Abstract
BACKGROUND AND PURPOSE: Little is known about the metabolic properties of brain edema associated with tumors. This work was conducted on the basis of the assumption that, in the presence of intra-axial and extra-axial brain tumors, the white matter involved by the edema is a site of metabolic change that involves the structure of the myelin sheath.
MATERIALS AND METHODS: Thirteen patients comprised our cohort affected by intra-axial and extra-axial cerebral tumors with a peritumoral T2-weighted MR signal hyperintensity as a result of edema, where MR spectroscopy showed no increase in choline-containing compounds. Measurements on proton MR spectroscopy (1H-MR spectroscopy) were performed with a 3T whole-body scanner with use of a point-resolved spectroscopy sequence for localization (TR, 2000 ms; TE, 35 ms), and the metabolites were quantified with the SAGE method. Peak intensities of the main metabolites were expressed as ratios of one another and were compared with values obtained in the white matter of the left frontal region in a control group of 16 healthy volunteers.
RESULTS: Choline-to-creatine (Cho/Cr) and myo-inositol-to-creatine (mIns/Cr) signal intensity ratios were normal in all patients. N-acetylaspartate-to-creatine (NAA/Cr) and N-acetylaspartate-to-choline (NAA/Cho) ratios decreased in 4 patients. Glutamate plus glutamine-to-creatine (Glx/Cr) was increased in 10 patients. A resonance peak at 3.44 ppm, strongly suggesting the presence of glucose, was detected in all but 1 patient. Lactate was detected in 12 patients and lipids in 5. Moreover, the resonances that pertained to the aliphatic amino acids valine, leucine, and isoleucine were present in 12 patients.
CONCLUSIONS: Our findings on MR spectroscopy confirmed the hypothesis that in the edema surrounding brain tumors, an energy-linked metabolic alteration was associated with injury to the myelin sheath.
Brain tumors are very often associated with perilesional edema. The pathophysiologic mechanisms at the base of its formation1,2 are related to ischemia caused by compression of the tumor,3 stasis followed by venous congestion,4 and excretory-secretory phenomena.5–8 Although the pathogenesis of the edema differs according to whether the tumor is intra-axial or extra-axial, ultrastructural studies have shown that, in both cases, the edema is vasogenic.9 The advent of functional MR techniques such as diffusion and perfusion imaging and spectroscopy has opened the door to in vivo dynamic and metabolic assessments.10–13 MR spectroscopy studies have investigated brain edema in experimental models14,15 and in the clinical setting with the use of 1.5T systems.16–22 MR spectroscopy has been boosted further by the clinical application of high-field-strength devices by offering the advantages of better signal-to-noise ratio and increased spectral resolution, thereby disclosing more metabolites and extending the range of metabolic information.23
The aim of our study was to investigate by 3T 1H-MR spectroscopy the metabolic properties of the brain tissue involved by the edema associated with intra-axial and extra-axial tumors and characterized by signal hyperintensity in MR T2-weighted sequences, to look for a specific metabolic pattern in the edema. Indeed, our hypothesis is that the structure of the white matter involved by metabolic alteration, more or less reversible, is precisely the myelin. This study was conducted on the basis of the reasonable assumption that the areas of the brain we examined were not affected by proliferation of neoplastic cells, because no increase in choline (Cho)-containing compounds was detected.
Patients and Methods
Subjects
Thirteen patients (8 men and 5 women) comprised our cohort. They were between 35 and 67 years old (mean age, 53 ± 8 years) with intra-axial or extra-axial supratentorial tumors, all with perilesional edema in which no increase in Cho relative content was detected by MR spectroscopy. We obtained written informed consent from all patients before we began the examination, and our local institutional review board approved the study.
Eleven patients underwent surgery with the following histologic diagnoses: 7 glioblastomas, 1 metastasis, and 3 meningiomas. Two patients, not surgically treated, were affected by metastases because of the presence of a primary distant tumor with multiple brain lesions. According to Chernov et al,12 the extension of perilesional edema evaluated by T2-weighted MR images was graded as mild in 2 patients, moderate in 6, and severe in 5. All patients received dexamethasone as the only anti-edema drug.
MR Protocol
We performed all MR imaging and localized single-voxel 1H-MR spectroscopy measurements with a 3T whole-body scanner (GE Medical Systems, Milwaukee, Wis). Our standard routine clinical protocol involved use of the standard 8-channel phased array head coil, which allowed the best signal-to-noise ratio for MR imaging and MR spectroscopy.
MR imaging was performed with T2-weighted fast spin-echo (FSE) sequences (TR, 4200 ms; TE, 93 ms; NEX, 2; 24-cm field of view; 512 × 512 matrix; 4-mm sections), fluid-attenuated inversion recovery (FLAIR) sequences (TR, 9002 ms; TE, 91 ms; NEX, 2; 24-cm field of view; 320 × 320 matrix; 4-mm sections) in the axial plane, and T1-weighted spin-echo (SE) sequences (TR, 560 ms; TE, 18 ms; NEX, 2; 24-cm field of view; 384 × 224 matrix; 4-mm sections) in the sagittal and coronal planes before administration of a contrast agent and coronal and axial planes after administration of a contrast agent.
1H-MR spectra were acquired before administration of the contrast agent with a point-resolved spectroscopy sequence (PRESS) for localization, with TR 2000 ms and TE 35 ms, 128 acquisitions, and a 3-pulse chemical shift selection suppression (CHESS) sequence to provide water suppression.
For each spectrum, we collected 16 additional acquisitions with unsuppressed water for phase correction of the metabolite spectra. We used automated optimization of gradient shimming, transmitter pulse power, and water suppression. In all cases, the quality of the shimming obtained in the voxel was controlled by the spectral line width (full width of half maximum in Hz) of the unsuppressed water, obtained by the automated optimization sequence before scanning.
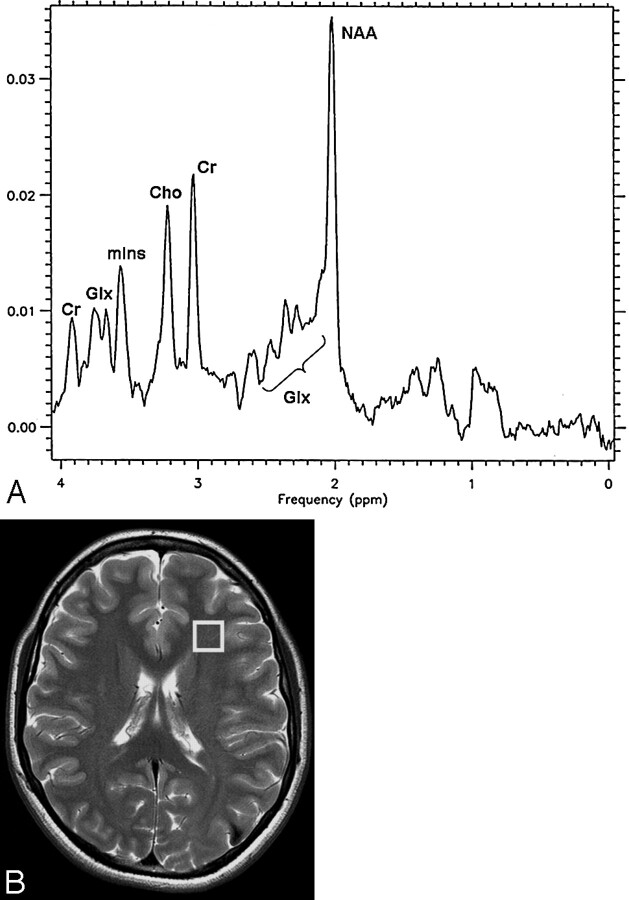
The value of the peak intensities of the main metabolites, N-acetylaspartate (NAA, 2.02 ppm), choline-containing compounds (Cho, 3.22 ppm), creatine and phosphocreatine (Cr, 3.03 ppm), myo-inositol (mIns, 3.56 and 4.06 ppm), and glutamate plus glutamine (Glx, 2.1–2.5 ppm) were expressed as ratios of one another, in the form of NAA/Cr, NAA/Cho, Cho/Cr, mIns/Cr, and Glx/Cr. We compared these ratios with the values obtained in the white matter of the left frontal region of 16 healthy volunteers between 25 and 67 years old (mean age, 40.7 ± 9 years): (NAA/Cr, 1.8 ± 0.3; NAA/Cho, 2 ± 0.4; Cho/Cr, 0.9 ± 0.2; mIns/Cr, 0.6 ± 0.2; and Glx/Cr, 1.8 ± 0.2). Figure 1 shows a T2-weighted FSE axial image of a normal brain with the selected volume of interest (VOI) (B), and the corresponding 1H-MR spectrum (A).
Fig 1.
A T2-weighted FSE axial image of a normal brain.
A, Localized proton spectrum from the VOI (8 cm3) in the white matter of the left frontal region of 16 healthy volunteers, recorded with use of the PRESS sequence at 3T (TR, 2000 ms; TE, 35 ms). (NAA indicates N-acetylaspartate; Cho, choline-containing compounds; Cr, creatine and phosphocreatine; mIns, myo-inositol; Glx, glutamate plus glutamine.)
B, Location of voxel used for localized proton spectra in healthy volunteers. A T2-weighted FSE axial image (TR, 4200 ms; TE, 93 ms; NEX, 2).
The VOI, which varied in size from 1.2 to 8 cm3, was accurately positioned on T2-weighted FSE axial and T1-weighted SE coronal and sagittal planes over the white matter involved by edema. The size of the voxel and location were chosen in an attempt to minimize the contribution of neoplastic and cerebral tissue without alteration in signal intensity.
Analysis of Spectra
We analyzed all MR spectra with the Spectral Analysis Program (SAGE; GE Medical Systems) with the following steps. After the 8-channel signals were combined, the estimated phase correction of the unsuppressed water signal intensity was used to phase-correct the corresponding metabolite (water suppressed) signal intensity. The water-suppressed signal intensity was subtracted from the unsuppressed one, and the “pure water signal intensity” obtained was scaled and subtracted from the suppressed signal intensity to obtain the final metabolite spectrum. After performing a Gaussian apodization, we applied the Fourier transform to the data and interpolated the data to obtain a resolution of 2055/4096 Hz per point. Chemical shifts were referenced to the signal intensity of Cho at 3.22 ppm, and peak integration of the real part of the spectrum for NAA, Cho, Cr, mIns, and Glx was performed. In particular, the signal intensity amplitude of the Glx complex was estimated in the frequency range of 2.1–2.5 ppm on the basis of phantom spectra of solution with 25 mmol/L glutamate and glutamine at physiologic pH.
With regard to the other metabolites detected in our spectra and manually selected, we arbitrarily assigned lactate (Lac, 1.33 ppm), glucose (Glc, 3.44, 3.78 ppm), and lipids (Lip, 1.3, 0.9 ppm) to 1 of 3 grades: low (+), medium (++), or high (+++), on the basis of the ratio of the integral of the metabolite peak to the integral of the unsuppressed water peak.24 Instead, for valine (Val, 1.00 ppm), leucine (Leu, 0.98 ppm), isoleucine (Ile, 1.04 and 0.9 ppm), and alanine (Ala, 1.47 ppm), we only determined the presence or absence of these metabolites.
Statistical Method
We analyzed the NAA/Cr, NAA/Cho, Cho/Cr, mIns/Cr, and Glx/Cr ratios with the Kruskal-Wallis test and determined the level of significance at a P value of < .05.
Results
Table 1 summarizes the NAA/Cr, NAA/Cho, Cho/Cr, mIns/Cr, and Glx/Cr ratios obtained in the edema of the 13 patients. The signal intensity ratios of Cho/Cr and mIns/Cr were normal in all patients.
Table 1:
Ratios of the main metabolites detected in the cerebral edema of 13 patients*
| NAA/Cr | NAA/Cho | Cho/Cr | mIns/Cr | Glx/Cr | |
|---|---|---|---|---|---|
| Glioblastomas (pt no.) | |||||
| 1 | 1.7 | 1.6 | 1.1 | 0.8 | 1.8 |
| 2 | 0.9† | 0.9† | 1 | 0.6 | 1.6 |
| 3 | 2 | 2.2 | 0.9 | 0.7 | 2.2† |
| 4 | 1† | 1† | 1 | 0.6 | 2.1† |
| 5 | 1.2† | 1.4† | 0.9 | 0.7 | 2.6† |
| 6 | 1.5 | 1.7 | 0.9 | 0.5 | 2 |
| 7 | 1.5 | 1.9 | 0.8 | 0.5 | 2.8† |
| Metastases (pt no.) | |||||
| 8 | 1.8 | 1.7 | 1 | 0.4 | 2.8† |
| 9 | 1.9 | 2.3 | 0.8 | 0.4 | 3† |
| 10 | 1.6 | 1.4† | 1.1 | 0.4 | 2.7† |
| Meningiomas (pt no.) | |||||
| 11 | 1.8 | 1.9 | 0.9 | 0.4 | 3.2† |
| 12 | 1.9 | 1.8 | 1 | 0.7 | 3.7† |
| 13 | 1.4† | 1.8 | 0.8 | 0.4 | 2.5† |
Note:—NAA indicates N-acetylaspartate; Cr, creatine and phosphocreatine; Cho, choline- containing compounds; Glx, glutamate plus glutamine; mIns, myo-inositol.
Ratios of the main metabolites are detected by PRESS sequences (TR, 2000 ms; TE, 35 ms) in edema surrounding 7 glioblastomas, 3 metastases, and 3 meningiomas. These values were compared with those obtained in the white matter of the left frontal region with use of the same technique in 16 healthy volunteers between 25 and 67 years old (mean age, 40.7 ± 9 years). The normal values are NAA/Cr 1.8 ± 0.3; NAA/Cho 2 ± 0.4; Cho/Cr 0.9 ± 0.2; mIns/Cr 0.6 ± 0.2 and Glx/Cr 1.8 ± 0.2.
Altered values.
The NAA/Cr ratio was decreased in 4 (30.8%) patients: 3 with glioblastomas and 1 with meningioma. The NAA/Cho ratio was decreased in 4 (30.8%) patients: 3 with glioblastomas and 1 with metastasis. The Glx/Cr ratio was increased in 10 (77%) patients: 4 with glioblastomas, all with metastases and meningiomas.
Analysis of the NAA/Cr, NAA/Cho, Cho/Cr, and Glx/Cr ratios with the Kruskal-Wallis test failed to reveal statistically significant values associated with the different types of tumors (NAA/Cr, P = .28; NAA/Cho, P = .33; Cho/Cr, P = .94; Glx/Cr, P = .05). Instead, there was a significant difference among the 3 groups with regard to the mIns/Cr ratio (P = .03 < .05). The significant difference according to the usual statistical tests for multiple comparisons was between the gliomas and metastases.
As shown in Table 2, the resonances of the aliphatic amino acids Val, Leu, and Ile were present in 12 (92.3%) patients: 6 with glioblastomas, all with metastases and meningiomas. Ala was detected in the edema of 2 (15.4%) patients affected by a glioblastoma and a meningioma.
Table 2:
Detection of metabolites normally not found in the human brain
| Glc 3.44 ppm* | Lac* | Lip* | Other Metabolites* | |
|---|---|---|---|---|
| Glioblastomas (pt no.) | ||||
| 1 | ++ | + | Val, Leu, Ile | |
| 2 | + | + | Val, Leu, Ile | |
| 3 | + | ++ | + | Val, Leu, Ile, Ala |
| 4 | + | + | Val, Leu, Ile | |
| 5 | ++ | + | ||
| 6 | + | + | + | Val, Leu, Ile |
| 7 | + | Val, Leu, Ile | ||
| Metastases (pt no.) | ||||
| 8 | ++ | + | Val, Leu, Ile | |
| 9 | + | + | Val, Leu, Ile | |
| 10 | + | + | Val, Leu, Ile | |
| Meningiomas (pt no.) | ||||
| 11 | + | ++ | + | Val, Leu, Ile |
| 12 | +++ | ++ | Val, Leu, Ile | |
| 13 | ++ | + | + | Val, Leu, Ile, Ala |
Note:—Glc indicates glucose; Lac, lactate; Lip, lipids; Val, valine; Leu, leucine; Ile, isoleucine; and Ala, alanine.
Peak resonances of Glc, Lac, and Lip detected and arbitrarily assigned to 1 of 3 grades, low (+), medium (++), or high (+++) on the basis of the ratio of the integral of the metabolite peak to the integral of unsuppressed water peak. For Val, Leu, Ile, and Ala, only their presence is indicated.
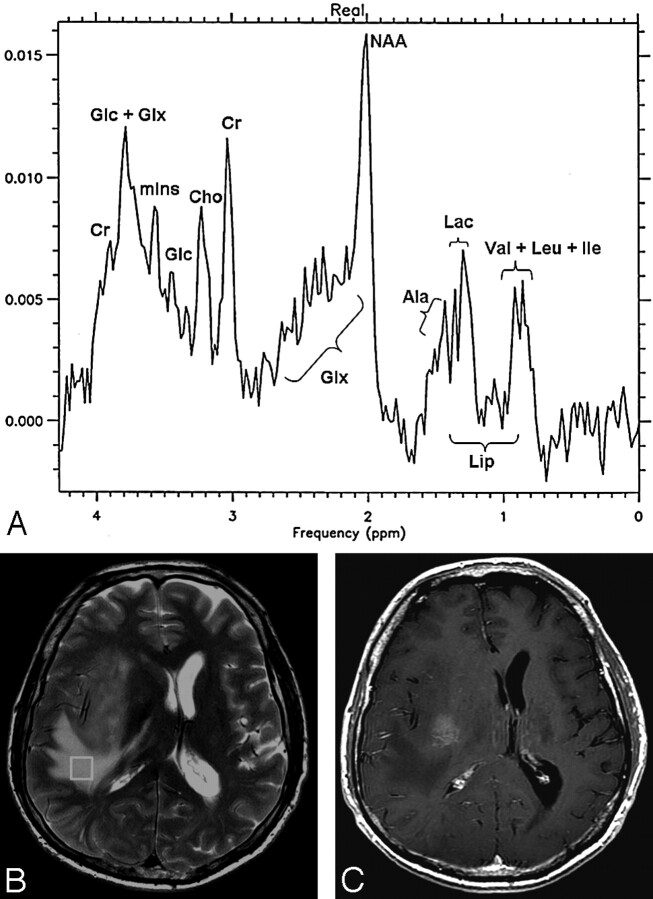
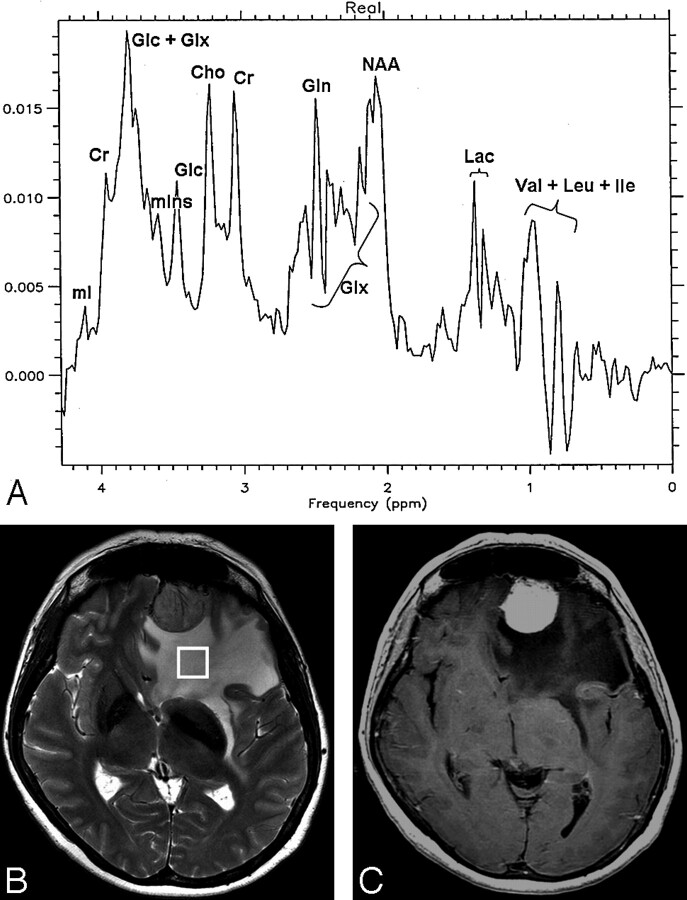
Lac was found in variable amounts in 12 (92.3%) patients: 6 with glioblastomas, all with metastases and meningiomas, and Lip in 5 (35.5%) patients: 3 with glioblastomas and 2 with meningiomas. Figures 2A and 3A show the MR spectra corresponding to the edematous tissue in patient 3, affected by a glioblastoma (Fig 2, B-C), and in patient 12, affected by a meningioma (Fig 3, B-C).
Fig 2.
A glioblastoma in patient 3.
A, Localized proton spectrum of the edema surrounding the large temporofrontal glioblastoma. The NAA/Cr, NAA/Cho, Cho/Cr, and mIns/Cr ratios are in the normal range. The Glx/Cr ratio is increased to 2.2 (normal value, 1.8 ± 0.2). Glc (3.78 and 3.44 ppm), Lac, Lip, Val, Leu, Ile, and Ala are detected.
B, Axial FSE T2-weighted image (TR, 4200 ms; TE, 93 ms; NEX, 2) with the location of the voxel (6.4 cm3) in the edematous tissue.
C, Axial SE T1-weighted image (TR, 560 ms; TE, 18 ms) after administration of contrast agent. At this level, the upper part of the tumor can be seen.
Fig 3.
A meningioma in patient 12.
A, Localized proton spectrum of the edema surrounding the meningioma. The NAA/Cho, Cho/Cr, and mIns/Cr ratios are in the normal range. NAA/Cr is decreased to 1.4 (normal value, 1.8 ± 0.3), and Glx/Cr is increased to 2.5 (normal value, 1.8 ± 0.2). Glc, Lac, Lip, Val, Leu, and Ile are detected.
B, Axial FSE T2-weighted image (TR, 4200 ms; TE, 93 ms; NEX, 2) with the voxel (8 cm3) location in the edematous tissue.
C, Axial SE T1-weighted image (TR, 560 ms; TE, 18 ms) after administration of contrast agent with strong enhancement of the tumor.
As shown in Fig 3A, in the region of the resonances between 2.1 and 2.5 ppm, relative to the Glx complex, a strong signal intensity centered at 2.44 ppm was present and assigned to glutamine.25 This resonance was present in noticeable intensity in 6 (46.2%) patients: 1 with glioblastoma, 2 with metastases, and all with meningiomas.
Moreover, an intense broad signal intensity between 3.6 and 3.8 ppm was found in all patients. This resonance usually arises from the α-hydrogens of amino acids (such as, for example, Glx), from mIns and other metabolites, such as mannitol and Glc. Mannitol, not present in the healthy brain, can be detected if administered as an anti-edema drug, but we can exclude its presence in our spectra because the patients did not receive it.
The presence of Glc was confirmed by the second peak of this metabolite at 3.44 ppm,26,27 as clearly shown in Figs 2A and 3A. However, taurine also had a resonance peak at 3.4 ppm. Nevertheless, we excluded the presence of this metabolite because the relative concentration of Cho was normal in all our patients. In fact, the second peak of taurine at 3.2 ppm, if present, would determine the increase in the relative concentration of Cho. As a consequence, Glc was also detected in 12 (92.3%) patients: 6 with glioblastomas, all with metastases and meningiomas.
Discussion
Edematous brain tissue associated with extra-axial tumors, such as meningiomas or metastatic neoplasms, usually is not involved by tumoral infiltrations. Conversely, in the case of gliomas, the surrounding edematous area is infiltrated by tumoral cells, even beyond the area of enhancement.28 On this basis, Chiang et al29 were able to differentiate by MR spectroscopy between high-grade gliomas and solitary metastases on the basis of their different Cho/Cr ratios.
In our cohort, it should be noted that the Cho/Cr signal intensity ratio was normal in the edematous tissue of all the patients and also in the edema associated with glioblastomas. This means that in the analyzed VOIs, no evidence of cell proliferation was found, which allowed us to assert that the patients had only edema.
The most recurrent metabolic findings associated with cerebral peritumoral edema are decreased NAA and the appearance of lactate (Lac).12,19,20,22 In particular, Chernov et al12 examined peritumoral brain tissue and found a significant reduction in the NAA/Cr and NAA/Cho ratios and a more frequent presence of Lac compared with the healthy brain. In their study, the NAA level was lowest in cases with a higher content of Lac in the lesion than in the perilesional brain tissue, which suggests that Lac diffuses from tumoral into peritumoral tissue. In the opinion of these authors, the decrease in NAA might have corresponded to the neuronal alteration responsible for associated epilepsy.
Also in our study, nearly all the patients (92.3%) had Lac in the edema, whereas the decrease in the relative concentration of NAA occurred only in 30.8% of the patients. Although we cannot exclude that the reduction in the relative concentration of NAA correlates with the presence of Lac, we have to take into account also that the administration of dexamethasone may be responsible for the metabolic alteration itself.30
In our cases, MR spectroscopy of the areas of the tumor (data not shown) revealed that Lac was present in 5 neoplasms (all meningiomas and 2 glioblastomas). In the spectra of the other tumors, lactate was not assessable because of the presence of intense resonances of mobile lipids related to extensive tumoral necrosis. However, its presence could not be excluded. Therefore, we cannot rule out that Lac in edema is the result of diffusion from the tumor, according to Chernov.12
To our knowledge, there are no published studies of MR spectroscopy reporting the presence of Glc in brain edema. Although this metabolite has been detected on 1H-MR spectroscopy in patients with diabetes,31,32 none of the patients in our cohort had diabetes mellitus. Therefore, Glc seems to be a feature of edema.
Both Glc and Lac are claimed to be integral and important entities of metabolism of cerebral energy.33 Lac plays an important role in the metabolism of cerebral oxidative energy, as does Glc.33,34 Lac is oxidized by neuronal mitochondria, and its presence inhibits the consumption of Glc, which therefore accumulates in the tissue.33 This theory would account for the concomitant presence of Lac and Glc in 11 (84.6%) of our patients.
A very important metabolic change, because it was found in nearly all our patients, was the increase in the Glx complex. This alteration is correlated with neuronal loss and demyelination.35 Danielsen and Ross36 claimed that if glutamate exceeds the appropriate concentration as a neurotransmitter, its surplus leads to a toxic effect. It follows that the glutamine-synthetase enzyme would increase to protect the astrocytes by removing the excess glutamate, and this would account for the increase in glutamine that we have seen in our spectra.
In addition, the resonances assigned to mobile lipids were found in 5 (38.5%) of our patients. The presence of lipids in 1H-MR spectra is correlated with changes in the cell membrane with a mobilization of membrane phospholipids.37 Danielsen and Ross36 reported that lipids are also associated with myelin, and their mobilization could be related to a structural myelin impairment.
Our study also detected peaks relative to the amino acids Val, Leu, and Ile in 92.3% of patients. A 1H-MR spectroscopy on people with multiple sclerosis (MS) reported that these substances also correlated with demyelination in MS plaques.35 Other MS studies have reported an increased Cho/Cr ratio in plaque during acute disease,38 and this alteration was related to severe inflammation without demyelination.39 The fact that the Cho/Cr ratio was normal in all of our patients does not rule out the possibility that the metabolic changes that we documented were associated with tissue damage without inflammation.
If Lac is an energy source for neuronal mitochondria, according to Schurr's theory,33 the accumulation of Glc and Glx could have a toxic effect with structural myelin impairment, as testified by the presence of amino acids and lipids. This global metabolic alteration can be reversible after resection of a tumor, whereas an irreversible change in the structure of the myelin sheath could explain the persistent MR T2-weighted signal hyperintensity that failed to return to normal after tumor resection.40 In fact, demyelinization can occur secondary to a local mass effect, as reported by Heinz41 in the case of a patient with a calcified meningioma. This meningioma showed a large hypoattenuated area around the tumor on CT, and results of a biopsy showed demyelinization. When signal hyperintensity on MR T2-weighted images persists after tumor resection, irreversible and more severe structural myelin impairment can be hypothesized.
Conclusions
This study supported the hypothesis that brain tissue involved by edema has intrinsic metabolic properties. 3T 1H-MR spectroscopy revealed substances such as Glc and amino acids not previously revealed by a lower magnetic field. Our findings suggest that the edema spectra are indicative of energy-linked metabolic damage associated with structural injury, which may or may not be reversible. As the literature correlates these changes to myelin injury, we suggest that myelin is the most involved structural component.
Acknowledgments
We thank Prof. Patrizia Agati of the University of Bologna for her important contribution to the statistical analysis.
References
- 1.Klatzo I. Presidential address. Neuropathological aspects of brain edema. J Neuropathol Exp Neurol 1967;26:1–14 [DOI] [PubMed] [Google Scholar]
- 2.Kimelberg HK. Current concepts of brain edema. Review of laboratory investigation. J Neurosurg 1995;83:1051–59 [DOI] [PubMed] [Google Scholar]
- 3.Tatagiba M, Mirzai S, Samii M. Peritumoral blood flow in intracranial meningiomas. Neurosurgery 1991;28:400–04 [DOI] [PubMed] [Google Scholar]
- 4.Hiyama H, Kubo O, Tajika Y, et al. Meningiomas associated with peritumoral venous stasis: three types on cerebral angiogram. Acta Neurochir (Wien) 1994;129:31–38 [DOI] [PubMed] [Google Scholar]
- 5.Philippon J, Foncin JF, Grob R, et al. Cerebral edema associated with meningiomas: possible role of a secretory-excretory phenomenon. Neurosurgery 1984;14:295–301 [DOI] [PubMed] [Google Scholar]
- 6.Constantini S, Tamir J, Gomori MJ, et al. Tumor prostaglandin levels correlate with edema around supratentorial meningiomas. Neurosurgery 1993;33:204–10; discussion 211 [PubMed] [Google Scholar]
- 7.Benzel EC, Gelder FB. Correlation between sex hormone binding and peritumoral edema in intracranial meningiomas. Neurosurgery 1988;23:169–74 [DOI] [PubMed] [Google Scholar]
- 8.Kalkanis SN, Carroll RS, Zhang J, et al. Correlation of vascular endothelial growth factor messenger RNA expression with peritumoral vasogenic cerebral edema in meningiomas. J Neurosurg 1996;85:1095–101 [DOI] [PubMed] [Google Scholar]
- 9.Bitzer M, Klose U, Geist-Barth B, et al. Alterations in diffusion and perfusion in the pathogenesis of peritumoral brain edema in meningiomas. Eur Radiol 2002;12:2062–76 [DOI] [PubMed] [Google Scholar]
- 10.Krabbe K, Gideon P, Wagn P, et al. MR diffusion imaging of human intracranial tumors. Neuroradiology 1997;39:483–89 [DOI] [PubMed] [Google Scholar]
- 11.Henry RG, Vigneron DB, Fischbein NJ, et al. Comparison of relative cerebral blood volume and proton spectroscopy in patients with treated gliomas. AJNR Am J Neuroradiol 2000;21:357–66 [PMC free article] [PubMed] [Google Scholar]
- 12.Chernov MF, Kubo O, Hayashi M, et al. Proton MRS of the peritumoral brain. J Neurol Sci 2005;228:137–42 [DOI] [PubMed] [Google Scholar]
- 13.Law M, Cha S, Knopp EA, et al. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 2002;222:715–21 [DOI] [PubMed] [Google Scholar]
- 14.Braun KP, Dijkhuizen RM, de Graaf RA, et al. Cerebral ischemia and white matter edema in experimental hydrocephalus: a combined in vivo MRI and MRS study. Brain Res 1997;757:295–98 [DOI] [PubMed] [Google Scholar]
- 15.Rooney WD, Ebisu T, Mancuso A, et al. Metabolite 1H relaxation in normal and hyponatremic brain. Magn Reson Med 1996;35:688–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Son BC, Park CK, Choi BG, et al. Metabolic changes in pericontusional oedematous areas in mild head injury evaluated by 1H MRS. Acta Neurochir Suppl 2000;76:13–16 [DOI] [PubMed] [Google Scholar]
- 17.Kamada K, Houkin K, Iwasaki Y, et al. In vivo proton magnetic resonance spectroscopy for metabolic changes of human brain edema. Neurol Med Chir (Tokyo) 1994;34:676–81 [DOI] [PubMed] [Google Scholar]
- 18.Kamada K. [Sequential observations of brain edema with proton magnetic resonance imaging and spectroscopy.] Hokkaido Iqaku Zasshi 1996;71:105–22 [PubMed] [Google Scholar]
- 19.Domingo Z, Rowe G, Blamire AM, et al. Role of ischaemia in the genesis of oedema surrounding meningiomas assessed using magnetic resonance imaging and spectroscopy. Br J Neurosurg 1998;12:414–18 [DOI] [PubMed] [Google Scholar]
- 20.Chumas P, Condon B, Oluoch-Olunya D, et al. Early changes in peritumorous oedema and contralateral white matter after dexamethasone: a study using proton magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry 1997;62:590–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamada K, Moller M, Saguer M, et al. A combined study of tumor-related brain lesions using MEG and proton MR spectroscopic imaging. J Neurol Sci 2001;186:13–21 [DOI] [PubMed] [Google Scholar]
- 22.Sijens PE, Oudkerk M. 1H chemical shift imaging characterization of human brain tumor and edema. Eur Radiol 2002;12:2056–61 [DOI] [PubMed] [Google Scholar]
- 23.Di Costanzo A, Trojsi F, Tosetti M, et al. High-field proton MRS of human brain. Eur J Radiol 2003;48:146–53 [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Chang KH, Song IC, et al. Brain abscess and brain tumor: discrimination with in vivo H-1 MR spectroscopy. Radiology 1997;204:239–45 [DOI] [PubMed] [Google Scholar]
- 25.Moore CM, Wardrop M, deB Frederick B, et al. Topiramate raises anterior cingulate cortex glutamine levels in healthy men; a 4.0 T magnetic resonance spectroscopy study. Psychopharmacology (Berl) 2006;188:236–43 [DOI] [PubMed] [Google Scholar]
- 26.Opstad KS, Provencher SW, Bell BA, et al. Detection of elevated glutathione in meningiomas by quantitative in vivo 1H MRS. Magn Reson Med 2003;49:632–37 [DOI] [PubMed] [Google Scholar]
- 27.Rothman DL. Studies of metabolic compartmentation and glucose transport using in vivo MRS. NMR Biomed 2001;14:149–60 [DOI] [PubMed] [Google Scholar]
- 28.Daumas-Duport C, Monsaigheon V, Blond S, et al. Serial stereotactic biopsies and CT scan gliomas: correlative study in 100 astrocytomas, oligo-astrocytomas and oligodendrocytomas. J Neurooncol 1987;4:317–28 [DOI] [PubMed] [Google Scholar]
- 29.Chiang IC, Kuo YT, Lu CY, et al. Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 2004;46:619–27 [DOI] [PubMed] [Google Scholar]
- 30.Chumas P, Condon B, Oluoch-Olunya D, et al. Early changes in peritumorous oedema and contralateral white matter after dexamethasone: a study using proton magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry 1997;62:590–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron FJ, Kean MJ, Wellard RM, et al. Insights into the acute cerebral metabolic changes associated with childhood diabetes. Diabet Med 2005;22:648–53 [DOI] [PubMed] [Google Scholar]
- 32.Kreis R, Ross BD. Cerebral metabolic disturbances in patients with subacute and chronic diabetes mellitus: detection with proton MR spectroscopy. Radiology 1992;184:123–30 [DOI] [PubMed] [Google Scholar]
- 33.Schurr A. Lactate: the ultimate cerebral oxidative energy substrate? J Cereb Blood Flow Metab 2006;26:142–52 [DOI] [PubMed] [Google Scholar]
- 34.Serres S, Bezancon E, Franconi JM, et al. Ex vivo NMR study of lactate metabolism in rat brain under various depressed states. J Neurosci Res 2005;79:19–25 [DOI] [PubMed] [Google Scholar]
- 35.Sharma R. Serial amino-neurochemicals analysis in progressive lesion analysis of multiple sclerosis by magnetic resonance imaging and proton magnetic resonance spectroscopic imaging. Magn Reson Med Sci 2002;1:169–73 [DOI] [PubMed] [Google Scholar]
- 36.Danielsen ER, Ross B. Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases. New York: Marcel Dekker;1999. :35–36
- 37.Negendank W. Studies of brain tumors by MRS: a review. NMR Biomed 1992;5:303–24 [DOI] [PubMed] [Google Scholar]
- 38.Landtblom AM, Sjoqvist L, Soderfeldt B, et al. Proton MR spectroscopy and MR imaging in acute and chronic multiple sclerosis –ringlike appearances in acute plaques. Acta Radiol 1996;37:278–87 [DOI] [PubMed] [Google Scholar]
- 39.Brenner RE, Munro PM, Williams SC, et al. The proton NMR spectrum in acute EAE: the significance of the change in the Cho:Cr ratio. Magn Reson Med 1993;29:737–45 [DOI] [PubMed] [Google Scholar]
- 40.Stevens JM, Ruiz JS, Kendall BE. Observation on peritumoral oedema in meningioma. Part I: Distribution, spread and resolution of vasogenic oedema seen on computed tomography. Neuroradiology 1983;25:71–80 [DOI] [PubMed] [Google Scholar]
- 41.Heinz ER. Normal and abnormal white matter. In: Rosenberg RN, ed. Clinical Neurosciences. London: Churchill Livingstone;1984. :606