Abstract
Bioprosthetic heart valves (BPHVs) have fundamentally changed the treatment of valvular heart disease. Despite the continuous progress of BPHVs, from early valve designs for use in surgical replacement to the rapidly evolving use of transcatheter replacement techniques and designs, valve dysfunction and degeneration remain fundamental issues. Current guidelines and proposed standard definitions of BPHV dysfunction and degeneration outline the importance of imaging. Imaging plays a key role in understanding valve degeneration, including clinical imaging to identify transvalvular gradients, leaflet thickening, thrombosis, calcification, and restricted or reduced leaflet motion. Similarly, translational imaging approaches—including micro-CT, high-speed video, computational modeling, and high-resolution microscopy—and histologic analysis are crucial to understanding mechanisms of valve degeneration and factors that may contribute to valve dysfunction. This article provides an overview of valve dysfunction and degeneration and the role of imaging.
© RSNA, 2019
Summary
Valve dysfunction and degeneration remain fundamental problems of bioprosthetic heart valves; key processes involved in dysfunction and degeneration and associated imaging findings are reviewed.
Key Points
■ Structural valve degeneration is a key issue in both surgical and transcatheter bioprosthetic valves.
■ Definitions of valve dysfunction and structural valve degeneration vary, but all detail processes such as thrombus and calcification that result in valve dysfunction.
■ Clinical imaging, including CT and echocardiography, are essential to identifying valve degeneration, although controversies remain on the significance of associated findings such as leaflet thickening.
■ Translational imaging approaches provide insight into cellular mechanisms of valve degeneration and development of new imaging techniques.
Introduction
Valvular heart disease is associated with shortened life span, deterioration of quality of life, and economic burden and is estimated to impact 2.5% of the population in industrialized countries. This percentage reflects a decreasing prevalence in rheumatic disease and increasing prevalence of degenerative disease, with mitral regurgitation and aortic stenosis being the most common manifestations. Notably, valvular heart disease is a societal health issue that will only grow as the population ages. For example, with the number of people older than 75 years expected to double by 2050 in the United States alone and more than half of those aged 70 years or older having heart valve disease, the incidence of valvular heart disease is expected to rise (1–5). Furthermore, improving care for children with congenital and genetic valve defects has resulted in an increasing prevalence of adults living with congenital valve disease (6). While guidelines outline the complex management of patients with valvular disease, valve replacement has revolutionized treatment and improved patient outcomes (7).
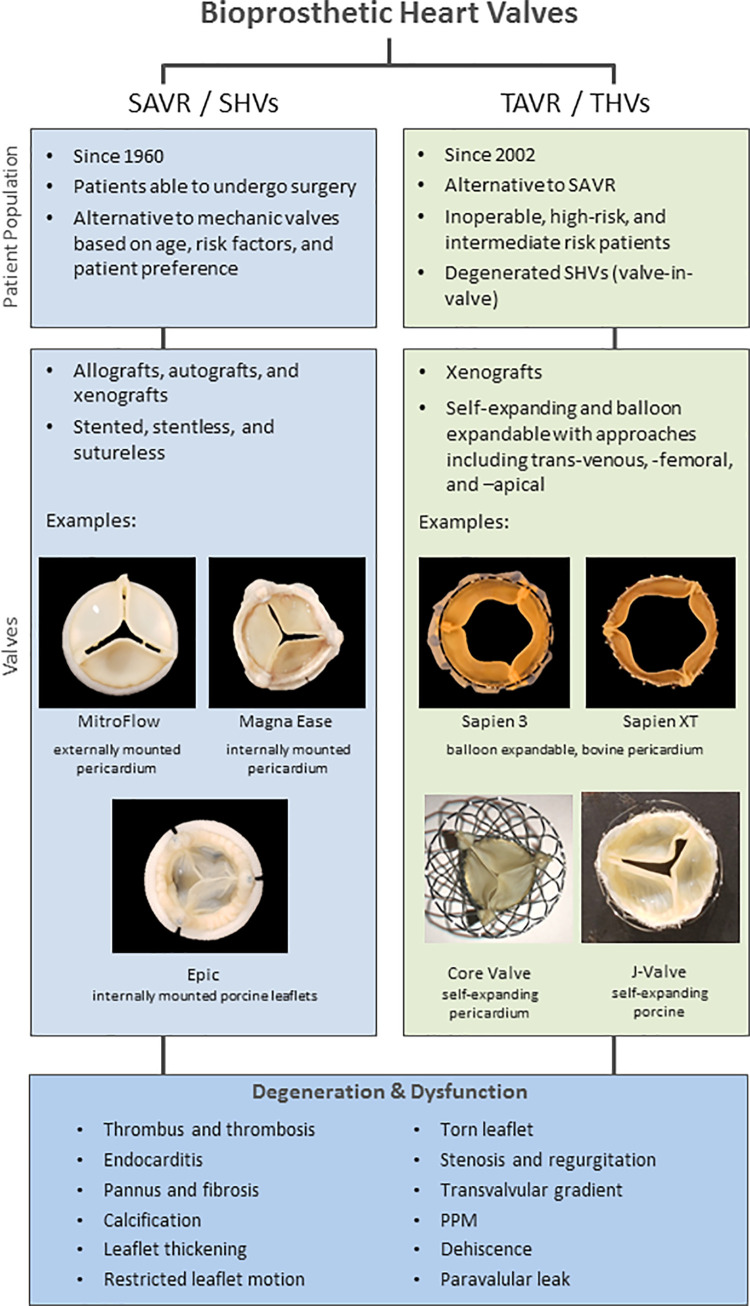
Valve replacements generally fall into two main categories based on procedure: surgical valve replacement and, more recently, transcatheter valve replacement. Although replacements are used in various anatomic locations, much focus is placed on their use in surgical aortic valve replacement and transcatheter aortic valve replacement. Figure 1 provides a brief overview of valves used in both transcatheter and surgical aortic valve replacements. First reported in 1960, surgical aortic valve replacement uses mechanical or bioprosthetic surgical heart valves (SHVs) including allografts, autografts, and xenografts made of pericardium or porcine leaflets or complete valves (8–10). Bioprosthetic SHVs serve as an alternative to mechanical valves that require lifelong anticoagulation and thus incur increased patient risk. The 2017 American College of Cardiology/American Heart Association guidelines (7) indicate reasonable use of bioprosthetic SHVs over mechanical valves in patients older than 70 years or in patients aged 50–70 years based on risk factors and patient preference.
Figure 1:
Image shows overview of bioprosthetic heart valves used in transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR). Manufacturer information is as follows: MitroFlow (Sorin, USA), MagnaEase (Edwards Lifesciences, USA), Sapien 3 and Sapien XT (Edwards Lifesciences), Epic (St. Jude Medical, USA), Core Valve (Medtronic, USA), and J-Valve (JC Medical, USA). PPM = patient prosthesis mismatch, SHV = surgical heart valve, THV = transcatheter heart valve.
In 2002, transcatheter aortic valve replacement was introduced through a transvenous approach by using bioprosthetic transcatheter heart valves (THVs). This was followed by a transarterial approach, and it has rapidly become standard of care for patients considered to be at high or intermediate surgical risk (11–15). Although initial trials were performed in patients with clinically significant comorbidities and risk factors (ie, those at high risk for surgery or unable to undergo surgery), transcatheter aortic valve replacement is rapidly expanding to include patients at low risk, as well as valve-in-valve transcatheter aortic valve replacement procedures to place THVs inside of degenerated SHVs (16,17). THVs are predominately made with pericardial leaflets attached to a variety of frame designs that allow the THVs to be crimped onto a catheter and placed by using a transcatheter approach with either a balloon-expandable or self-expanding design.
Both surgical and transcatheter bioprosthetic heart valves (BPHVs) (THVs and SHVs) are subject to dysfunction and degeneration owing to their bioprosthetic nature with a great deal of focus being placed on defining, monitoring for, and predicting valve degeneration. In addition, there is a growing need to better understand the risk factors and mechanisms of degeneration. Valve degeneration is a particular point of focus as valve replacement moves toward treatment of younger patients with greater life expectancies. Below, we provide an overview of BPHV dysfunction and degeneration and associated imaging methods. While this generally includes endocarditis, in-depth discussion of infection as well as acute complications following replacement are beyond the scope of the review. Instead, we aim to highlight clinical conundrums of BPHV degeneration and to present translational and fundamental science imaging approaches to understand mechanisms of degeneration and develop new clinical approaches and imaging techniques.
Bioprosthetic Valve Degeneration and Dysfunction
Past Definitions of BPHV Degeneration and Dysfunction
BPHV degeneration has had many definitions but is generally defined as a permanent process causing hemodynamic dysfunction in the form of stenosis or regurgitation requiring reintervention. Historically, the clinical definition of degeneration depended on the need for surgical reintervention with outcomes of BPHVs reported as survival with freedom from reintervention (9,18). This approach has obvious flaws, because there are many patients with worsening BPHV function who are not candidates for redo surgery owing to comorbid illnesses. This historical approach has also failed to provide detailed clinical information needed for insight into the mechanism of degeneration. Such information is key because degeneration is a varied and complex process constituting interplay of mechanical forces, patient anatomy, and biologic and cellular processes. To help meet this clinical need, new definitions of BPHV degeneration have been proposed and aim to evaluate known processes in intrinsic BPHV degeneration—such as calcification, fibrosis, and leaflet tears—as well as external factors associated with dysfunction and/or degeneration including infection, thrombus, patient prosthesis mismatch, paravalvular leak, and valve positioning. Figure 2 shows factors associated with valve dysfunction and degeneration observable at the time of BPHV explant.
Figure 2:
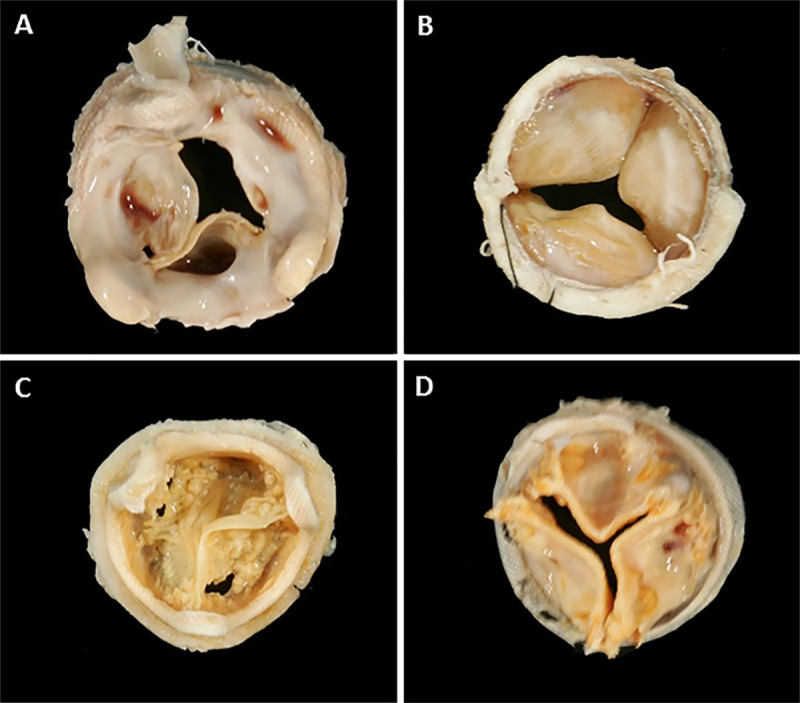
Images show examples of features of bioprosthetic heart valves (BPHVs) pathology seen at gross examination at explant. BPHV explants demonstrate, A, large amounts of fibrosis with areas of thrombus on aortic aspect of surgical heart valve (SHV) with pericardial leaflets; B, fibrosis on ventricular aspect on SHV with pericardial leaflets; and calcified SHVs with, C, leaflet tears in valves with porcine and, D, pericardial leaflets.
Current Definitions and Rates of BPHV Degeneration and Dysfunction
The most recent document detailing BPHV degeneration and dysfunction is endorsed by the European Association of Percutaneous Cardiovascular Interventions, the European Society of Cardiology, and the European Association for Cardio-Thoracic Surgery. Capodanno et al (18) propose a standard definition of structural valve degeneration (SVD) as permanent valve dysfunction resulting from those factors both intrinsic and extrinsic to the BPHV leading to degeneration and/or hemodynamic dysfunction. However, this proposed definition places endocarditis and valve thrombus in separate categories and notably differentiates hemodynamic valve deterioration from morphologic deterioration. Furthermore, Capodanno et al propose use of the term bioprosthetic valve failure, which integrates SVD etiology and clinical consequences. The authors propose that this classification will help prevent placing too much emphasis on BPHV-related outcomes in asymptomatic patients. These definitions also align nicely with other proposed standard definitions of SVD including those recently outlined in the white paper by Dvir et al (9,18,19), which proposes three stages of SVD based on severity. Figure 3 summarizes key points of definitions by Capodanno et al and Dvir et al.
Figure 3:
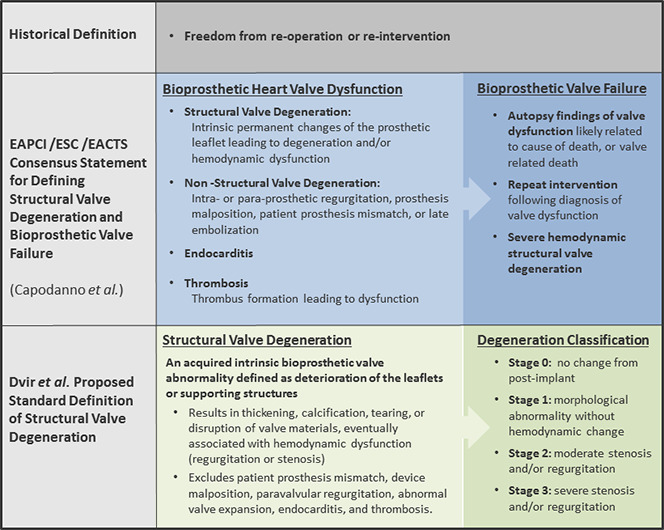
Definitions of bioprosthetic heart valve dysfunction and degeneration. EACTS = European Association for Cardio-Thoracic Surgery, EAPCI = European Association of Percutaneous Cardiovascular Interventions, ESC = European Society of Cardiology.
Reported rates of BPHV dysfunction and degeneration vary. Such variation may be linked to the differing definitions of BPHV dysfunction and degeneration used in studies, as well as the varying patient populations and valve types. From prior meta-analyses, SVD in porcine and pericardial SHVs is noted to start at approximately 8 years following implantation with SVD increasing greatly at 10 years following implantation (20–22). However, early SVD is an important clinical problem that has to date not been adequately quantified in scope due to issues of defining SVD as discussed previously. Fortunately, there is growing awareness of the issue and with the advent of transcatheter valve-in-valve therapies, there is now a treatment strategy for even older patients with SVD. With the growing interest has come new data on the durability of THVs and SHVs. In the recently published NOTION (Nordic aortic valve intervention) trial (23), investigators evaluated the rates of valve failure and SVD in a low-risk population randomized between surgical aortic valve replacement and transcatheter aortic valve replacement and aimed to classify degeneration and valve failure according to current proposed standard definitions. The rates of valve failure were similar in SHVs and THVs (6.7% vs 7.5%) through 6 years, but there was a significantly higher rate of SVD in SHVs compared with THVs (24.0% vs 4.8%).
Imaging of BPHVs
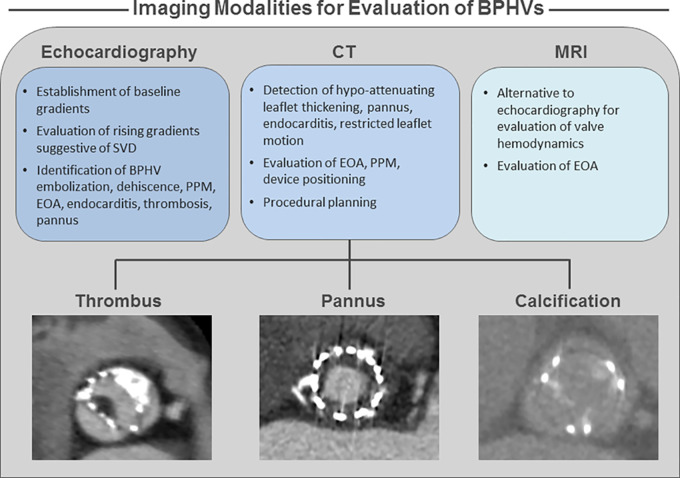
Imaging plays a central role in investigating valve function and pathology (Fig 4). Transthoracic echocardiography (24) is standard for investigation of valve function with guidelines recommending transthoracic echocardiography performed after both surgical aortic valve replacement and transcatheter aortic valve replacement. Recommendations for timing of baseline and follow-up imaging vary depending on procedure type (transcatheter aortic valve replacement vs surgical aortic valve replacement), but allow for monitoring of transvalvular hemodynamic changes suggestive of stenosis or regurgitation with detailed criteria for use of echocardiography to define BPHV function outlined in current proposed definitions of BPHV degeneration (9,19,25,26). Recently, imaging-based definitions of valve degeneration have been introduced based on baseline echocardiographic gradients and interval change in gradients, with a rise in gradient suggestive of SVD and SVD severity. For example, Dvir et al (19) outline 10 mm Hg and/or an absolute gradient greater than 20 mm Hg to be suggestive of SVD with the severity of SVD defined based on the severity or worsening of the valvular gradient evaluated in combination with other echocardiographic parameters including Doppler velocity index, effective orifice area, peak flow velocity, acceleration time, ratio of acceleration time to left ventricular ejection time, and characterization of the transvalvular flow envelope. In addition, echocardiography can also be used in the evaluation of valve positioning including regurgitation, paravalvular leak, embolization and dehiscence, patient prosthesis mismatch through measurement of effective orifice area, endocarditis, thrombosis, and pannus (7,19,26).
Figure 4:
Image shows clinical imaging approaches for bioprosthetic heart valve (BPHV) degeneration and dysfunction. EOA = effective orifice area, PPM = patient prosthesis mismatch, SVD = structural valve degeneration.
MRI provides an alternative to echocardiography for assessing valvular hemodynamics in patients with BPHVs and has been shown to be relatively safe for imaging at both 1.5-T and 3.0-T for a number of valve types. MRI may be useful when echocardiography is limited by imaging windows, although BPHVs can cause artifact on MR images. However, effective orifice area calculated from MRI has been shown to correlate to effective orifice area calculated by using echocardiography in both normal and dysfunctional BPHVs (27–31).
The role of CT in the proposed criteria for defining BPHV deterioration is more limited than is echocardiography. CT can potentially be used for depiction of restricted leaflet motion, endocarditis, leaflet thickening, and calcification (7,19). Given the excellent spatial resolution, most advocate for CT to be primarily used to identify leaflet thickening and/or calcification, with restriction being more easily and thoroughly characterized at echocardiography. Unfortunately, owing to the limited capacity for tissue characterization, CT is incapable of differentiating thrombus from fibrotic valve thickening but can be helpful in differentiating these processes from pannus, which typically spares the leaflets and is circumferential below the valve level. These are commonly the three differential considerations in the setting of rising valve gradients. In clinical practice, CT should be considered in patients with rising gradients, with a trial of anticoagulation now recommended for those with findings of leaflet thickening. However, should we consider CT to play a role in BPHV dysfunction as it relates to procedural planning? Factors such as evaluation of effective orifice area and patient prosthesis mismatch, device malpositioning, paravalvular leak, and embolization are factors to consider in BPHV dysfunction and/or degeneration. However, use of CT for preprocedural planning can reduce rates of such factors through providing annular sizing and guidance on device expansion and placement (32,33).
BPHV Thrombus and Leaflet Thickening
Current definitions of SVD exclude BPHV thrombus and/or thrombosis, and rates of BPHV thrombosis vary depending on the definition used. However, subclinical leaflet thrombus depictable at routine CT following valve replacement is thought to be more frequent. Recent data from noninvasive imaging suggest that BPHVs used in both surgical aortic valve replacement and transcatheter aortic valve replacement are prone to thrombus formation, which can be observed as hypoattenuating leaflet thickening, or HALT, on both porcine and pericardial BPHVs (34–37). HALT may cause impaired leaflet movement that can lead to hemodynamic compromise. Recent work on explanted transcatheter BPHVs shows that leaflet thickening results from a progressive pathologic process wherein thrombus formation is followed by valve fibrosis and eventual calcification at later time points of implantation that contributed to valve dysfunction (38–40). However, do these findings mean we should image more frequently to identify HALT? What is the significance? Can imaging provide more details on the nature of HALT and its potential implications? Can we differentiate fibrosis and thrombus and discern the potential of such thrombus as a nidus for calcification for cellular process that may degenerate the BPHV? Should patients with asymptomatic HALT undergo anticoagulation? Lack of anticoagulation at discharge was recently found to be a predictor of THV hemodynamic deterioration and notable ongoing trials seek to study this further. Conversely, risk is associated with anticoagulation, a point highlighted by the recent early halting of the GALILEO (global study comparing a rivaroxaban-based antithrombotic strategy to an antiplatelet-based strategy after transcatheter aortic valve replacement to optimize clinical outcomes) trial evaluating rivaroxaban in patients with transcatheter aortic valve replacement (27).
Conundrums of Current SVD Definitions
Although establishing standardized definitions of SVD is a step forward, it is important to note that these definitions fail to incorporate the diverse biologic processes that cause SVD. Current proposed definitions of SVD aim to document clinical manifestations of valve failure but will not shed light on early aspects of these processes needed to determine cause and effect to potentially elucidate mechanisms and enable strategies to help prevent SVD onset. For example, while morphologic SVD can include leaflet thickening and calcification, does calcification and/or thickening appreciable at CT necessarily equate to calcification grossly observed at autopsy or histologic analysis of explanted valves? Moreover, in defining morphologic thickening, what is the best approach—imaging or ex vivo analysis? Akin to imaging, no current guidelines for a threshold of pathologic thickening at explant at autopsy or surgery exist, and typical fixation of valves at explant makes in-depth leaflet function studies following explant a logistical challenge yet to be reported in the literature.
From an imaging perspective, should we instead aim to use imaging modalities that help to detect calcification earlier or monitor more closely for factors that may promote calcification such as thrombus? Furthermore, definitions of SVD are proposed to apply to all BPHVs and patients despite clear differences including valve design, flow dynamics, leaflet compositions, fixation processes, implantation procedures, risk factors, comorbidities, and sex.
SVD Insights from Translational and Fundamental Science Imaging
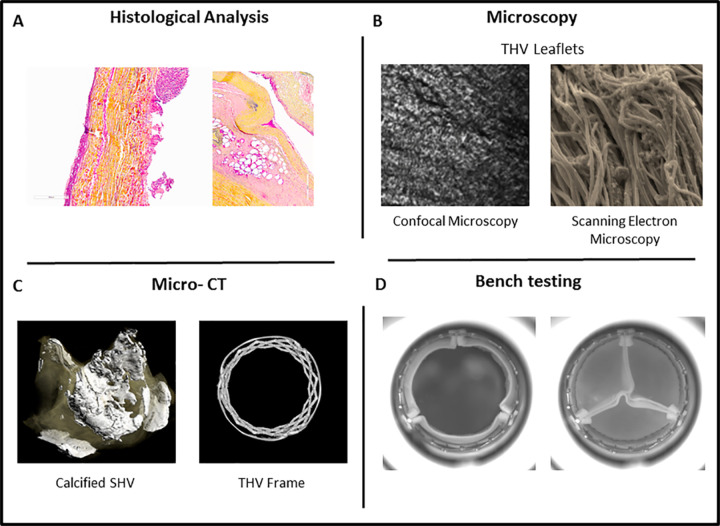
Defining and determining mechanisms of BPHV degeneration to improve patient outcomes and valve durability and provide insight to imaging conundrums is arguably limited by a number of factors, including available imaging modalities and their resolution. However, translational and fundamental scientific imaging approaches continue to provide invaluable insight and the basis on which to plan future investigations (Fig 5). For example, recent studies (38,41,42) outline a timeline for key features of THV pathology by using histologic analysis and microscopic imaging approaches, including confocal microscopy and scanning electron microscopy, to demonstrate the presence of early thrombus, followed by fibrosis and calcification of THVs around 4 years. These studies support current clinical imaging studies suggesting that valve thrombus detectable with clinical imaging may relate to long-term degeneration (including at later time points) such as calcification. Furthermore, micro-CT of explanted SHVs combined with fluorine 18 sodium fluoride micro-PET/CT imaging, proposed to help detect areas of microcalcification indicative of tissue degeneration that occurs before the onset of the macrocalcification, correlates well with histologic evidence of BPHV degeneration. Moreover, clinical application of this PET/CT imaging approach was able to increase ability to help detect calcification and enable prediction of forthcoming hemodynamic dysfunction (43).
Figure 5:
Image shows translational and fundamental scientific imaging approaches to study bioprosthetic heart valve (BPHV) degeneration. A, Histologic cross-section of pericardial BPHV leaflet (left) and porcine leaflet (right) from explanted valves. B, Confocal microscopy and scanning electron microscopy of transcatheter heart valve (THV) leaflets to evaluate collagen structure within leaflet. C, Micro-CT of calcified explanted surgical heart valve (SHV) (left) and micro-CT of THV frame (right). D, Representative images of THV undergoing bench testing to assess hemodynamics in pulse duplicator.
Bench studies of BPHVs may also help to understand cellular processes associated with valve dysfunction. Use of high-resolution imaging (eg, high-speed cameras, video, and micro-CT) and computational modeling have produced a more in-depth understanding of valve function and flow dynamics, which may impact BPHV pathology (eg, the identification of stagnation zones related to thrombus formation). Moreover, such bench studies of valve positioning, sizing and overexpansion, and device selection in valve-in-valve procedures have shed light on how procedural factors may impact hemodynamics, leaflet geometry, and mechanical stresses placed on the valve—an important consideration given that typically mineralization (eg, calcification) is increased as sites of mechanical stress (44–50).
Conclusion
BPHVs have seen a relatively rapid evolution from initial surgical aortic valve replacement interventions in the 1960s to the rapid clinical integration of transcatheter aortic valve replacement and THVs starting in 2002. However, valve degeneration remains a fundamental problem of all BPHVs. Definitions of degeneration including SVD vary but generally outline phenotypes of dysfunction observable clinically and relying on clinical imaging including CT, MRI, and echocardiography. While controversies remain on defining degeneration and the significance of imaging findings, translational and fundamental imaging techniques continue to provide in-depth analysis to establish timelines of SVD and elucidate mechanisms leading to degeneration, as well as insight into associated risk factors. Furthermore, new imaging modalities and approaches are aimed at reducing rates of degeneration and enabling prediction of degeneration and providing clinical insight into the complex associated biologic processes, which will ultimately improve patient outcomes. Therefore, overall, although BPHV degeneration is a key issue, multimodality imaging and thoughtful investigations are leading the way in helping to define, document, and determine the mechanisms of degeneration that will ultimately lead to improved valve durability and improved outcomes.
Disclosures of Conflicts of Interest: S.L.S. disclosed no relevant relationships. P.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Circle Cardiovascular Imaging and has grants/grants pending with Abbott, Edwards Lifesciences, Medtronic, and Neovasc; institution received research support from Edwards. Other relationships: disclosed no relevant relationships. J.A.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Circle Cardiovascular Imaging and HeartFlow; institution has grants/grants pending with Abbott, Edwards Lifesciences, Medtronic, and Neovasc; institution received research support from Edwards. Other relationships: disclosed no relevant relationships.
Abbreviations:
- BPHV
- bioprosthetic heart valve
- SHV
- surgical heart valve
- SVD
- structural valve degeneration
- THV
- transcatheter heart valve
References
- 1.Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol 2014;30(9):962–970. [DOI] [PubMed] [Google Scholar]
- 2.Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol 2011;8(3):162–172. [DOI] [PubMed] [Google Scholar]
- 3.Moore M, Chen J, Mallow PJ, Rizzo JA. The direct health-care burden of valvular heart disease: evidence from US national survey data. Clinicoecon Outcomes Res 2016;8:613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borer J. The challenge: about the Heart Valve Society of America. Heart Valve Society of America. http://www.heartvalvesocietyofamerica.org/more.html. Published 2017. Accessed July 1, 2017. [Google Scholar]
- 5.van Geldorp MW, Heuvelman HJ, Kappetein AP, et al. Quality of life among patients with severe aortic stenosis. Neth Heart J 2013;21(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triedman JK, Newburger JW. Trends in congenital heart disease: the next decade. Circulation 2016;133(25):2716–2733. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70(2):252–289. [DOI] [PubMed] [Google Scholar]
- 8.Harken DE, Soroff HS, Taylor WJ, Lefemine AA, Gupta SK, Lunzer S. Partial and complete prostheses in aortic insufficiency. J Thorac Cardiovasc Surg 1960;40:744–762. [PubMed] [Google Scholar]
- 9.Rodriguez-Gabella T, Voisine P, Puri R, Pibarot P, Rodés-Cabau J. Aortic bioprosthetic valve durability: incidence, mechanisms, predictors, and management of surgical and transcatheter valve degeneration. J Am Coll Cardiol 2017;70(8):1013–1028. [DOI] [PubMed] [Google Scholar]
- 10.Piazza N, Bleiziffer S, Brockmann G, et al. Transcatheter aortic valve implantation for failing surgical aortic bioprosthetic valve: from concept to clinical application and evaluation (part 1). JACC Cardiovasc Interv 2011;4(7):721–732. [DOI] [PubMed] [Google Scholar]
- 11.Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106(24):3006–3008. [DOI] [PubMed] [Google Scholar]
- 12.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363(17):1597–1607. [DOI] [PubMed] [Google Scholar]
- 13.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374(17):1609–1620. [DOI] [PubMed] [Google Scholar]
- 14.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364(23):2187–2198. [DOI] [PubMed] [Google Scholar]
- 15.Webb JG, Chandavimol M, Thompson CR, et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 2006;113(6):842–850. [DOI] [PubMed] [Google Scholar]
- 16.Capodanno D, Leon MB. Upcoming TAVI trials: rationale, design and impact on clinical practice. EuroIntervention 2016;12(Y):Y51–Y55. [DOI] [PubMed] [Google Scholar]
- 17.Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation 2012;126(19):2335–2344. [DOI] [PubMed] [Google Scholar]
- 18.Capodanno D, Petronio AS, Prendergast B, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017;38(45):3382–3390. [DOI] [PubMed] [Google Scholar]
- 19.Dvir D, Bourguignon T, Otto CM, et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation 2018;137(4):388–399. [DOI] [PubMed] [Google Scholar]
- 20.Foroutan F, Guyatt GH, O’Brien K, et al. Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: systematic review of observational studies. BMJ 2016;354:i5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, Furnary AP, Li HF, Grunkemeier GL. Bioprosthetic aortic valve durability: a meta-regression of published studies. Ann Thorac Surg 2017;104(3):1080–1087. [DOI] [PubMed] [Google Scholar]
- 22.Puvimanasinghe JP, Steyerberg EW, Takkenberg JJ, et al. Prognosis after aortic valve replacement with a bioprosthesis: predictions based on meta-analysis and microsimulation. Circulation 2001;103(11):1535–1541. [DOI] [PubMed] [Google Scholar]
- 23.Søndergaard L, Ihlemann N, Capodanno D, et al. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J Am Coll Cardiol 2019;73(5):546–553. [DOI] [PubMed] [Google Scholar]
- 24.Chen MY, Rochitte CE, Arbab-Zadeh A, et al. Prognostic value of combined CT angiography and myocardial perfusion imaging versus invasive coronary angiography and nuclear stress perfusion imaging in the prediction of major adverse cardiovascular events: the CORE320 multicenter study. Radiology 2017;284(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009;22(9):975–1014; quiz 1082–1084. [DOI] [PubMed] [Google Scholar]
- 26.Pislaru SV, Nkomo VT, Sandhu GS. Assessment of prosthetic valve function after TAVR. JACC Cardiovasc Imaging 2016;9(2):193–206. [DOI] [PubMed] [Google Scholar]
- 27.Windecker S, Tijssen J, Giustino G, et al. Trial design: rivaroxaban for the prevention of major cardiovascular events after transcatheter aortic valve replacement: Rationale and design of the GALILEO study. Am Heart J 2017;184:81–87. [DOI] [PubMed] [Google Scholar]
- 28.Shellock FG. Prosthetic heart valves and annuloplasty rings: assessment of magnetic field interactions, heating, and artifacts at 1.5 Tesla. J Cardiovasc Magn Reson 2001;3(4):317–324. [DOI] [PubMed] [Google Scholar]
- 29.Saeedi M, Thomas A, Shellock FG. Evaluation of MRI issues at 3-Tesla for a transcatheter aortic valve replacement (TAVR) bioprosthesis. Magn Reson Imaging 2015;33(4):497–501. [DOI] [PubMed] [Google Scholar]
- 30.Shellock FG. Biomedical implants and devices: assessment of magnetic field interactions with a 3.0-Tesla MR system. J Magn Reson Imaging 2002;16(6):721–732. [DOI] [PubMed] [Google Scholar]
- 31.Maragiannis D, Jackson MS, Flores-Arredondo JH, et al. Functional assessment of bioprosthetic aortic valves by CMR. JACC Cardiovasc Imaging 2016;9(7):785–793. [DOI] [PubMed] [Google Scholar]
- 32.Wilson R, McNabney C, Weir-McCall JR, Sellers S, Blanke P, Leipsic JA. Transcatheter Aortic and Mitral Valve Replacements. Radiol Clin North Am 2019;57(1):165–178. [DOI] [PubMed] [Google Scholar]
- 33.Blanke P, Weir-McCall JR, Achenbach S, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI) / transcatheter aortic valve replacement (TAVR): An expert consensus document of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2019;13(1):1–20. [DOI] [PubMed] [Google Scholar]
- 34.Leetmaa T, Hansson NC, Leipsic J, et al. Early aortic transcatheter heart valve thrombosis: diagnostic value of contrast-enhanced multidetector computed tomography. Circ Cardiovasc Interv 2015;8(4):e001596. [DOI] [PubMed] [Google Scholar]
- 35.Pache G, Blanke P, Zeh W, Jander N. Cusp thrombosis after transcatheter aortic valve replacement detected by computed tomography and echocardiography. Eur Heart J 2013;34(46):3546. [DOI] [PubMed] [Google Scholar]
- 36.Dalén M, Sartipy U, Cederlund K, et al. Hypo-attenuated leaflet thickening and reduced leaflet motion in sutureless bioprosthetic aortic valves. J Am Heart Assoc 2017;6(8):e005251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sondergaard L, De Backer O, Kofoed KF, et al. Natural history of subclinical leaflet thrombosis affecting motion in bioprosthetic aortic valves. Eur Heart J 2017;38(28):2201–2207. [DOI] [PubMed] [Google Scholar]
- 38.Sellers SL, Turner CT, Sathananthan J, et al. Transcatheter aortic heart valves: histological analysis providing insight to leaflet thickening and structural valve degeneration. JACC Cardiovasc Imaging 2019;12(1):135–145. [DOI] [PubMed] [Google Scholar]
- 39.Del Trigo M, Muñoz-Garcia AJ, Wijeysundera HC, et al. Incidence, timing, and predictors of valve hemodynamic deterioration after transcatheter aortic valve replacement: multicenter registry. J Am Coll Cardiol 2016;67(6):644–655. [DOI] [PubMed] [Google Scholar]
- 40.Hansson NC, Grove EL, Andersen HR, et al. Transcatheter aortic valve thrombosis: incidence, predisposing factors, and clinical implications. J Am Coll Cardiol 2016;68(19):2059–2069. [DOI] [PubMed] [Google Scholar]
- 41.Yahagi K, Ladich E, Kutys R, et al. Pathology of balloon-expandable transcatheter aortic valves. Catheter Cardiovasc Interv 2017;90(6):1048–1057. [DOI] [PubMed] [Google Scholar]
- 42.Yahagi K, Torii S, Ladich E, et al. Pathology of self-expanding transcatheter aortic valves: findings from the CoreValve US pivotal trials. Catheter Cardiovasc Interv 2018;91(5):947–955. [DOI] [PubMed] [Google Scholar]
- 43.Cartlidge T, White A, Van Beek E, Newby D, Dweck M. 18F-fluoride positron emission tomography: computed tomography angiography predicts bioprosthetic valve degeneration. J Am Coll Cardiol 2018;71(11 Suppl):A1452. [Google Scholar]
- 44.Sathananthan J, Sellers SL, Fraser R, et al. Impact of implant depth on hydrodynamic function with the ACURATE neo transcatheter heart valve following valve-in-valve transcatheter aortic valve replacement in Mitroflow bioprosthetic valves: an ex-vivo bench study. EuroIntervention 2018 Nov 27. pii: EIJ-D-18-00947. [Epub ahead of print] [DOI] [PubMed]
- 45.Sathananthan J, Sellers S, Barlow A, et al. Overexpansion of the SAPIEN 3 transcatheter heart valve: an ex vivo bench study. JACC Cardiovasc Interv 2018;11(17):1696–1705. [DOI] [PubMed] [Google Scholar]
- 46.Marx P, Kowalczyk W, Demircioglu A, et al. The fluid dynamical performance of the Carpentier-Edwards PERIMOUNT Magna Ease prosthesis. BioMed Res Int 2018;2018:5429594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohli K, Wei ZA, Yoganathan AP, Oshinski JN, Leipsic J, Blanke P. Transcatheter mitral valve planning and the Neo-LVOT: utilization of virtual simulation models and 3D printing. Curr Treat Options Cardiovasc Med 2018;20(12):99. [DOI] [PubMed] [Google Scholar]
- 48.Wei ZA, Sonntag SJ, Toma M, Singh-Gryzbon S, Sun W. Computational fluid dynamics assessment associated with transcatheter heart valve prostheses: a position paper of the ISO working group. Cardiovasc Eng Technol 2018;9(3):289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Midha PA, Raghav V, Sharma R, et al. The fluid mechanics of transcatheter heart valve leaflet thrombosis in the neosinus. Circulation 2017;136(17):1598–1609. [DOI] [PubMed] [Google Scholar]
- 50.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg 2005;79(3):1072–1080. [DOI] [PubMed] [Google Scholar]