different metabolic disorders were identified on cUS, and cUS and MR imaging findings were consistent. cUS was better for detecting GLCs and LSV, and MR imaging better for diffuse, peripheral white matter changes; delayed myelination; and abnormalities in neuronal migration. Neonatal cUS is a reliable tool for the early bedside detection of abnormality highly suggestive of a metabolic disorder.
Acknowledgments
We are grateful to S.J. Counsell, J.M. Allsop, J. Fitzpatrick, N. Blanken, and M.v.d. Lee for their technical support.
Footnotes
This research was undertaken with financial support from the Doctor Catharina van Tussenbroek Foundation, the Academy of Medical Science, the Health Foundation, and Philips Medical Systems.
References
- 1.Burton BK. Inborn errors of metabolism in infancy: a guide to diagnosis. Pediatrics 1998;102:69–77 [DOI] [PubMed] [Google Scholar]
- 2.Leonard JV, Morris AAM. Diagnosis and early management of inborn errors of metabolism presenting around the time of birth. Acta Paediatr 2006;95:6–14 [DOI] [PubMed] [Google Scholar]
- 3.Patay Z, Robertson NJ, Cox IJ. Metabolic disorders in the neonate. In: Rutherford MA, ed. MRI of the Neonatal Brain. Philadelphia: WB Saunders;2002;315–47
- 4.Khong PL, Lam BCC, Tung HKS, et al. MRI of neonatal encephalopathy. Clin Radiol 2003;58:833–44 [DOI] [PubMed] [Google Scholar]
- 5.de Vries LS, Gunardi H, Barth PG, et al. The spectrum of cranial ultrasound and magnetic resonance imaging abnormalities in congenital cytomegalovirus infection. Neuropediatrics 2004;35:113–19 [DOI] [PubMed] [Google Scholar]
- 6.Daneman A, Epelman M, Blaser S, et al. Imaging of the brain in full-term neonates: does sonography still play a role? Pediatr Radiol 2006;36:636–46 [DOI] [PubMed] [Google Scholar]
- 7.Leijser LM, de Vries LS, Cowan FM. Using cerebral ultrasound effectively in the newborn infant. Early Hum Dev 2006;82:827–35 [DOI] [PubMed] [Google Scholar]
- 8.van der Knaap MS, Valk J, de Neeling N, et al. Pattern recognition in magnetic resonance imaging in white matter disorders in children and young adults. Neuroradiology 1991;33:478–93 [DOI] [PubMed] [Google Scholar]
- 9.Kohlschlütter A. Neuroradiological and neurophysiological indices for neurometabolic disorders. Eur J Pediatr 1994;153:S90–93 [DOI] [PubMed] [Google Scholar]
- 10.Yamagata T, Yano S, Okabe I, et al. Ultrasonongraphy and magnetic resonance imaging in Leigh disease. Pediatr Neurol 1990;6:326–29 [DOI] [PubMed] [Google Scholar]
- 11.Wariyar UK, Welch RJ, Milligan DWA, et al. Sonographic and pathological features of callosal hypoplasia in non-ketotic hyperglycinaemia. Arch Dis Child 1990;65:670–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichihashi K, Yano S, Kobayashi S, et al. Serial imaging of Menkes disease. Neuroradiology 1990;32:56–59 [DOI] [PubMed] [Google Scholar]
- 13.Chi CS, Mak CS, Shian WJ. Leigh syndrome with progressive ventriculomegaly. Pediatr Neurol 1994;10:244–46 [DOI] [PubMed] [Google Scholar]
- 14.Samson JF, Barth PG, de Vries JI, et al. Familial mitochondrial encephalopathy with fetal ultrasonographic ventriculomegaly and intracerebral calcifications. Eur J Pediatr 1994;153:510–16 [DOI] [PubMed] [Google Scholar]
- 15.Russel IM, van Sonderen L, van Straaten HLM, et al. Subependymal germinolytic cysts in Zellweger syndrome. Pediatr Radiol 1995;25:254–55 [DOI] [PubMed] [Google Scholar]
- 16.Fariello G, Dionisi-Vici C, Orazi C, et al. Cranial ultrasonography in maple syrup urine disease. AJNR Am J Neuroradiol 1996;17:311–15 [PMC free article] [PubMed] [Google Scholar]
- 17.Shian WJ, Chi CS, Mak SC. Neuro-image in infants and children with mitochondrial disorders. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1996;37:96–102 [PubMed] [Google Scholar]
- 18.Brun N, Robitaille Y, Grignon A, et al. Pyruvate carboxylase deficiency: prenatal onset of ischemia-like brain lesions in two sibs with the acute neonatal form. Am J Med Genet 1999;84:94–101 [PubMed] [Google Scholar]
- 19.al-Essa M, Dhaunsi GS, Rashed M, et al. Zellweger syndrome in Saudi Arabia and its distinct features. Clin Pediatr 1999;38:77–86 [DOI] [PubMed] [Google Scholar]
- 20.Forstner R, Hoffmann GF, Gassner I, et al. Glutaric aciduria type I: ultrasonographic demonstration of early signs. Pediatr Radiol 1999;29:138–43 [DOI] [PubMed] [Google Scholar]
- 21.Hogeveen M, Blom HJ, van Amerongen M, et al. Hyperhomocysteinemia as risk factor for ischemic and hemorrhagic stroke in newborn infants. J Pediatr 2002;141:429–31 [DOI] [PubMed] [Google Scholar]
- 22.Cavalleri F, Berardi A, Burlina AB, et al. Diffusion-weighted MRI in maple syrup urine disease encephalopathy. Neuroradiology 2002;44:499–502 [DOI] [PubMed] [Google Scholar]
- 23.Paupe A, Bidat L, Sonigo P, et al. Prenatal diagnosis of hypoplasia of the corpus callosum in association with non-ketotic hyperglycinemia. Ultrasound Obstet Gynecol 2002;20:616–19 [DOI] [PubMed] [Google Scholar]
- 24.Viola A, Confort-Gouny S, Ranjeva JP, et al. MR imaging and MR spectroscopy in rhizomelic chondrodysplasia punctata. AJNR Am J Neuroradiol 2002;23:480–83 [PMC free article] [PubMed] [Google Scholar]
- 25.Brüggemann LW, Groenendaal F, Ristoff E, et al. Glutathione synthetase deficiency associated with antenatal cerebral bleeding. J Inherit Metab Dis 2004;27:275–76 [DOI] [PubMed] [Google Scholar]
- 26.van Straaten HLM, van Tintelen JP, Trijbels JMF, et al. Neonatal lactic acidosis, complex I/IV deficiency, and fetal cerebral disruption. Neuropediatrics 2005;36:193–99 [DOI] [PubMed] [Google Scholar]
- 27.Cowan FM. Sedation for magnetic resonance scanning of infants and young children. In: Whitwam JG, McCloy RF, eds. Principles and Practice of Sedation. London: Blackwell Healthcare;1998. :206–13
- 28.Mercuri E, Guzzetta A, Laroche S, et al. Neurologic examination of preterm infants at term age: comparison with term infants. J Pediatr 2003;142:647–55 [DOI] [PubMed] [Google Scholar]
- 29.Dubowitz L, Ricci D, Mercuri E. The Dubowitz neurological examination of the full-term newborn. MRDD Res Rev 2005;11:52–60 [DOI] [PubMed] [Google Scholar]
- 30.Patel PJ, Kolawole TM, Malabarey TM, et al. Adrenoleukodystrophy: CT and MRI findings. Pediatr Radiol 1995;25:256–58 [DOI] [PubMed] [Google Scholar]
- 31.Barkovich AJ, Peck WW. MR of Zellweger syndrome. AJNR Am J Neuroradiol 1997;18:1163–70 [PMC free article] [PubMed] [Google Scholar]
- 32.Steinlin M, Blaser S, Boltshauser E. Cerebellar involvement in metabolic disorders: a pattern-recognition approach. Neuroradiology 1998;40:347–54 [DOI] [PubMed] [Google Scholar]
- 33.Sue CM, Crimmins DS, Soo YS, et al. Neuroradiological features of six kindreds with MELAS tRNA(Leu) A3243G point mutation: implications for pathogenesis. J Neurol Neurosurg Psychiatry 1998;65:233–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valanne L, Ketonen L, Majander A, et al. Neuroradiologic findings in children with mitochondrial disorders. AJNR Am J Neuroradiol 1998;19:369–77 [PMC free article] [PubMed] [Google Scholar]
- 35.van Beynum IM, Smeitink JA, den Heijer M, et al. Hyperhomocysteinemia: a risk factor for ischemic stroke in children. Circulation 1999;99:2070–72 [DOI] [PubMed] [Google Scholar]
- 36.de Koning TJ, de Vries LS, Groenendaal F, et al. Pontocerebellar hypoplasia associated with respiratory-chain defects. Neuropediatrics 1999;30:93–95 [DOI] [PubMed] [Google Scholar]
- 37.van Hove JL, Kishnani PS, Demaerel P, et al. Acute hydrocephalus in nonketotic hyperglycemia. Neurology 2000;54:754–56 [DOI] [PubMed] [Google Scholar]
- 38.Willis TA, Davidson J, Gray RG, et al. Cytochrome oxidase deficiency presenting as birth asphyxia. Dev Med Child Neurol 2000;42:414–17 [DOI] [PubMed] [Google Scholar]
- 39.al-Essa MA, Rashed MS, Bakheet SM, et al. Glutaric aciduria type II: observations in seven patients with neonatal- and late-onset disease. J Perinatol 2000;20:120–28 [DOI] [PubMed] [Google Scholar]
- 40.de Vries BB, van 't Hoff WG, Surtees RA, et al. Diagnostic dilemmas in four infants with nephrotic syndrome, microcephaly and severe developmental delay. Clin Dysmorphol 2001;10:115–21 [DOI] [PubMed] [Google Scholar]
- 41.Savasta S, Comi GP, Perini MP, et al. Leigh disease: clinical, neuroradiologic, and biochemical study of three new cases with cytochrome c oxidase deficiency. J Child Neurol 2001;16:608–13 [DOI] [PubMed] [Google Scholar]
- 42.Viola A, Chabrol B, Nicoli F, et al. Magnetic resonance spectroscopy study of glycine pathways in nonketotic hyperglycinemia. Pediatr Res 2002;52:292–300 [DOI] [PubMed] [Google Scholar]
- 43.Barone R, Parano E, Trifiletti RR, et al. White matter changes mimicking a leukodystrophy in a patient with mucopolysaccharidosis: characterization by MRI. J Neurol Sci 2002;195:171–75 [DOI] [PubMed] [Google Scholar]
- 44.Farina L, Chiapparini L, Uziel G, et al. MR findings in Leigh syndrome with COX deficiency and SURF-1 mutations. AJNR Am J Neuroradiol 2002;23:1095–100 [PMC free article] [PubMed] [Google Scholar]
- 45.Zand DJ, Simon EM, Pulitzer SB, et al. In vivo pyruvate detected by MR spectroscopy in neonatal pyruvate dehydrogenase deficiency. AJNR Am J Neuroradiol 2003;24:1471–74 [PMC free article] [PubMed] [Google Scholar]
- 46.Picker JD, Puga AC, Levy HL, et al. Arginase deficiency with lethal neonatal expression: evidence for the glutamine hypothesis of cerebral edema. J Pediatr 2003;142:349–52 [DOI] [PubMed] [Google Scholar]
- 47.Caruso PA, Poussaint TY, Tzika AA, et al. MRI and 1H MRS findings in Smith-Lemli-Opitz syndrome. Neuroradiology 2004;46:3–14 [DOI] [PubMed] [Google Scholar]
- 48.Parmar H, Sitoh YY, Ho L. Maple syrup urine disease: diffusion-weighted and diffusion-tensor magnetic resonance imaging findings. J Comput Assist Tomogr 2004;28:93–97 [DOI] [PubMed] [Google Scholar]
- 49.Sakai M, Inoue Y, Oba H, et al. Age dependence of diffusion-weighted magnetic resonance imaging findings in maple syrup urine disease encephalopathy. J Comput Assist Tomogr 2005;29:524–27 [DOI] [PubMed] [Google Scholar]
- 50.Pellicer A, Cabanas F, Perez-Higueras A, et al. Neural migration disorders studied by cerebral ultrasound and colour Doppler flow imaging. Arch Dis Child 1995;73:F55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hung PC, Wang HS. Hemimegalencephaly: cranial sonographic findings in neonates. J Clin Ultrasound 2005;33:243–47 [DOI] [PubMed] [Google Scholar]
- 52.Leijser LM, Srinivasan L, Rutherford MA, et al. Structural linear measurements in the newborn brain: accuracy of cranial ultrasound compared to MRI. Pediatr Radiol 2007;May 8; [Epub ahead of print] [DOI] [PubMed]