Abstract
Purpose
To compare the accuracy of semiautomated flow tracking with that of semiautomated valve tracking in the quantification of mitral valve (MV) regurgitation from clinical four-dimensional (4D) flow MRI data obtained in patients with mild, moderate, or severe MV regurgitation.
Materials and Methods
The 4D flow MRI data were retrospectively collected from 30 patients (21 men; mean age, 61 years ± 10 [standard deviation]) who underwent 4D flow MRI from 2006 to 2016. Ten patients had mild MV regurgitation, nine had moderate MV regurgitation, and 11 had severe MV regurgitation, as diagnosed by using semiquantitative echocardiography. The regurgitant volume (Rvol) across the MV was obtained using three methods: indirect quantification of Rvol (RvolINDIRECT), semiautomated quantification of Rvol using valve tracking (RvolVALVE), and semiautomated quantification of Rvol using flow tracking (RvolFLOW). A second observer repeated the measurements. Aortic valve flow was quantified as well to test for intervalve consistency. The Wilcoxon signed rank test, orthogonal regression, Bland-Altman analysis, and coefficients of variation were used to assess agreement among measurements and between observers.
Results
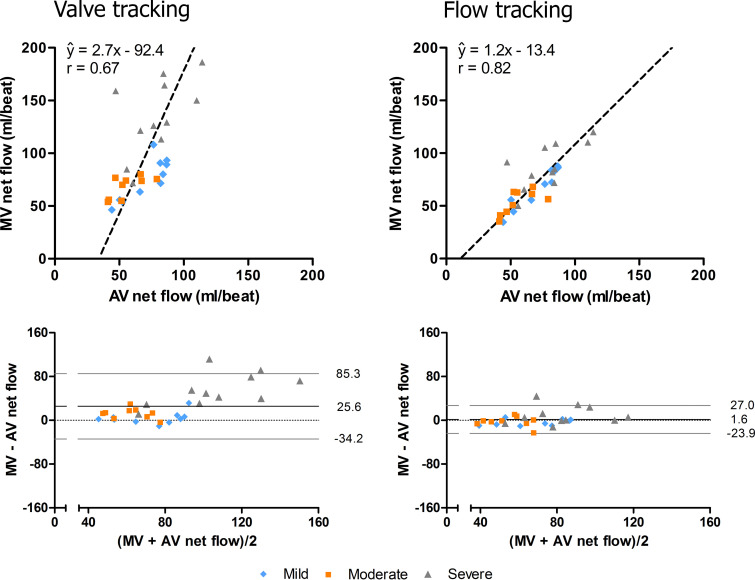
RvolFLOW was higher (median, 24.8 mL; interquartile range [IQR], 14.3–45.7 mL) than RvolVALVE (median, 9.9 mL; IQR, 6.0–16.9 mL; P < .001). Both RvolFLOW and RvolVALVE differed significantly from RvolINDIRECT (median, 19.1 mL; IQR, 4.1–47.5 mL; P = .03). RvolFLOW agreed more with RvolINDIRECT (ŷ = 0.78x + 12, r = 0.88) than with RvolVALVE (ŷ = 0.16x + 8.1, r = 0.53). Bland-Altman analysis revealed underestimation of RvolVALVE in severe MV regurgitation. Interobserver agreement was excellent for RvolFLOW (r = 0.95, coefficient of variation = 27%) and moderate for RvolVALVE (r = 0.72, coefficient of variation = 57%). Orthogonal regression demonstrated better intervalve consistency for flow tracking (ŷ = 1.2x − 13.4, r = 0.82) than for valve tracking (ŷ = 2.7x − 92.4, r = 0.67).
Conclusion
Flow tracking enables more accurate 4D flow MRI–derived MV regurgitation quantification than valve tracking in terms of agreement with indirect quantification and intervalve consistency, particularly in severe MV regurgitation.
Supplemental material is available for this article.
© RSNA, 2020
Summary
In patients with varying degrees of mitral valve regurgitation, four-dimensional flow MRI in combination with semiautomated flow tracking facilitates reproducible absolute regurgitant volume quantification with improved accuracy compared with semiautomated valve tracking.
Key Points
■ In patients with mitral valve (MV) regurgitation, semiautomated flow tracking enables more accurate four-dimensional flow MRI–based quantification of absolute regurgitant volume than semiautomated valve tracking, particularly in severe MV regurgitation.
■ Four-dimensional flow MRI–based MV regurgitation quantification is promising and potentially valuable for improving clinical triage of patients with MV regurgitation when used in addition to conventional MRI acquisitions for the evaluation of ventricular volumes and MV morphologic characteristics.
Introduction
Therapeutic decision-making in patients with mitral valve (MV) regurgitation is predominantly based on presenting symptoms and MV regurgitation severity. Transthoracic echocardiography is the method of choice for the assessment of MV regurgitation severity and is based on an integrated approach that uses a broad spectrum of measures, including valve morphologic characteristics, regurgitant jet characteristics, an estimate of the regurgitant volume (Rvol) using the proximal isovelocity surface area method, vena contracta width, pulmonary vein systolic flow reversal, and left ventricular (LV) dimensions (1,2). For patients in whom transthoracic echocardiographic examinations are unreliable because of poor acoustic windows or poor alignment of the transducer beam with the regurgitant jet, cardiac MRI is recommended for the diagnostic and prognostic assessment of MV regurgitation (2,3). Furthermore, a growing body of literature suggests that MRI could improve diagnosis and surgical timing in patients with MV regurgitation compared with transthoracic echocardiography (4–6). MRI is more accurate and reproducible than echocardiography in the assessment of ventricular volumes and flow across the heart valves (7,8). Although quantification of Rvol is possible with echocardiography, it has been associated with poor-to-moderate inter- and intraobserver agreement (4,9). Cardiac MRI–derived Rvol is associated with high interobserver agreement and is an independent predictor of the future need for surgery and postsurgical LV remodeling, with more predictive power than echocardiographic assessment (4,5). Moreover, in a cohort of 258 asymptomatic patients with moderate-to-severe primary MV regurgitation who received treatment on the basis of echocardiographic findings, quantification of Rvol with MRI resulted in reclassification of 24% of patients and led to better prognostic assessment in terms of indication for MV surgery or in terms of death (6).
The current state-of-the-art MRI method to quantify MV regurgitation makes use of two different acquisition techniques: two-dimensional (2D) phase-contrast MRI (2D flow MRI) across the aortic valve (AV) and short-axis cine MRI (balanced steady-state free precession [bSSFP]) of the LV. Combined, these acquisitions allow for calculation of Rvol across the MV by subtracting the AV forward flow (as measured with 2D flow MRI) from the LV stroke volume (LVSV [as measured with bSSFP]). This indirect method is required because the MV has a high degree of annular motion, which is not accounted for with use of 2D flow MRI in a fixed slice across the MV.
With the advent of four-dimensional (4D) flow MRI, it has become possible to quantify three-directional blood flow in three dimensions over time and, thus, to perform flow quantification during postprocessing, using measurement planes that follow the motion of the heart valves. This technique is also known as retrospective valve tracking (10). It has been shown that 4D flow MRI in combination with retrospective valve tracking enables accurate blood flow measurements across all four heart valves (11,12). Quantification of MV regurgitation at the level of the valve is challenging, however, as regurgitant flow is characterized by high blood velocity, turbulence, and incoherent flow at the valvular level, resulting in higher orders of motion. In such regions, protons within the same acquired voxel can have different velocities, canceling out the composite signal (intravoxel phase dispersion), which in turn leads to signal loss and an underestimation of Rvol (13–16). A suggested solution is to measure in the left atrium at a distance of 1–2 cm from the regurgitant orifice, and perpendicular to the regurgitant jet, an approach called flow tracking (17,18). Thus far, this type of analysis has only been performed in patients with mild or moderate MV regurgitation and has not been performed in patients with severe MV regurgitation. In addition, it is not known to what extent flow tracking can improve measurement accuracy compared with valve-tracking analysis. The purpose of this study was to compare semiautomated flow tracking with semiautomated valve tracking for quantification of Rvol from clinical 4D flow MRI data obtained in a group of patients with mild, moderate, or severe MV regurgitation. We hypothesized that use of semiautomated flow tracking is more accurate than use of semiautomated valve tracking for measurement of Rvol across the MV. A secondary objective was to compare 4D flow MRI–based severity classification with echocardiography-based classification of mild, moderate, and severe MV regurgitation.
Materials and Methods
Caas MR Solutions software was provided by Pie Medical Imaging (Maastricht, the Netherlands). Data inclusion and analysis were controlled by authors not employed by Pie Medical Imaging.
Study Population
Thirty-four patients with MV regurgitation diagnosed with echocardiography who underwent cardiothoracic MRI including 4D flow MRI for assessment of MV regurgitation were retrospectively selected from local research databases in a consecutive manner (Leiden UMC and UMC Utrecht, the Netherlands). Examinations were performed between 2006 and 2016, and we ensured that the studies also included cine bSSFP MRI (two-chamber, three-chamber, four-chamber, coronal aorta view, and short-axis stack) and 2D flow MRI of the ascending aorta. Further inclusion criteria were data set compatibility with the postprocessing software, a sufficiently large field of view to perform flow tracking, the absence of severe velocity aliasing in the 4D flow MRI data, and the absence of shunt flow, as net flow differences between the MV and AV would invalidate intervalve consistency tests. Three data sets were excluded because of insufficient field-of-view coverage, and one data set was excluded because of severe velocity aliasing. Thirty patients were included (21 men [mean age, 60 years ± 9 {standard deviation}; nine women [mean age, 64 years ± 12]; mean age, 61 years ± 10). Sample size was determined on the basis of prior knowledge of the number of available severe MV regurgitation data sets (n = 11), making sure that mild, moderate, and severe MV regurgitation groups were approximately equal in size. The patients imaged at Leiden UMC had secondary mild (n = 10) or moderate MV regurgitation (n = 9) and cardiomyopathy. Patients imaged at UMC Utrecht had asymptomatic primary severe MV regurgitation (n = 11). Patients with mild or moderate MV regurgitation had previously been included in research studies on the application of manual and semiautomated valve tracking in patients with various heart diseases (11,19). However, those studies did not include patients with severe MV regurgitation, nor did they investigate the diagnostic utility of semiautomated flow tracking.
Severity grading was based on semiquantitative echocardiographic examinations with scores for valves with abnormal morphologic characteristics, visually assessed regurgitant jet characteristics, vena contracta width, presence of pulmonary vein systolic flow reversal, and LV dimensions (20). Echocardiographic scoring of patients with severe MV regurgitation was also performed using a recently proposed scoring index (21) by three cardiologists (S.M.B., S.A.J.C., and G.P.B., with 10, 12, and 2 years of experience in transthoracic echocardiography and transesophageal echocardiography, respectively). The cardiologists unanimously confirmed the presence of severe MV regurgitation. Institutional medical ethical approval was obtained for the study, and all patients provided written informed consent.
Data Acquisition
MRI scans were acquired with 1.5-T MRI systems (Intera and Ingenia; Philips Healthcare, Best, the Netherlands). The 2D cine bSSFP was performed at a spatial resolution of 1.25 × 1.25 × 8.00 mm3 to 1.56 × 1.56 × 10.00 mm3 and in 30 cardiac phases. The 2D flow MRI measurements were performed at the level of the midascending aorta at a spatial resolution of 1.25 × 1.25 × 8.00 mm2 to 1.37 × 1.37 × 8.00 mm2. The number of cardiac phases was 20 for the group with severe MV regurgitation and 40 for the groups with mild or moderate MV regurgitation. This was due to a difference in cardiac MRI protocols between the hospitals. Both acquisitions were electrocardiographically gated and performed during breath holds. Patients with mild or moderate MV regurgitation underwent MRI and echocardiography on the same day. Patients with severe MV regurgitation underwent the examinations several days or weeks apart (median, 20 days; interquartile range [IQR], 5–81 days).
Four-dimensional flow MRI was performed using retrospective electrocardiographic gating and during free breathing with the following parameters: three-directional velocity encoding, 150–280 cm/sec; repetition time msec/echo time msec, 14/3.3 to 8.3/4.3; and flip angle, 10°. For acceleration, echo-planar imaging was used with a factor of five and a sensitivity-encoding factor of two (10). The acquired spatial resolution was 2.90 × 3.80 × 6.00 mm3 for patients with mild or moderate MV regurgitation and 3.43 × 3.66 × 3.50 mm3 for those with severe MV regurgitation. The through-plane spatial resolution was higher in patients with severe MV regurgitation to mitigate phase dispersion in eccentric and angulated regurgitation jets. The reconstructed spatial resolutions were 1.45 × 1.45 × 6.00 mm3 (mild and moderate) and 2.89 × 2.89 × 3.50 mm3 (severe), and the field of view was 370 × 370 × 48 mm3 (mild and moderate) and 370 × 370 × 63 mm3 (severe). Thirty cardiac phases were measured, resulting in reconstructed temporal resolutions of 21–39 msec.
Data Analysis
Three methods were used to quantify Rvol across the MV: indirect quantification of Rvol (RvolINDIRECT), semiautomated quantification of Rvol using valve tracking (RvolVALVE), and semiautomated quantification of Rvol using flow tracking (RvolFLOW). Analyses were performed by C.P.S.B., who had 2.5 years of experience in cardiac 4D flow MRI analysis and access to the patients’ echocardiography-based severity grades.
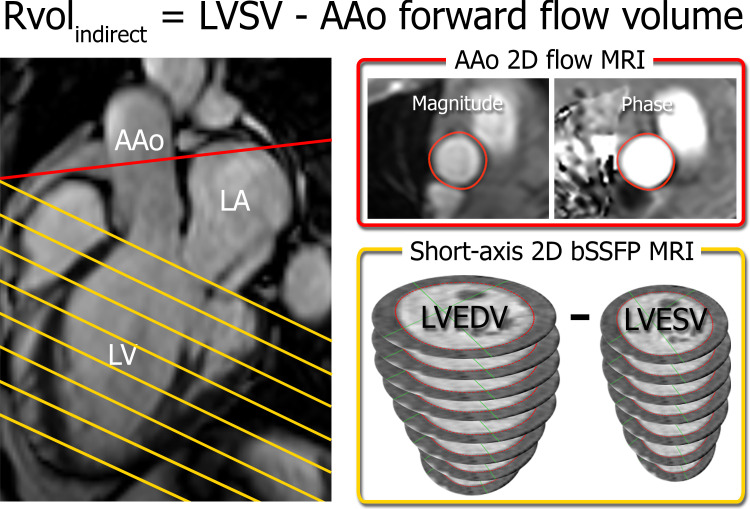
Indirect quantification.—Caas MR Ventricular analysis software (version 4.3, Pie Medical Imaging) was used to contour endocardial borders at end diastole (LV end-diastolic volume) and end systole (LV end-systolic volume) on short-axis bSSFP images to determine LVSV (LVSV = LV end-diastolic volume − LV end-systolic volume). Calculation of LVSV included apex-to-base volume correction based on manually drawn long-axis (two-chamber and four-chamber) endo- and epicardial contours. Forward flow across the ascending aorta was determined from 2D flow MRI (Caas MR Flow version 1.1, Pie Medical Imaging), and RvolINDIRECT was determined by subtracting the ascending aorta forward flow from the LVSV (Fig 1). RvolINDIRECT was chosen as a reference standard because of its good prognostic value and reproducibility (4–8).
Figure 1:
Indirect quantification of regurgitant volume (RvolINDIRECT) by using short-axis balanced steady-state free precession (bSSFP) MRI in the left ventricle (LV) (yellow) and two-dimensional (2D) flow MRI in the ascending aorta (AAo) (red). Slice locations are indicated on a three-chamber bSSFP image (left). Semiautomated contouring of the aortic flow area (top right) and the LV endocardial borders at end diastole and end systole (bottom right) allows for quantification of the ascending aorta forward flow volume and LV stroke volume (LVSV) and calculation of RvolINDIRECT. LA = left atrium, LVEDV = LV end-diastolic volume, LVESV = LV end-systolic volume.
Valve tracking.—Valve tracking was performed with 2D cine bSSFP images (Caas MR Solutions version 5.1 - 4D flow; Pie Medical Imaging). The MV was tracked on two- and four-chamber cine views. After manually identifying the location of the MV annulus by selecting two points in each view at a single phase, the motion of the valve was tracked automatically throughout the cardiac cycle. If correction was needed, the automated tracking was repeated starting from a different cardiac phase. Next, color-coded 4D flow MRI–derived in-plane velocities were projected onto the moving long-axis cine views to check for possible misalignment between the 4D flow MRI data and cine images, which could be corrected for by means of translation. Next, a time-resolved three-dimensional plane was reconstructed and mapped to the 4D flow MRI data, and an initial four-point contour was generated automatically on the basis of the landmark points that were used for tracking in the two- and four-chamber cine views. Visual feedback was provided by means of a color-coded 4D flow MRI through-plane velocity overlay and used to make manual adjustments to the contour. Finally, forward and backward blood flow were quantified in milliliters per heartbeat and corrected for through-plane valve motion based on the tracked valve. For every cardiac phase, streamlines were generated from within the contour to allow three-dimensional visualization superimposed on the long-axis cine views.
In the case of MV prolapse that caused the regurgitant orifice to be located in the atrium and not in the annular plane, an additional measurement was obtained in which the valve-tracking plane was moved to the level of the regurgitant orifice during regurgitation. This correction was performed to anticipate the possibility of Rvol underestimation merely due to a spatial mismatch between the location of measurement and the regurgitant orifice.
Flow tracking.—Flow tracking was only performed when regurgitation was present (ie, during systole). Color-coded 4D flow MRI–derived in-plane velocities were projected onto the moving long-axis cine views and served to identify the location of the MV regurgitation jet on a two-chamber and four-chamber cine bSSFP image (Caas MR Solutions, version 5.1 - 4D flow). By clicking in the MV regurgitation jet, 1–2 cm distal to the regurgitant orifice inside the left atrium, a measurement plane was generated and automatically angulated perpendicular to the direction of the regurgitant jet. In the reformatted measurement plane, an initial four-point contour was generated automatically and manually adjusted when needed on the basis of the 4D flow MRI velocity map. Both tracking methods included automatic velocity-aliasing correction and velocity-offset correction, performed by fitting a linear plane through the intensities of automatically detected stationary tissue voxels in the phase-contrast images. The phase-contrast values of the linear plane were subsequently subtracted from the original images (22).
All three above-mentioned methods to determine Rvol were repeated by a second observer, J.J.M.W., with 15 years of experience in cardiac 4D flow MRI analysis, to test for reproducibility. For validation purposes, the first observer also quantified forward and backward flow across the AV by means of semiautomated valve tracking and, when appropriate, flow tracking, to test for intervalve consistency on the basis of the principle of conservation of mass (MV forward flow − MV backward flow [referred to as Rvol] = AV forward flow − AV backward flow). Valve tracking of the AV was performed on coronal and three-chamber cine bSSFP images.
The 4D flow MRI–based severity grades were compared with echocardiography-based grades after applying prespecified cutoff values (≥30 and ≥60 mL for moderate and severe MV regurgitation, respectively) (2,20).
Statistical Analysis
Statistical testing was performed by using SPSS Statistics (version 25.0; IBM, Armonk, NY) by C.P.S.B. Normality testing was performed by using a Shapiro-Wilk test. Agreement among RvolINDIRECT, RvolVALVE, and RvolFLOW was evaluated using a Friedman test and post hoc Wilcoxon signed rank tests. The agreement between MV and AV net flow was assessed by using a Wilcoxon signed rank test. Orthogonal regression and Bland-Altman analysis were used to further assess the agreement among the observed variables and to evaluate interobserver agreement. Coefficients of variation, defined as the standard deviation of the interobserver differences in Rvol divided by the mean Rvol between both observers, were also determined. The Pearson correlation coefficient is denoted by r, and P < .05 was considered indicative of a statistically significant difference. The 4D flow MRI–based and echocardiography-based severity classifications were compared by using categorical scatterplots and contingency tables.
Results
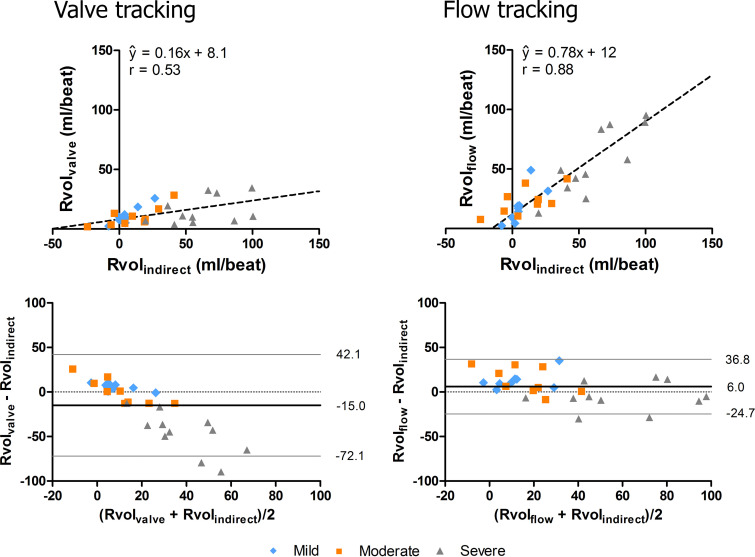
Semiautomated valve tracking and flow tracking allowed for quantification of Rvol in all patients with MV regurgitation. Figure 2 shows a representative example. The results are shown in Figure 3, as plotted against RvolINDIRECT measurements. Significant differences were found among RvolINDIRECT, RvolVALVE, and RvolFLOW (P < .001). Overall, RvolFLOW was higher (median, 24.8 mL; IQR, 14.3–45.7 mL) than RvolVALVE (median, 9.9 mL; IQR, 6.0–16.9 mL; P < .001). Both RvolFLOW and RvolVALVE differed significantly from RvolINDIRECT (median, 19.1 mL; IQR, 4.1–47.5 mL; P = .03 for both). Orthogonal regression revealed better agreement between RvolFLOW and RvolINDIRECT (ŷ = 0.78x + 12, r = 0.88) compared with RvolVALVE and RvolINDIRECT (ŷ = 0.16x + 8.1, r = 0.53). Bland-Altman analysis revealed a trend toward underestimation of RvolVALVE in severe MV regurgitation (Fig 3 [bottom]).
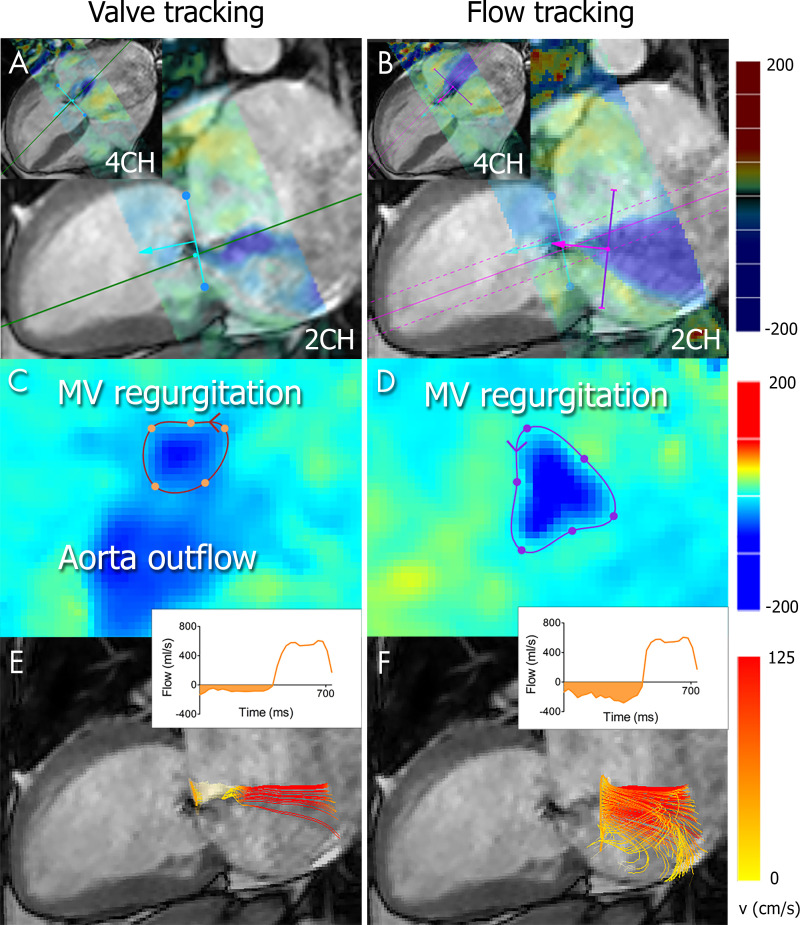
Figure 2:
Regurgitant volume quantification with four-dimensional (4D) flow MRI in a 38-year-old woman with severe mitral valve (MV) regurgitation diagnosed with echocardiography. A, B, The location of the MV annulus is identified on two-chamber (2CH) and four-chamber (4CH) cine balanced steady-state free precession images, followed by, A, automatic valve tracking throughout the cardiac cycle. During regurgitation, an additional plane is initialized to enable, B, flow tracking at a 1–2-cm distance from the regurgitant orifice and perpendicular to the direction of the regurgitant jet. Colors represent in-plane 4D flow MRI velocities projected onto the long-axis cine views. C, D, The 4D flow MRI through-plane velocity measurements are projected onto the valve-tracking or flow-tracking plane, allowing for detailed contouring of the flow region of interest. E, F, Time-resolved streamlines are generated from within the contour, and the flow is quantified (inset).
Figure 3:
Orthogonal regression (top) and Bland-Altman (bottom) plots of regurgitant volume (Rvol) measured with valve tracking (RvolVALVE) (left) and with flow tracking (RvolFLOW) (right) versus indirectly quantified Rvol (RvolINDIRECT) (RvolINDIRECT = left ventricular stroke volume − ascending aorta). Mean differences and 95% limits of agreement are indicated by the black and gray lines in the Bland-Altman plots. There is better agreement between RvolFLOW and RvolINDIRECT than between RvolVALVE and RvolINDIRECT.
According to orthogonal regression, interobserver agreement was excellent for RvolINDIRECT (r = 0.91, coefficient of variation = 48%), moderate for RvolVALVE (r = 0.72, coefficient of variation = 57%), and excellent for RvolFLOW (r = 0.95, coefficient of variation = 27%). Bland-Altman plots of the interobserver differences can be found in Figure E1 (supplement). Limits of agreement were widest for RvolINDIRECT. The largest mean difference was observed for RvolFLOW.
Initially, a large interobserver difference (64 mL) was seen in one patient with severe MV regurgitation. After discussing the analysis with the first observer, the second observer revised his findings. The reason for the initial discrepancy was the presence of multiple jets, parts of which were not identified by the second observer because of high angulation of the measurement plane close to the border of the field of view. After revision, a difference of 32 mL between the observers remained as a result of differently angulated measurement planes with respect to the multiple jets. Videos of the analyses of the two observers can be found in Movies 1 and 2 (supplement).
Movie 1:
Flow quantification from 4D flow MRI in a 59-year-old man with severe MR and multiple regurgitation jets by observer 1. Mitral valve (MV) forward flow is quantified using valve tracking. MV regurgitant flow is quantified using flow tracking. A) 4D flow MRI streamlines of forward and backward MV flow, with 2-chamber cine bSSFP background image for anatomic reference. B) Color-coded projection of 4D flow MRI through-plane velocities onto the valve tracking plane (during forward flow) and flow tracking plane (during regurgitation), which was used to contour the flow region of interest for every timestep. During MV regurgitation, aorta outflow is also visible in the velocity projection. C) 4D flow MRI streamlines of forward and backward MV and AV flow, with 3-chamber cine bSSFP background image for anatomic reference. MV regurgitation jet shears along the septal side of the left atrial wall, close to the aortic root. AV regurgitation was also quantified using flow tracking.
Movie 2:
Flow quantification from 4D flow MRI in a 59-year-old man with severe MR and multiple regurgitation jets by observer 2. A-B) Original RvolFLOW measurement, which was 64 mL lower than observer 1’s measurement. Not all regurgitation jets are captured because the measurement plane extends outside the field-of-view. During MV regurgitation, aortic and pulmonary outflow are also visible in the velocity projection. C-D) Revised RvolFLOW measurement, which is still 32 mL lower than observer 1’s measurement due to differently angulated measurement planes between the observers.
Eight patients with severe MV regurgitation had MV prolapse that caused the regurgitant orifice to be located in the atrium and not in the annular plane. Moving the valve tracking plane to the level of the regurgitant orifice in these cases improved the agreement between RvolVALVE and RvolINDIRECT, albeit only modestly (ŷ = 0.28x + 7.6, r = 0.75). Jet eccentricity was observed in seven patients with severe MV regurgitation and three patients with moderate MV regurgitation, in whom jets impinged on the left atrial wall in all but three patients. Furthermore, five patients with severe MV regurgitation had multiple jets. In two of these patients, the jets left the regurgitant orifice in different directions; thus, it was not possible to place the measurement plane perpendicular to all jets.
Figure 4 demonstrates the forward flow measurements across the MV and AV, which were used to test for intervalve consistency. Orthogonal regression demonstrated better intervalve consistency for flow tracking than for valve tracking in terms of the correlation coefficient (r = 0.82 vs 0.67, respectively) and the slope being closer to 1 and the intercept being closer to 0 (1.2 vs 2.7 and −13.4 vs −92.4, respectively) (Fig 5). There was a statistically significant difference (ie, inconsistency) between MV net flow (ie, forward flow − Rvol) and AV net flow when Rvol was quantified using valve tracking (MV net flow: median, 80.1 mL, IQR = 70.2–121.3 mL; AV net flow: median, 67.0 mL, IQR = 52.3–84.0 mL; P < .001) but not when Rvol was quantified using flow tracking (MV net flow: median, 69.4 mL, IQR = 55.5–85.0 mL; AV net flow: median, 67.0 mL, IQR, 52.3–84.0 mL; P = .85). This finding was especially apparent in the group with severe MV regurgitation, as shown in the Bland-Altman plots (Fig 5 [bottom]), and can be explained by an underestimation of Rvol using valve tracking.
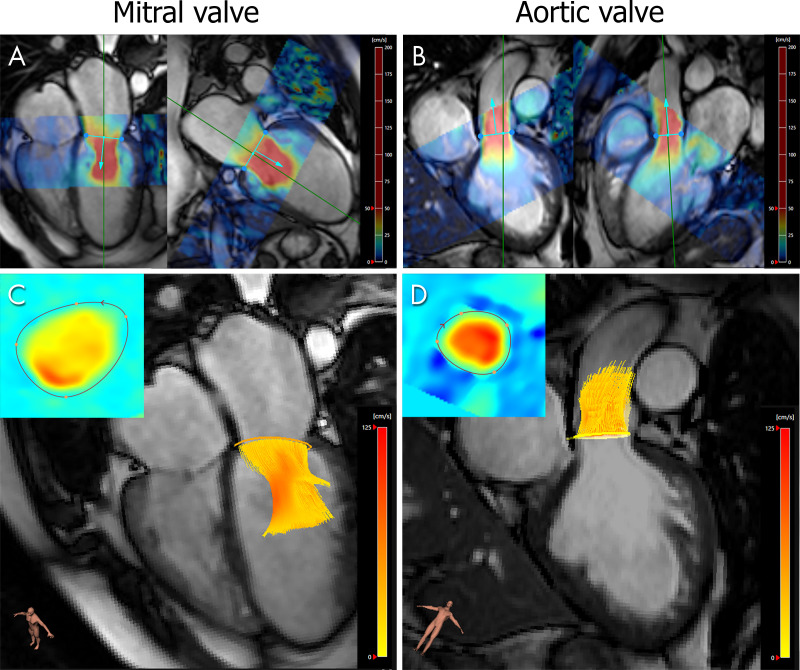
Figure 4:
Forward flow quantification with four-dimensional (4D) flow MRI across the mitral valve (MV) and aortic valve in a 45-year-old man with moderate MV regurgitation diagnosed with echocardiography. A, B, Semiautomated valve tracking on two orthogonal long-axis cine balanced steady-state free precession images for each valve. Colors represent the in-plane velocity measured with 4D flow MRI. C, D, The 4D flow MRI through-plane velocity measurements are projected onto the valve-tracking plane (inset), and time-resolved streamlines are generated from within the contour.
Figure 5:
Orthogonal regression (top) and Bland-Altman (bottom) plots of mitral valve (MV) net flow measured with valve tracking (left) and flow tracking (right) versus aortic valve (AV) net flow measured with valve tracking. Mean differences and 95% limits of agreement are indicated by the black and gray lines in the Bland-Altman plots. Flow tracking demonstrates better agreement between MV net flow and AV net flow than valve tracking.
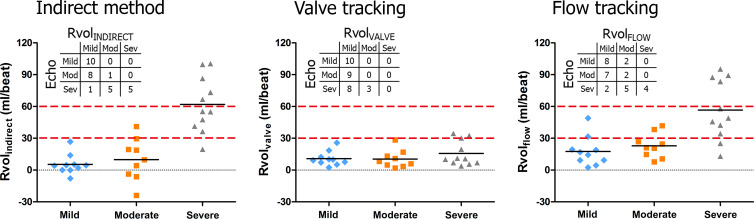
There was a substantial overlap of MRI-derived Rvol measurements among mild, moderate, and severe MV regurgitation as diagnosed by using semiquantitative echocardiography. Figure 6 shows that none of the methods (eg, flow tracking, valve tracking, or the indirect method) provided a sharp distinction between the severity groups. Moreover, it is shown that adoption of absolute cutoff values used in quantitative echocardiography (2,20) would cause most moderate and severe MV regurgitation cases to be reclassified as a lower class (Fig 6 [inset]).
Figure 6:
Regurgitant volume (Rvol) measured using the indirect method (left), four-dimensional (4D) flow MRI in combination with valve tracking (middle), and 4D flow MRI in combination with flow tracking (right), divided into classes of severity on the basis of semiquantitative echocardiographic findings. Black horizontal lines represent Rvol means per severity class. Dashed red lines indicate cutoff values for mild mitral valve (MV) regurgitation (Rvol <30 mL), moderate MV regurgitation (Rvol 30–59 mL), and severe MV regurgitation (Rvol >60 mL) used in quantitative echocardiography. Adoption of these cutoff values would cause most moderate and severe MV regurgitation cases to be reclassified to a lower class. Inset contingency tables provide comparison of MRI-based severity classification resulting from cutoff values of 30 and 60 mL with echocardiography-based classification. Neither flow tracking nor valve tracking or the indirect method enable a sharp distinction among the severity groups.
Discussion
In this study, we quantified MV regurgitation from clinical 4D flow MRI data by means of semiautomated flow tracking and semiautomated valve tracking. Flow tracking provided more accurate quantification of MV regurgitation than valve tracking in terms of agreement with indirect quantification and consistency of net flow over the MV and AV, particularly in severe MV regurgitation. Interobserver analysis demonstrated excellent reproducibility for flow tracking and moderate reproducibility for valve tracking.
Several factors may underlie the observation that valve tracking did not allow for accurate Rvol measurements in severe MV regurgitation, whereas flow tracking did. Contributing factors may be valve morphologic characteristics, dynamic jets or jet eccentricity, and high flow velocities leading to signal loss as a result of intravoxel phase dispersion and incoherent flow effects. By moving the measurement plane away from the valve to a region of more coherent flow and lower velocities, we were able to minimize signal loss. Apart from signal loss, flow displacement effects might also explain why flow tracking captured severe regurgitation jets better than valve tracking. Flow displacement occurs when relatively long echo times, like those used in an echo-planar imaging readout, cause spatial information to be encoded at a later time point than velocity information. In high-velocity regurgitation jets, this effect may result in misregistration of velocities in the regurgitant orifice to a location more upstream along the regurgitant jet. On the basis of the velocity encoding and echo time used in the group with severe MV regurgitation (180 cm/sec and 4.0 msec, respectively, in nine of 11 patients with severe MV regurgitation), this displacement can theoretically measure up to 0.7 cm. Considering the 0.35-cm interslice distance and the 1–2-cm distance between the valve-tracking and flow-tracking plane, it is possible that flow displacement contributed to the difference between RvolVALVE and RvolFLOW in severe MV regurgitation jets. Future studies with different 4D flow MRI acquisition strategies are warranted to provide more insight into the benefits of flow tracking in MV regurgitation.
In the interobserver analysis, two measures of reproducibility can be discerned: systematic bias (which was largest for RvolFLOW) and overall variability (which was largest for RvolINDIRECT). The observed variability in RvolINDIRECT can be attributed to variability in LVSV measurements. The systematic bias in RvolFLOW measurements was, in retrospect, due to a systematic difference in how the observers contoured the regurgitant flow areas (first observer contoured a wider area than second observer). Semiautomatic contour definition based on, for example, velocity isolines may in the future resolve the systematic bias as observed in this study.
The presence of multiple regurgitation jets was found to introduce interobserver variability: The three largest interobserver differences in this study were observed in patients with severe MV regurgitation and multiple jets. These findings bring to light an important challenge in the accurate quantification of severe MV regurgitation and multiple jets. Automatic detection of MV regurgitation jets and multiple planes of measurement potentially further reduce this observer variation.
An advantage of flow tracking over valve tracking in the studied cohort is the fact that the region of interest could generally be better separated from the simultaneous aortic outflow. It is of note that although the through-plane spatial resolution was relatively high in patients with severe MV regurgitation (3.50 mm vs 6.00 mm in patients with mild or moderate MV regurgitation), the in-plane resolution was slightly poorer (3.43 × 3.63 mm2 vs 2.90 × 3.80 mm2 in patients with mild or moderate MV regurgitation), which might have caused RvolVALVE measurements in patients with severe MV regurgitation to be affected more by phase dispersion–induced signal loss. MV prolapse is another potential reason for Rvol underestimation with valve tracking. However, in the current study, we found that moving the valve-tracking plane to the level of the regurgitant orifice only subtly improved the measurement.
Recent studies have reported discordance between MRI-based and echocardiography-based assessment of MV regurgitation (4,6). In our study, we also found that MRI-based Rvol measurements did not relate well to echocardiography-based severity grades. It should be considered that echocardiography-based and MRI-based severity assessment relied on different parameters. Echocardiographic evaluation did not include quantification of Rvol, which was in fact the only MRI parameter considered. However, a combination of MRI-derived parameters may have higher prognostic value than Rvol alone. Rvol has, for instance, been shown to strongly correlate with LV end-diastolic volume as well as with postsurgical decreases in LV end-diastolic volume (4,23). In addition, LV end-systolic volume has been shown to improve specificity in MRI-based prognostication of patients with severe MV regurgitation as well as (indirectly quantified) Rvol (6), and the left atrial volume indexed to the body surface ratio has been identified as a predictor of long-term outcome in primary organic MV regurgitation (24,25). Other parameters that could be considered for prognostic purposes in addition to Rvol are systolic pulmonary flow reversal and regurgitant jet eccentricity, both of which are taken into account in echocardiography-based grading. Finally, impaired LV strain is a promising imaging biomarker of early myocardial dysfunction in patients with MV regurgitation (26,27).
The higher prognostic power that MRI-derived Rvol was found to have over other MRI- or echocardiographically derived measures (4–6) underlines the importance of taking this parameter into account in surgical decision-making. Untreated severe MV regurgitation is associated with poor survival, whereas timely intervention results in improved outcomes (6,28). Furthermore, the 2017 Euro Heart Survey and the Olmsted County study have shown that surgical treatment is being denied in up to 49% of symptomatic patients with severe MV regurgitation, mainly as a result of referrals for surgery being made too late, leading to increased morbidity and mortality (29,30). Timely Rvol quantification by using MRI may improve surgical timing in MV regurgitation, although it is not yet part of clinical guidelines. Compared with the indirect method, which is still more widely available, 4D flow MRI in combination with flow tracking is advantageous in (a) the ability to not only quantify but also visualize the (regurgitant) blood flow, in order to get a better understanding of the cause of the regurgitation, and (b) the ability to perform measurements across all heart valves, allowing for intervalve-consistency testing. Further studies in large cohorts with the correlation of clinical outcomes are important to strengthen the role of MRI-derived Rvol in clinical practice. We would like to stress that we do not consider the indirect method to be the reference standard, although it is the best reference currently available.
Previous reports on 4D flow MRI–derived quantification of MV regurgitation have demonstrated its feasibility in mild and moderate MV regurgitation with valve tracking but have not demonstrated its feasibility in severe MV regurgitation (11,17). Our study shows that 4D flow MRI in combination with flow tracking enables accurate quantification even in severe MV regurgitation. Because of the volumetric nature of the acquisition, eccentric and dynamic regurgitation jets can be captured without knowledge of the regurgitation pattern before image acquisition (which is required in 2D flow MRI) and without operator dependency or geometric or directional restrictions during acquisition planning, unlike in echocardiography. The acquisition is less sensitive to physiologic variability than the indirect method, which requires multiple breath holds at different moments in time. In 4D flow MRI, measurements at different locations are all influenced equally by physiologic variability, as the final image series represents an average over all the measured cardiac cycles.
A number of limitations of our study should be noted. First, there was a difference in the cause of MV regurgitation between the patient groups. Patients with severe MV regurgitation had primary asymptomatic MV regurgitation as a result of an intrinsically abnormal MV, whereas those with mild or moderate MV regurgitation had secondary MV regurgitation. It is likely that complex valve morphologic characteristics in primary MV regurgitation complicated MV regurgitation quantification because of consequent jet eccentricity and complexity. Future studies on quantification of primary MV regurgitation of varying severity grades are warranted. Furthermore, patients with severe MV regurgitation underwent MRI and echocardiography several days or weeks apart, whereas those with mild or moderate MV regurgitation underwent the examinations on the same day. The accuracy of the flow measurements may have been limited by the relatively low spatial resolution and long echo time. Another limitation was the anisotropic voxel size of the 4D flow MRI acquisitions. This could have affected measurement accuracy in eccentric regurgitation jets angulated to the basal plane of the heart, to which the acquisition volume was planned parallel. With regard to the placement of the flow-tracking plane perpendicular to the regurgitant jet, it is important to note that, in theory, nonperpendicular placement should not result in different measurement results, as the decrease in through-plane velocities is compensated for by an increase in the flow area. In practice, however, partial volume effects can cause errors, and nonperpendicular measurement results in poorer definition of the flow area of interest because of more diffuse boundaries. The impact of the plane angulation was not explored in our study, nor was the impact of the distance to the regurgitant orifice. In the case of multiple jets, the measurement plane was as much as possible placed perpendicular to the largest jet. In the case of impingement to the left atrial wall, the measurement was obtained before the area of impingement if possible and was otherwise obtained along the left atrial wall.
In conclusion, semiautomated flow tracking enables more accurate Rvol quantification in patients with MV regurgitation than semiautomated valve tracking, particularly in severe MV regurgitation. Whether the use of 4D flow MRI in combination with semiautomated flow tracking can be used to improve prognostication in patients with MV regurgitation must be investigated in future studies.
SUPPLEMENTAL FIGURES
Disclosures of Conflicts of Interest: C.P.S.B. Activities related to the present article: data analysis software was provided by Pie Medical Imaging. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. J.J.M.W. disclosed no relevant relationships. J.P.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is employed by Pie Medical Imaging. Other relationships: institution has patent pending with Pie Medical Imaging. G.P.B. disclosed no relevant relationships. S.A.J.C. disclosed no relevant relationships. S.M.B. disclosed no relevant relationships. A.J.N. disclosed no relevant relationships. T.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution receives payment for lectures including service on speakers bureaus from Philips Healthcare and Bayer Healthcare. Other relationships: disclosed no relevant relationships. P.v.O. disclosed no relevant relationships. R.N.P. disclosed no relevant relationships.
Abbreviations:
- AV
- aortic valve
- bSSFP
- balanced steady-state free precession
- 4D
- four-dimensional
- IQR
- interquartile range
- LV
- left ventricle
- LVSV
- LV stroke volume
- MV
- mitral valve
- Rvol
- regurgitant volume
- RvolFLOW
- semiautomated quantification of Rvol using flow tracking
- RvolINDIRECT
- indirect quantification of Rvol
- RvolVALVE
- semiautomated quantification of Rvol using valve tracking
- 2D
- two-dimensional
References
- 1.Zamorano JL, Fernández-Golfín C, González-Gómez A. Quantification of mitral regurgitation by echocardiography. Heart 2015;101(2):146–154. [DOI] [PubMed] [Google Scholar]
- 2.Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30(4):303–371. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;38(36):2739–2791. [DOI] [PubMed] [Google Scholar]
- 4.Uretsky S, Gillam L, Lang R, et al. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol 2015;65(11):1078–1088. [DOI] [PubMed] [Google Scholar]
- 5.Myerson SG, d’Arcy J, Christiansen JP, et al. Determination of clinical outcome in mitral regurgitation with cardiovascular magnetic resonance quantification. Circulation 2016;133(23):2287–2296. [DOI] [PubMed] [Google Scholar]
- 6.Penicka M, Vecera J, Mirica DC, Kotrc M, Kockova R, Van Camp G. Prognostic implications of magnetic resonance-derived quantification in asymptomatic patients with organic mitral regurgitation: comparison with doppler echocardiography-derived integrative approach. Circulation 2018;137(13):1349–1360. [DOI] [PubMed] [Google Scholar]
- 7.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 2000;21(16):1387–1396. [DOI] [PubMed] [Google Scholar]
- 8.Authors/Task Force members ; Elliott PM, Anastasakis A, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35(39):2733–2779. [DOI] [PubMed] [Google Scholar]
- 9.Biner S, Rafique A, Rafii F, et al. Reproducibility of proximal isovelocity surface area, vena contracta, and regurgitant jet area for assessment of mitral regurgitation severity. JACC Cardiovasc Imaging 2010;3(3):235–243. [DOI] [PubMed] [Google Scholar]
- 10.Westenberg JJM, Roes SD, Ajmone Marsan N, et al. Mitral valve and tricuspid valve blood flow: accurate quantification with 3D velocity-encoded MR imaging with retrospective valve tracking. Radiology 2008;249(3):792–800. [DOI] [PubMed] [Google Scholar]
- 11.Kamphuis VP, Roest AAW, Ajmone Marsan N, et al. Automated cardiac valve tracking for flow quantification with four-dimensional flow MRI. Radiology 2019;290(1):70–78. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao A, Tariq U, Alley MT, Lustig M, Vasanawala SS. Inlet and outlet valve flow and regurgitant volume may be directly and reliably quantified with accelerated, volumetric phase-contrast MRI. J Magn Reson Imaging 2015;41(2):376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urchuk SN, Plewes DB. Mechanisms of flow-induced signal loss in MR angiography. J Magn Reson Imaging 1992;2(4):453–462. [DOI] [PubMed] [Google Scholar]
- 14.Gatenby JC, McCauley TR, Gore JC. Mechanisms of signal loss in magnetic resonance imaging of stenoses. Med Phys 1993;20(4):1049–1057. [DOI] [PubMed] [Google Scholar]
- 15.Westenberg JJ, van der Geest RJ, Wasser MNJ, et al. Objective stenosis quantification from post-stenotic signal loss in phase-contrast magnetic resonance angiographic datasets of flow phantoms and renal arteries. Magn Reson Imaging 1998;16(3):249–260. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira PF, Gatehouse PD, Mohiaddin RH, Firmin DN. Cardiovascular magnetic resonance artefacts. J Cardiovasc Magn Reson 2013;15(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calkoen EE, Westenberg JJ, Kroft LJ, et al. Characterization and quantification of dynamic eccentric regurgitation of the left atrioventricular valve after atrioventricular septal defect correction with 4D Flow cardiovascular magnetic resonance and retrospective valve tracking. J Cardiovasc Magn Reson 2015;17(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myerson SG, Francis JM, Neubauer S. Direct and indirect quantification of mitral regurgitation with cardiovascular magnetic resonance, and the effect of heart rate variability. MAGMA 2010;23(4):243–249. [DOI] [PubMed] [Google Scholar]
- 19.Marsan NA, Westenberg JJM, Ypenburg C, et al. Quantification of functional mitral regurgitation by real-time 3D echocardiography: comparison with 3D velocity-encoded cardiac magnetic resonance. JACC Cardiovasc Imaging 2009;2(11):1245–1252. [DOI] [PubMed] [Google Scholar]
- 20.Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007;28(2):230–268. [DOI] [PubMed] [Google Scholar]
- 21.Jansen R, Hart EA, Peters M, et al. An easy-to-use scoring index to determine severity of mitral regurgitation by 2D echocardiography in clinical practice. Echocardiography 2017;34(9):1275–1283. [DOI] [PubMed] [Google Scholar]
- 22.Walker PG, Cranney GB, Scheidegger MB, Waseleski G, Pohost GM, Yoganathan AP. Semiautomated method for noise reduction and background phase error correction in MR phase velocity data. J Magn Reson Imaging 1993;3(3):521–530. [DOI] [PubMed] [Google Scholar]
- 23.Uretsky S, Supariwala A, Nidadovolu P, et al. Quantification of left ventricular remodeling in response to isolated aortic or mitral regurgitation. J Cardiovasc Magn Reson 2010;12(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Tourneau T, Messika-Zeitoun D, Russo A, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol 2010;56(7):570–578. [DOI] [PubMed] [Google Scholar]
- 25.Rusinaru D, Tribouilloy C, Grigioni F, et al. Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: results from a large international multicenter study. Circ Cardiovasc Imaging 2011;4(5):473–481. [DOI] [PubMed] [Google Scholar]
- 26.Schiros CG, Dell’Italia LJ, Gladden JD, et al. Magnetic resonance imaging with 3-dimensional analysis of left ventricular remodeling in isolated mitral regurgitation: implications beyond dimensions. Circulation 2012;125(19):2334–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed MI, Gladden JD, Litovsky SH, et al. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction >60%. J Am Coll Cardiol 2010;55(7):671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suri RM, Vanoverschelde JL, Grigioni F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013;310(6):609–616. [DOI] [PubMed] [Google Scholar]
- 29.Dziadzko V, Clavel MA, Dziadzko M, et al. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet 2018;391(10124):960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28(11):1358–1365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.