Abstract
Coronary CT angiography (CCTA) has evolved into a first-line diagnostic test for the investigation of chest pain. Despite advances toward standardizing the reporting of CCTA through the Coronary Artery Disease Reporting and Data System (or CAD-RADS) tool, the prognostic value of CCTA in the earliest stages of atherosclerosis remains limited. Translational work on the bidirectional interplay between the coronary arteries and the perivascular adipose tissue (PVAT) has highlighted PVAT as an in vivo molecular sensor of coronary inflammation. Coronary inflammation is dynamically associated with phenotypic changes in its adjacent PVAT, which can now be detected as perivascular attenuation gradients at CCTA. These gradients are captured and quantified through the fat attenuation index (FAI), a CCTA-based biomarker of coronary inflammation. FAI carries significant prognostic value in both primary and secondary prevention (patients with and without established coronary artery disease) and offers a significant improvement in cardiac risk discrimination beyond traditional risk factors, such as coronary calcium, high-risk plaque features, or the extent of coronary atherosclerosis. Thanks to its dynamic nature, FAI may be used as a marker of disease activity, with observational studies further suggesting that it tracks the response to anti-inflammatory interventions. Finally, radiotranscriptomic studies have revealed complementary radiomic patterns of PVAT, which detect more permanent adverse fibrotic and vascular PVAT remodeling, further expanding the value of PVAT phenotyping as an important readout in modern CCTA analysis.
© RSNA, 2021
Summary
Perivascular adipose tissue mapping by means of the coronary CT angiography (CCTA)–derived fat attenuation index is a recently developed method to noninvasively detect coronary inflammation and atherosclerotic changes at CCTA, with incremental prognostic value over and above traditional risk factors.
Essentials
■ There is an unmet need for biomarkers that noninvasively visualize coronary inflammation and track responses to anti-inflammatory interventions in atherosclerosis.
■ Perivascular adipose tissue (PVAT) acts as an in vivo biosensor of coronary inflammation by modifying its composition in response to inflammatory signals.
■ The fat attenuation index detects coronary inflammation by quantifying dynamic spatial changes in PVAT attenuation.
■ Further radiotranscriptomic analyses reveal additional imaging biomarkers linked to adverse PVAT remodeling with important diagnostic and prognostic implications in coronary artery disease.
Introduction
Coronary artery disease (CAD) as a precursor of myocardial infarction (MI) has a significant impact on survival and quality of life and is further associated with a significant health-related economic burden (1). Traditionally, functional tests (ischemia tests) were the first-line examinations for the investigation of stable chest pain (2). As opposed to functional imaging, coronary CT angiography (CCTA) provides “anatomic” information by detecting coronary atherosclerotic plaques, even when these are not associated with significant myocardial ischemia. Following the results from large observational studies (3–5) and randomized trials (eg, the Scottish CT of the Heart trial) (4), CCTA is now widely accepted as a first-line investigational tool for the detection of CAD according to both national (National Institute for Health and Care Excellence) and international (European Society of Cardiology) guidelines (1,2). Recent studies have estimated that the adoption of the National Institute for Health and Care Excellence guidelines in the United Kingdom may save up to £16 million in health care expenditure by proposing CCTA as a first-line diagnostic test for the investigation of stable chest pain (1,6). This shift is also reflected in the most recent European Society of Cardiology guidelines, in which CCTA has been upgraded to a class I recommendation in the investigation of chronic coronary syndromes, so that noninvasive functional imaging and CCTA are both accepted as appropriate first-line tests. Despite that, CCTA and functional imaging are not necessarily interchangeable. For instance, early “asymptomatic” stages of CAD that are not associated with significant ischemia may be detected with CCTA but not with functional imaging (2). In terms of flow-limiting stenosis, the CT-derived fractional flow reserve adds functional dimension to CCTA by providing a noninvasive assessment of the hemodynamic significance of coronary atherosclerosis (7), as the CT-derived fractional flow reserve is strongly related with the invasive reference standard measurement of the fractional flow reserve (8,9).
In the absence of ischemia, the optimal diagnostic approach and downstream management for “low-risk” individuals at the earliest stages of coronary atherosclerosis remains unclear. The coronary artery calcium (CAC) score provides a widely used tool for the early detection of atherosclerosis in asymptomatic individuals, with well-established prognostic value in primary prevention (10). However, despite its value as a risk modifier, the CAC score cannot exclude the presence of noncalcified (yet possibly high-risk) plaques (11) and seems to increase when patients receive treatments that lower cardiovascular risk, such as statins (12). Notably, studies suggest that approximately 50% of MI events occur in patients without obstructive coronary atherosclerosis when vulnerable plaques rupture or erode (13). By imaging the plaque anatomy, rather than its downstream hemodynamic effects (such as in functional imaging), CCTA has facilitated a deeper understanding of the “unstable plaques,” focusing on their distinct structural and morphologic characteristics (ie, high-risk plaque [HRP] features). However, there remains an unmet need to develop more specific, dynamic biomarkers to detect such vulnerable patients and plaques. In an effort to address this need, we have developed the fat attenuation index (FAI), a CT biomarker that detects vascular inflammation by analyzing the three-dimensional changes of CT attenuation from the perivascular adipose tissue (PVAT) around diseased coronary artery segments (14).
CCTA in Clinical Practice
Current state-of-the-art CCTA interpretation for CAD consists of three pillars (7): first, luminal factors relating to the degree of luminal stenosis; second, hemodynamic factors reflecting the relationship of such lesions or stenoses to downstream ischemia (such as the fractional flow reserve); and, finally, vascular parameters describing the structure of the atherosclerotic plaque wall. The latter include domains such as calcification and HRP features (eg, low-attenuating plaque, positive remodeling, and spotty calcification), which may confer an increased risk of adverse cardiovascular events (15,16). Given variations in the reporting and interpretation of CCTA scans in the past, the Coronary Artery Disease Reporting and Data System(or CAD-RADS) tool was recently established to standardize the terminology in imaging reports (17). Another commonly used metric, the Agatston CAC score, calculated using noncontrast cardiac CT images, reflects the volume and attenuation of coronary calcifications and is a well-established, independent cardiovascular risk factor (18). However, its value is limited to primary prevention (10). The CAC score describes irreversible structural changes, and the amount of calcification even increases in response to medical treatment with statins. Therefore, its value in monitoring the effects of lipid-lowering therapies during secondary prevention is limited (19). Despite all of these features, there remains an unmet need to move beyond the structural plaque characteristics and detect coronary inflammation, a feature responsible for both plaque formation and plaque rupture.
The Role of Inflammation in Coronary Atherosclerosis
Atherosclerosis has long been known to be an inflammatory process (20). Inflammation is a key factor not only for the development but also for the progression of atherosclerotic plaques (21). The inflammatory hypothesis of atherothrombosis was elegantly demonstrated in the landmark Canakinumab Anti-inflammatory Thrombosis Outcome Study, in which treatment with canakinumab—a monoclonal antibody targeting IL-1β, a critical regulator of inflammation (22)—led to a significant reduction in major adverse cardiovascular events without affecting low-density lipoprotein levels in patients with a prior MI and persistently elevated levels of high-sensitivity C-reactive protein (23). The subsequent Colchicine Cardiovascular Outcomes Trial further showed that colchicine, when given to patients with a recent MI, reduces the incidence of ischemic cardiovascular events compared with placebo (24). The Low-Dose Colchicine trial expanded these benefits of low-dose colchicine to patients with chronic coronary disease (25). Of note, methotrexate given to patients with a prior MI did not affect inflammatory marker levels and failed to reduce the incidence of cardiovascular events (26).
Noninvasive detection of vascular inflammation has often been hailed as the holy grail of cardiovascular diagnostics because its timely and accurate detection would enable the early identification of “healthy” individuals at high risk of cardiovascular disease and could therefore guide a more personalized deployment of preventative and therapeutic strategies. For instance, PET/CT imaging is a noninvasive imaging modality that allows direct detection of inflammation in the coronary arteries (27). One of its main strengths relies on the ability to design highly specific radiotracers that can detect distinct biologic processes in the human body (28). The most commonly used PET tracer, fluorine 18 (18F) fluorodeoxyglucose, has been shown to correlate with vascular inflammation thanks to its uptake by metabolically active inflammatory cells residing in the vascular wall (28); however, its use in coronary imaging is limited by significant myocardial uptake (29). 18F–sodium fluoride is an excellent alternative that detects areas of active microcalcification and may be superior in identifying culprit plaques (29). Other PET markers, such as gallium 68–tetraazacyclododecane tetraacetic acid–octreotate, which binds to the somatostatin receptor subtype 2 found in macrophages, have shown promise in detecting atherosclerotic inflammation. In contrast to 18F-fluorodeoxyglucose, gallium 68–tetraazacyclododecane tetraacetic acid–octreotate and 18F-sodium fluoride are not affected by myocardial uptake, thus making them promising candidates in coronary imaging (28). However, PET/CT imaging in general has several inherent limitations, such as high costs, high radiation exposure, long acquisition times, and a limited spatial resolution (28,30). Therefore, there is an unmet need to build cost-effective biomarkers that noninvasively visualize coronary inflammation and change in response to medical treatments that target vascular inflammation.
PVAT as a “Biosensor” of Vascular Inflammation
Coronary arteries are surrounded by a layer of fat, namely the PVAT. Translational studies suggest a very close anatomic and physiologic relationship between the two structures, in a way that it is now suggested by many that PVAT may be considered part of the vessel. PVAT consists of three main components: lipid-containing cells as adipocytes, stromal cells (eg, preadipocytes), and interstitial tissue (31). Given the absence of a well-defined anatomic barrier, we have recently defined PVAT as the adipose tissue located within a radial distance from the outer vessel wall equal to the diameter of the adjacent vessel (32). PVAT interacts with the arterial wall in a bidirectional manner, and this distinguishes it from more systemic depots, such as the total epicardial adipose tissue or subcutaneous adipose tissue (14,31).
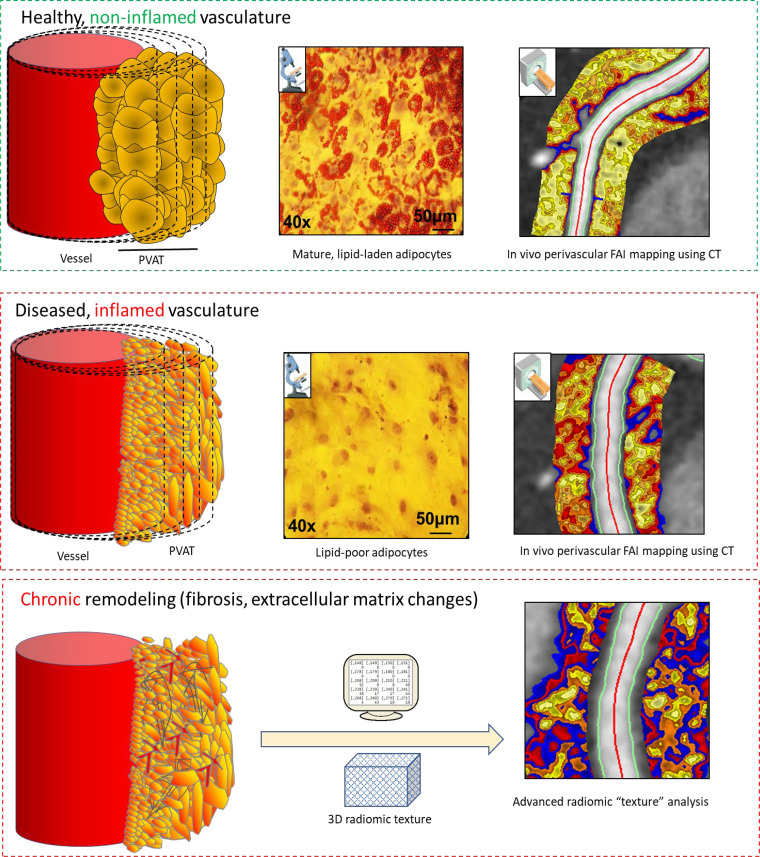
In our recent translational work, we attempted to shed light on the complex interplay between the vascular wall and its PVAT (14). We demonstrated the existence of a complex interplay between the two entities, in which PVAT modifies arterial inflammation and the arterial redox state through the release of bioactive adipokines (33). Just as important, however, is the inside-to-outside signaling. In the presence of vascular inflammation, human PVAT undergoes a phenotypic switch in response to proinflammatory stimuli (TNF-α, IL-6, and IFN-γ) released from the adjacent vessel in a paracrine manner, which seem to trigger lipolysis and block adipocyte differentiation, thus resulting in a shift from a lower to a higher water/lipid ratio (Fig 1). This phenotypic switch in PVAT therefore functions as a dynamic in vivo sensor of vascular inflammation.
Figure 1:
Inflammatory processes around the coronary arteries visualized by the fat attenuation index (FAI). In the presence of coronary inflammation, perivascular adipose tissue (PVAT) is characterized by a shift toward a greater aqueous and lower lipophilic content through inhibition of adipocyte differentiation and local activation of lipolysis. This phenotypic shift is reflected in CT attenuation gradients in the perivascular space detectable through the perivascular FAI. Perivascular adipocytes exposed to a low-inflammation environment versus a high-inflammation environment are characterized by a higher accumulation of intracellular lipid droplets, as indicated by the red color in the microscopy images, which correspond to Oil-Red-O staining. With chronic inflammation, there is eventual adverse fibrotic and other remodeling of the PVAT, which can be detected using advanced radiomic texture analysis of the perivascular fat. (Reprinted, with permission, from references 14 and 34.) 3D = three-dimensional.
Perivascular FAI Mapping as a Recently Developed Dimension in CCTA
In cardiac CT, adipose tissue attenuation falls within the window of −190 to −30 HU, whereas water has by definition an attenuation of zero (14,35). We have recently shown that adipocyte size and intracellular lipid content drive the balance between water and lipid content at a tissue level. Adipose tissue sites characterized by larger adipocytes shift the CT attenuation closer to −190 HU, whereas adipose tissue with small (and fat-free) adipocytes is expected to cause attenuation closer to −30 HU. This is the fundamental principle underlying the development of the perivascular FAI, a CT-derived metric that captures the balance between lipids and water within PVAT. Importantly, perivascular FAI detects coronary inflammation by measuring the spatial changes of attenuation of PVAT, which reflects the inflammatory burden of the adjacent vascular wall (7). The FAI can be measured around any segment of the coronary tree, but its original standardization was performed around prespecified segments of the proximal right coronary artery, left anterior descending artery, and left circumflex artery. To extract meaningful interpretations from the FAI, perivascular attenuation gradients need to be adjusted for local anatomy, biologic factors, scan settings, and reconstruction parameters (7,36). These steps are crucial, as measurement of the uncorrected perivascular attenuation may lead to misleading results (31).
The FAI has several advantages: it is not confounded by the extent of coronary calcification, it is independent of the grade of systemic inflammation in the patient (measured by high-sensitivity C-reactive protein), and it is not related to the severity of coronary stenosis (7,32). Moreover, the FAI seems to carry value in both primary and secondary prevention. As described in our prior work (32), the FAI independently predicts adverse cardiac events (ie, cardiac mortality) in patients with and those without established CAD at baseline (32).
Importantly, the FAI provides information about the three-dimensional composition of PVAT around the arterial wall, reflecting the magnitude of the inflammatory signals secreted from the vascular wall and diffused to the perivascular space. For this reason, the FAI is independent of commonly used anthropometric and volumetric indexes that instead reflect obesity and adipose tissue expansion. For instance, epicardial adipose tissue volume is closely linked to systemic and visceral obesity, and higher levels have been linked to a higher prevalence of CAD and plaque vulnerability (37). On the contrary, we have previously shown that the FAI is independent of systemic factors such as insulin resistance (14). Unlike other parameters, such as body mass index or waist-to-hip ratio, the FAI is not a marker of obesity but rather reflects the ability of perivascular fat to function as an in vivo molecular sensor of coronary inflammation (32).
More specifically, we recently explored the prognostic role of the FAI in real-life cohorts of patients undergoing clinically indicated CCTA in the Cardiovascular Risk Prediction Using CT (CRISP-CT) study (32). In a post hoc analysis of prospectively collected data from two CCTA cohorts, the first from Erlangen, Germany (n = 1872), and the second from Cleveland, Ohio, United States (n = 2040), higher pericoronary FAI values (reflective of a higher inflammatory burden) were positively associated with a higher risk of adverse cardiac events. These results were validated around the right coronary and left anterior descending arteries in both cohorts. Of note, perivascular FAI mapping was not predictive of noncardiac mortality in either population, highlighting the cardiac-specific nature of the biomarker. In further statistical analyses, an adjusted FAI value of −70.1 HU provided an optimal cutoff above which the risk for all-cause mortality (hazard ratio, 2.55; 95% CI: 1.65, 3.92; P < .0001), cardiac mortality (hazard ratio, 9.04; 95% CI: 3.35, 24.40; P < .0001), and acute coronary syndromes (hazard ratio, 5.08; 95% CI: 1.89, 13.61; P = .0012) seemed to increase. Furthermore, the FAI improved traditional risk prediction models that included age, sex, cardiovascular risk factors, HRP features, the extent of CAD, and the extent of coronary calcification (by means of the CAC score) (32). In CRISP-CT, the prognostic value of the perivascular FAI was independent of the volume of perivascular fat, whereas there was no statistical interaction with the degree of epicardial adipose tissue volume.
As a noninvasive metric linked to coronary inflammation, FAI also detects the presence of culprit lesions in patients presenting with acute MI, with culprit lesions exhibiting higher perivascular FAI values than stable reference plaque segments (14,38). Studies exploring the association of 18F-sodium fluoride uptake in coronary plaques with adjacent pericoronary adipose tissue attenuation have demonstrated a strong, almost collinear, association (r = 0.63 for the maximum standard uptake value and r = 0.68 for the target-to-background ratio; all P < .001) (39). Along these lines, even an unadjusted pericoronary adipose tissue attenuation may reliably distinguish culprit from nonculprit lesions at CCTA (38). A post hoc analysis of the CRISP-CT study specifically analyzed the incidence of cardiac mortality across population strata of high (vs low) FAI values as well as the presence or absence of HRP features. As shown in Figure 2, in the presence of a normal (low) FAI, HRP features were not associated with an increased cardiac risk. However, in the presence of an abnormally elevated FAI, HRP features identified a truly “high-risk” population with a 6.26-fold higher adjusted cardiac risk level compared with the low-FAI and HRP-negative reference group (40). Interestingly, recent evidence suggests that the perivascular FAI is also linked to functional characteristics of the adjacent coronary lesions, with new studies showing that higher coronary inflammation measured using perivascular fat attenuation is related with lower CT-derived fractional flow reserve values (41,42). Taken together, these findings suggest that vascular inflammation measured by this method is closely linked to adverse plaque remodeling and its hemodynamic consequences.
Figure 2:
A, Fat attenuation index (FAI) mapping stratifies the cardiac risk associated with high-risk plaque (HRP) features. B, In the presence of a normal FAI (low FAI [FAIlow]), HRP features are not associated with an increased cardiac risk in the Cardiovascular Risk Prediction Using CT study population. However, in the presence of an abnormally elevated FAI, HRP features identify a truly “high-risk” population with a 6.26-fold higher adjusted cardiac risk compared with the FAIlow/HRP-negative reference group. (Reprinted, with permission, from reference 40.) CCTA = coronary CT angiography.
It should further be noted that the FAI is not a static value but appears to be modifiable through anti-inflammatory and disease-modifying medical therapies. In a subanalysis of the CRISP-CT study, among patients who received initial treatment with statins or aspirin after coronary CCTA, FAI did not retain its significant association with subsequent adverse events (32). These findings were confirmed by other groups (43) who found the perivascular FAI to be a dynamic tool for monitoring the response to statin treatment, demonstrating a significant reduction in the pericoronary FAI around noncalcified and mixed plaques but not around calcified plaques. This might be due to the fact that statins stabilize vulnerable plaques by a reduction of the necrotic core (43,44).
Furthermore, the perivascular FAI exhibits a dynamic character and changes in response to anti-inflammatory and other therapeutic interventions. It has recently been shown that treating patients with psoriasis with biologic treatments (eg, anti-TNF-α, anti–IL-17, or anti–IL-12 or anti–IL-23), but not with nonbiologic treatments (eg, methotrexate or skin therapy), results in a significant reduction of coronary inflammation measured by the perivascular FAI within 12 months from treatment initiation (34). Whether these changes occur rapidly over the first few weeks of treatment or develop over several months remains unclear. It is important to understand that biologically, the FAI is driven by the balance between intracellular lipid content within perivascular adipocytes and the aqueous phase (intracellular and extracellular) within the same space. When an anti-inflammatory treatment is administered to target vascular inflammation, the perivascular FAI also changes following the “cooling” of the adjacent vascular wall or plaque. For this to happen, the adipocytes will need to change their phenotype and induce their differentiation, a process that takes at least 9 days in the basic science laboratory (14). In vivo, this process may take longer than that, and there is now a significant volume of evidence to suggest that we expect differences within the first 5 weeks from the withdrawal of the inflammatory stimulation coming from a ruptured plaque during an acute coronary syndrome; indeed, small but significant changes in the perivascular FAI around culprit lesions are observed within 5 weeks of a plaque rupture event (14). In another study, there were dramatic changes in the perivascular FAI (both around the culprit lesion and also in reference proximal segments of the coronary tree) 6 months after an acute coronary syndrome, suggesting that post-MI treatment not only reduces the inflammatory burden of the culprit lesion but also suppresses the overall inflammatory burden of the coronary tree (45). Importantly, approximately 23.5% of the patients with acute coronary syndrome fail to reduce their perivascular FAI, possibly identifying nonresponders to traditional guideline-directed medical therapy. Along these lines, an ongoing randomized clinical trial (Peri-vascular Adipose Tissue Inflammation Evaluated Using Coronary CT Angiography, or P-VECT) is expected to explore the value of a FAI-guided approach in initiating pharmacotherapy with atorvastatin and aspirin for cardiovascular risk reduction and tracking longitudinal changes in coronary inflammation among individuals who have high FAI levels at baseline but do not qualify for prevention on the basis of existing guidelines. Indeed, in a nonrandomized trial, the perivascular FAI around mixed or soft plaques changed significantly within 1 year of initiation of statin treatment, whereas the perivascular FAI around calcified plaques remained unchanged (40).
Radiomic Phenotyping of PVAT Heterogeneity
Although the FAI captures dynamic changes in coronary inflammation, it was not originally developed to detect more permanent features of perivascular fat remodeling, including fibrotic and microvascular remodeling, which may carry additive value in long-term risk prediction (45). Such subtle changes in PVAT composition are not reliably detectable by the human reader; however, advances in computational processing now enable the detection of both fine and coarse radiomic patterns in the pericoronary adipose tissue. Such shape-, attenuation-, and texture-related parameters represent the field of radiomics, and their subsequent link to the underlying gene-expression patterns has given birth to the field of cardiovascular “radiotranscriptomics” (45).
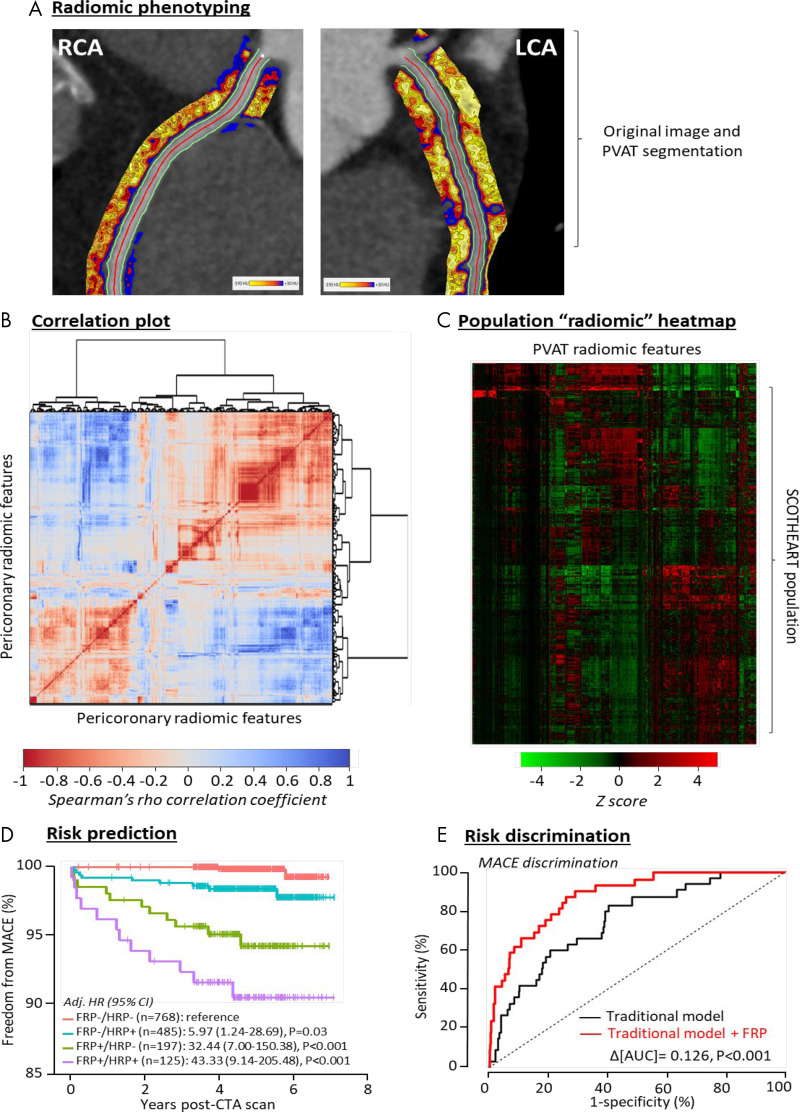
In our recent study, we successfully linked the CT radiomic profile of in vivo adipose tissue imaging with the gene-expression profile of biopsy specimens collected from the same patients. We demonstrated that wavelet-adjusted mean attenuation, a basis of the FAI, was the single best predictor of the expression of TNFA. However, additional radiomic features capturing the spatial heterogeneity and texture of adipose tissue were needed to better characterize the extent of adipose tissue fibrotic remodeling (as evidenced by the expression of COL1A1) and microvascular remodeling (as evidenced by the expression of the endothelial marker CD31). We then used a machine learning approach to develop a radiomic signature of pericoronary adipose tissue (Fig 3) that predicts adverse outcomes at 5 years after CCTA, which we were able to validate in 1575 eligible patients from the Scottish CT of the Heart trial. Importantly, the fat radiomic profile offered incremental prognostic value beyond HRP and CAC. Finally, in a proof-of-concept study, we showed that patients who had experienced an MI had significantly higher fat radiomic profile values than matched controls. Unlike the FAI, which changed dynamically in the 6 months after acute MI, the fat radiomic profile remained unchanged, further supporting its role as an imaging marker of more permanent PVAT remodeling in response to CAD.
Figure 3:
Radiomic phenotyping of coronary perivascular adipose tissue (PVAT). A, Radiomic mapping based on numerous shape-, attenuation-, and texture-related parameters in the pericoronary space of the right coronary artery (RCA) and left coronary artery (LCA). B, Correlation plot of all 1391 stable radiomic features in the Scottish CT of the Heart (SCOT-HEART) population (n = 1575 patients), with hierarchic clustering revealing distinct clusters of radiomic variance. C, Radiomic heatmap of the SCOT-HEART population. D, Prediction of major adverse cardiac events (MACE) in the SCOT-HEART trial for high versus low fat radiomic profile (FRP) values also stratified by the presence of high-risk plaques (HRPs). E, Improved MACE risk discrimination in the SCOT-HEART trial, with the use of the FRP performing over and above traditional risk factors as indicated by the change in the area under the curve (AUC). (Reprinted from reference 45.) Adj. HR = adjusted hazard ratio, CTA = CT angiography.
How to Use PVAT Phenotyping in Clinical Practice
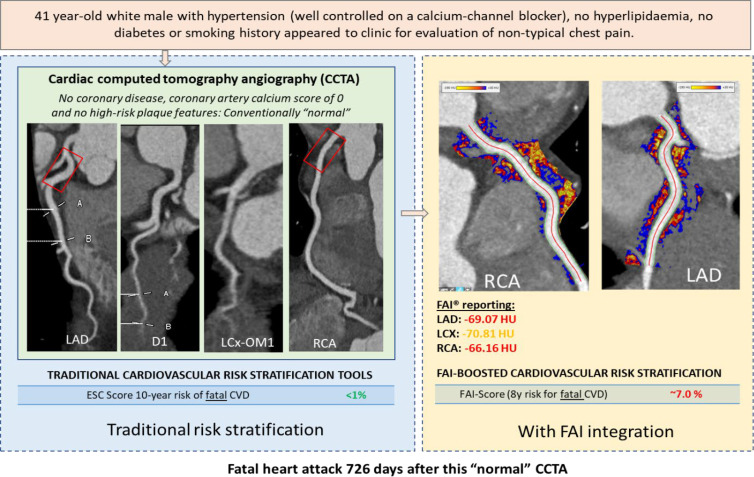
In clinical practice, 70%–80% of the patients who undergo CCTA as part of clinical care have no obstructive atherosclerosis, and the majority do not have any visible atheroma (46). However, it is widely accepted that 50% of heart attacks happen among people with minor disease (47). These people can be captured by quantifying the degree of vascular inflammation in their coronary arteries through PVAT imaging. Indeed, implementing perivascular fat phenotyping across this population reclassifies approximately 25% of the people with no disease (even those with zero calcium) because it captures the disease process years before it leads to calcification (32). These people can be targeted with measures of primary prevention (eg, initiation of statin treatment or increasing the dose of statins); even in patients who are already receiving maximum statin treatment, the use of the FAI may trigger the initiation of other prevention measures, such as anti–proproteinkonvertase subtilisin/kexin type 9 treatment, which lowers cholesterol in patients. Among those with coronary atherosclerosis, it can identify the “vulnerable plaques” and allow maximizing treatment strategies to prevent plaque rupture. The case presented in Figure 4 shows a 41-year-old male patient from the CRISP-CT study with a history of hypertension who presented with atypical chest pain and was referred to undergo CCTA for further diagnostic evaluation. Despite no evidence of CAD and a low European Society of Cardiology risk score for 10-year fatal cardiovascular disease, the patient experienced a fatal MI 726 days after his initial imaging. FAI mapping revealed elevated values around most coronary vessels, suggestive of coronary inflammation, with a FAI-guided risk score retrospectively reclassifying his risk of adverse cardiovascular events to a higher risk group.
Figure 4:
A case of fat attenuation index (FAI)–guided risk stratification. A 41-year-old male patient from the Cardiovascular Risk Prediction Using CT study with a history of hypertension who presented with atypical chest pain and was referred to undergo coronary CT angiography (CCTA) for further diagnostic evaluation. Left: Despite no evidence of coronary artery disease, a calcium score of zero, and a low European Society of Cardiology (ESC) risk score for 10-year fatal cardiovascular disease (CVD), the patient experienced a fatal myocardial infarction 726 days after his initial imaging. Right: FAI mapping revealed elevated values around most coronary vessels, suggestive of coronary inflammation, with a FAI-guided risk score retrospectively reclassifying his risk of adverse cardiovascular events to a higher risk group. This supports the notion that coronary inflammation captured by the perivascular FAI precedes the plaque formation and predicts events many years in advance, even before any sign of plaque or calcification is developed. D1 = first diagonal, LAD = left anterior descending artery, LCX = left circumflex artery, OM1 = first obtuse marginal artery, RCA = right coronary artery.
We have previously shown that following heart attacks, 80% of patients have a reduced degree of inflammation in the ruptured plaque within 6 months of the event with the application of secondary prevention measures (45). However, 20% of the ruptured plaques do not “cool down” within 6 months, and this may flag the patients who are more likely to have recurrent events, making them candidates for therapeutics that target inflammation (eg, colchicine) (25,48).
In everyday practice, clinicians are often bombarded with various clinical risk factors–derived risk scores, information about calcium scores, plaque information from CCTA, and information on systemic biomarkers. What is needed is systematic integration into a single risk profile to guide personalized treatment strategies. Indeed, a recently developed cloud-based platform (CaRi-cloud) aims to host Oxford’s proprietary risk calculator (CaRi-HEART), (Caristo Diagnostics) which integrates the information from the perivascular space, with other information being extracted from the images as well as from the patient’s clinical profile to provide a personalized risk prediction on which the clinicians can act. Because of the complexity of the analysis, the analysis can be done by using cloud computational power, and the results are projected against a large international database for interpretation. A research version of the platform is currently deployed in a large number of hospitals in the United Kingdom, Europe, the United States, and Asia, and clinicians’ feedback is now being collected to allow for optimization of its clinical implementation workflow. This technology is undergoing the final stages of regulatory clearance in Europe and the United States, and it is expected to be available for clinical use in early 2021.
Conclusion
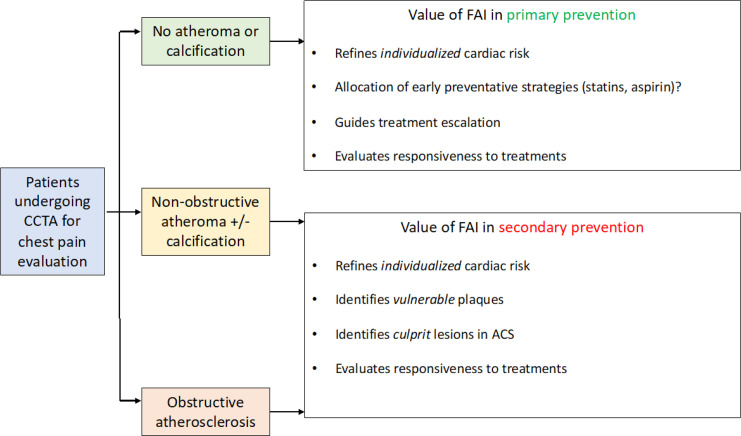
CCTA is now established as a first-line test in the investigation of suspected CAD. However, with its expanding use and adoption across different clinical settings, there is an urgent need to improve its sensitivity and specificity while also refining its diagnostic and prognostic value in the earliest stages of coronary atherosclerosis. We have recently introduced a translational research paradigm that led to the generation and validation of the perivascular FAI, a noninvasive marker that captures coronary inflammation thanks to the ability of PVAT to modify its composition in response to adjacent inflammatory stimuli. Several studies have demonstrated the reliability and prognostic value of perivascular FAI mapping, as well as its diagnostic utility in detecting active coronary microcalcification or inflammation and value in tracking the response to anti-inflammatory interventions (Fig 5). This review showcases how we can harness the role of PVAT as a real-life molecular sensor to develop CT-based imaging biomarkers that may improve the early detection and prevention of CAD in patients undergoing diagnostic assessment with CCTA. To further integrate traditional approaches with CT biomarkers to develop a one-stop-shop artificial intelligence tool for clinicians, large cardiac CT data sets are necessary for training purposes. To this end, The Oxford Risk Factors and Non-Invasive Imaging Study has been established and is currently recruiting patients to link cardiovascular events with information from CT images, adipose tissue, and blood samples (49). This important effort is expected to further advance the role of cardiac CT by integrating old and new biomarkers into clinical and diagnostic algorithms.
Figure 5:
The potential role of the fat attenuation index (FAI) in primary and secondary prevention. The FAI has clinical value in patients both without and with established coronary artery disease (primary and secondary prevention, respectively), in which it can help refine cardiovascular risk, allocate preventative interventions, guide treatment escalation, evaluate responsiveness to treatment and even identify vulnerable plaques. ACS = acute coronary syndromes, CCTA = coronary CT angiography.
Acknowledgments
Acknowledgments
The methods for the analysis of the perivascular fat attenuation index described and for the radiotranscriptomic profiling of adipose tissue are subject to patent US10695023B2 and patent applications PCT/GB2017/053262, GB2018/1818049.7, GR20180100490, and GR20180100510 through exclusive license to Caristo Diagnostics.
C.A. is supported by the British Heart Foundation (grants FS/16/15/32047 and TG/19/2/34831) and the National Institute for Health Research Oxford Biomedical Research Centre, Oxford, England.
Disclosures of Conflicts of Interest: L.V.K. disclosed no relevant relationships. E.K.O. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: shareholder of and has served as a consultant for Caristo Diagnostics; institution has patent involvement with Caristo Diagnostics (patent license transferred from the University of Oxford to Caristo Diagnostics). Other relationships: Licensed patents: The methods for analysis of the perivascular fat attenuation index described in this report are subject to patent applications PCT/GB2017/053262, GB2018/1818049.7, GR20180100490, and GR20180100510, licensed through exclusive license to Caristo Diagnostics; Patent royalties: the methods for analysis of the perivascular fat attenuation index described in this report are subject to patent applications PCT/GB2017/053262, GB2018/1818049.7, GR20180100490, and GR20180100510, licensed through exclusive license to Caristo Diagnostics. C.A. Activities related to the present article: institution received grant from British Heart Foundation. Activities not related to the present article: author is founder, shareholder and Chief Scientific Officer of Caristo Diagnostics, a CT image-analysis company; author received payment for lecture from Covance. Other relationships: Patents pending: The methods for analysis of the perivascular fat attenuation index described and radiotranscriptomic profiling of adipose tissue are subject to patent applications PCT/GB2017/053262, GB2018/1818049.7, GR20180100490, and GR20180100510, licensed through exclusive license to Caristo Diagnostics. Issued patents: The methods for analysis of the perivascular fat attenuation index described and radiotranscriptomic profiling of adipose tissue are subject to patent US10,695,023B2, licensed through exclusive license to Caristo Diagnostics; licensed patents: The methods for analysis of the perivascular fat attenuation index described and radiotranscriptomic profiling of adipose tissue are subject to patent US10,695,023B2, licensed through exclusive license to Caristo Diagnostics.
Abbreviations:
- CAC
- coronary artery calcium
- CAD
- coronary artery disease
- CCTA
- coronary CT angiography
- CRISP-CT
- Cardiovascular Risk Prediction Using Computed Tomography
- FAI
- fat attenuation index
- HRP
- high-risk plaque
- MI
- myocardial infarction
- PVAT
- perivascular adipose tissue
References
- 1.National Institute for Health and Care Excellence . Resource impact report: recent-onset chest pain of suspected cardiac origin: assessment and diagnosis (CG95). National Institute for Health and Care Excellence website. https://www.nice.org.uk/guidance/cg95/resources/resource-impact-report-2726121709. Published 2016. Accessed March 20, 2020. [PubMed] [Google Scholar]
- 2.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41(3):407–477. [DOI] [PubMed] [Google Scholar]
- 3.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372(14):1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newby DE, Williams M, Hunter A, et al. ; SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385(9985):2383–2391 [Published correction appears in Lancet 2015;385(9985):2354.]. [DOI] [PubMed] [Google Scholar]
- 5.Newby DE, Adamson PD, Berry C, et al. ; SCOT-HEART Investigators. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379(10):924–933. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Williams MC, Newby DE, Nicol ED. The updated NICE guidelines: cardiac CT as the first-line test for coronary artery disease. Curr Cardiovasc Imaging Rep 2017;10(5):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oikonomou EK, West HW, Antoniades C. Cardiac computed tomography: assessment of coronary inflammation and other plaque features. Arterioscler Thromb Vasc Biol 2019;39(11):2207–2219. [DOI] [PubMed] [Google Scholar]
- 8.Nørgaard BL, Hjort J, Gaur S, et al. Clinical use of coronary CTA-derived FFR for decision-making in stable CAD. JACC Cardiovasc Imaging 2017;10(5):541–550. [DOI] [PubMed] [Google Scholar]
- 9.Qiao HY, Tang CX, Schoepf UJ, et al. Impact of machine learning-based coronary computed tomography angiography fractional flow reserve on treatment decisions and clinical outcomes in patients with suspected coronary artery disease. Eur Radiol 2020;30(11):5841–5851. [DOI] [PubMed] [Google Scholar]
- 10.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol 2018;72(4):434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koulaouzidis G, Charisopoulou D, Jenkins PJ, Koulaouzidis A, McArthur T. Prevalence of noncalcified coronary plaque in patients with calcium score of 0: the silent enemy. Angiology 2013;64(3):205–210. [DOI] [PubMed] [Google Scholar]
- 12.Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol 2015;65(13):1273–1282. [DOI] [PubMed] [Google Scholar]
- 13.Fishbein MC, Siegel RJ. How big are coronary atherosclerotic plaques that rupture? Circulation 1996;94(10):2662–2666. [DOI] [PubMed] [Google Scholar]
- 14.Antonopoulos AS, Sanna F, Sabharwal N, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 2017;9(398):eaal2658. [DOI] [PubMed] [Google Scholar]
- 15.Williams MC, Moss AJ, Dweck M, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol 2019;73(3):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams MC, Kwiecinski J, Doris M, et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial (Scottish Computed Tomography of the Heart). Circulation 2020;141(18):1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) Coronary Artery Disease Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2016;10(4):269–281. [DOI] [PubMed] [Google Scholar]
- 18.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358(13):1336–1345. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulos N, Melek BH, Arepalli CD, et al. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: a substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning). J Am Coll Cardiol 2013;61(19):1956–1961. [DOI] [PubMed] [Google Scholar]
- 20.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105(9):1135–1143. [DOI] [PubMed] [Google Scholar]
- 21.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 1999;340(2):115–126. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regen 2019;39(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 24.Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381(26):2497–2505. [DOI] [PubMed] [Google Scholar]
- 25.Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020;383(19):1838–1847. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380(8):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christen T, Sheikine Y, Rocha VZ, et al. Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging. JACC Cardiovasc Imaging 2010;3(8):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarkin JM, Joshi FR, Rudd JHF. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol 2014;11(8):443–457. [DOI] [PubMed] [Google Scholar]
- 29.Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383(9918):705–713. [DOI] [PubMed] [Google Scholar]
- 30.Antonopoulos AS, Antoniades C. Perivascular fat attenuation index by computed tomography as a metric of coronary inflammation. J Am Coll Cardiol 2018;71(23):2708–2709. [DOI] [PubMed] [Google Scholar]
- 31.Antoniades C, Kotanidis CP, Berman DS. State-of-the-art review article. Atherosclerosis affecting fat: What can we learn by imaging perivascular adipose tissue? J Cardiovasc Comput Tomogr 2019;13(5):288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018;392(10151):929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margaritis M, Antonopoulos AS, Digby J, et al. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 2013;127(22):2209–2221. [DOI] [PubMed] [Google Scholar]
- 34.Elnabawi YA, Oikonomou EK, Dey AK, et al. Association of biologic therapy with coronary inflammation in patients with psoriasis as assessed by perivascular fat attenuation index. JAMA Cardiol 2019;4(9):885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooks RA. A quantitative theory of the Hounsfield unit and its application to dual energy scanning. J Comput Assist Tomogr 1977;1(4):487–493. [DOI] [PubMed] [Google Scholar]
- 36.Antoniades C, Antonopoulos AS, Deanfield J. Imaging residual inflammatory cardiovascular risk. Eur Heart J 2020;41(6):748–758. [DOI] [PubMed] [Google Scholar]
- 37.Groves EM, Erande AS, Le C, et al. Comparison of epicardial adipose tissue volume and coronary artery disease severity in asymptomatic adults with versus without diabetes mellitus. Am J Cardiol 2014;114(5):686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goeller M, Achenbach S, Cadet S, et al. Pericoronary adipose tissue computed tomography attenuation and high-risk plaque characteristics in acute coronary syndrome compared with stable coronary artery disease. JAMA Cardiol 2018;3(9):858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwiecinski J, Dey D, Cadet S, et al. Peri-coronary adipose tissue density is associated with 18F-sodium fluoride coronary uptake in stable patients with high-risk plaques. JACC Cardiovasc Imaging 2019;12(10):2000–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oikonomou EK, Desai MY, Marwan M, et al. Perivascular fat attenuation index stratifies cardiac risk associated with high-risk plaques in the CRISP-CT study. J Am Coll Cardiol 2020;76(6):755–757. [DOI] [PubMed] [Google Scholar]
- 41.Hoshino M, Yang S, Sugiyama T, et al. Peri-coronary inflammation is associated with findings on coronary computed tomography angiography and fractional flow reserve. J Cardiovasc Comput Tomogr 2020;14(6):483–489. [DOI] [PubMed] [Google Scholar]
- 42.Yu M, Dai X, Deng J, Lu Z, Shen C, Zhang J. Diagnostic performance of perivascular fat attenuation index to predict hemodynamic significance of coronary stenosis: a preliminary coronary computed tomography angiography study. Eur Radiol 2020;30(2):673–681. [DOI] [PubMed] [Google Scholar]
- 43.Dai X, Yu L, Lu Z, Shen C, Tao X, Zhang J. Serial change of perivascular fat attenuation index after statin treatment: insights from a coronary CT angiography follow-up study. Int J Cardiol 2020;319:144–149. [DOI] [PubMed] [Google Scholar]
- 44.Kwon O, Kang SJ, Kang SH, et al. Relationship between serum inflammatory marker levels and the dynamic changes in coronary plaque characteristics after statin therapy. Circ Cardiovasc Imaging 2017;10(7):e005934. [DOI] [PubMed] [Google Scholar]
- 45.Oikonomou EK, Williams MC, Kotanidis CP, et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur Heart J 2019;40(43):3529–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J 2015;36(8):475–481. [DOI] [PubMed] [Google Scholar]
- 47.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 2003;108(14):1664–1672. [DOI] [PubMed] [Google Scholar]
- 48.Vaidya K, Arnott C, Martínez GJ, et al. Colchicine therapy and plaque stabilization in patients with acute coronary syndrome: a CT coronary angiography study. JACC Cardiovasc Imaging 2018;11(2 Pt 2):305–316. [DOI] [PubMed] [Google Scholar]
- 49.The ORFAN study . The Oxford Cohort for Heart, Vessels & Fat website. https://oxhvf.com/orfan/overview/. Accessed March 21, 2020.



![A, Fat attenuation index (FAI) mapping stratifies the cardiac risk associated with high-risk plaque (HRP) features. B, In the presence of a normal FAI (low FAI [FAIlow]), HRP features are not associated with an increased cardiac risk in the Cardiovascular Risk Prediction Using CT study population. However, in the presence of an abnormally elevated FAI, HRP features identify a truly “high-risk” population with a 6.26-fold higher adjusted cardiac risk compared with the FAIlow/HRP-negative reference group. (Reprinted, with permission, from reference 40.) CCTA = coronary CT angiography.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6936/7977699/6f01bae54d13/ryct.2021200563.fig2.jpg)