Abstract
Coronary artery calcium (CAC) is a marker of overall coronary atherosclerotic burden in an individual. As such, it is an important tool in cardiovascular risk stratification and preventive treatment of asymptomatic patients with unclear cardiovascular disease risk. Several guidelines have recommended the use of CAC testing in shared decision making between the clinician and patient. With recent updates in clinical management guidelines and broad recommendations for CAC, there is a need for concise updated information on CAC interpretation on traditional electrocardiographically gated scans and nongated thoracic scans. Important points to report when interpreting CAC scans include: the absolute Agatston score and the age, sex, and race-specific CAC percentile; general recommendations on time-to-rescan for individuals with a CAC score of 0; the number of vessels with CAC; the presence of CAC in the left main coronary artery; and specific highlighting of individuals with very high CAC scores of greater than 1000. When risk factor information is available, the 10-year coronary heart disease risk can also be easily assessed using the free online Multi-Ethnic Study of Atherosclerosis risk score calculator. Recent improvements in standardizing the reporting of CAC findings across gated and nongated studies, such as the CAC Data and Reporting System, show promise for improving the widespread clinical value of CAC in clinical practice.
© RSNA, 2021
Summary
The increased use of coronary artery calcium (CAC) on both gated and nongated CT scans necessitates a summary of recent updates in CAC interpretation and recommendations for more unified CAC reporting as outlined in this review.
Essentials
■ Coronary artery calcium (CAC) should be reported as both an absolute Agatston score and an age-, sex-, and race-specific percentile score.
■ The recommended time to rescan for individuals with a CAC score of 0 is 5–7 years for individuals with low 10-year cardiovascular disease risk (< 5% risk), 3–5 years for individuals with intermediate risk (5%–20% risk), and 3 years for individuals with high risk (> 20% risk) or individuals with diabetes mellitus.
■ The CAC Data and Reporting System is a novel method of reporting CAC findings to unify CAC reporting on both electrocardiographically gated and nongated thoracic CT scans.
Introduction
Approximately 50% of all cardiovascular disease (CVD)–related deaths have no prior cardiac symptoms or diagnoses, making appropriate risk stratification in asymptomatic individuals of the utmost importance (1). Clinical CVD risk assessment is usually based on a guideline-endorsed stepwise approach that starts with the identification of clearly high-risk conditions, such as established atherosclerotic CVD (ASCVD) and diabetes, that necessitate therapy without the need for further testing (2). In the absence of these conditions, other risk factors are explored using risk estimation algorithms such as the Pooled Cohort Equation in the United States or the Systematic Coronary Risk Evaluation in Europe to calculate the 10-year risk of CVD, which can be categorized as “high,” “borderline/intermediate” or “low” (1,2). While those with high or low risk have a clear assessment of net benefit from preventive therapy, the intermediate-risk group remains a gray area, and the cost and adverse effects of initiating lifelong preventive therapy must be balanced by the potential benefit to the patient (3,4). The coronary artery calcium (CAC) scan is an important tool in cardiovascular risk stratification and determination of appropriate preventive therapy in these asymptomatic patients with intermediate or unclear CVD risk (2,5).
Historically, CAC was viewed as an estimator of the likelihood of obstructive coronary artery disease and might have led directly to cardiac catheterization (6). It is, however, more apt as a marker of overall atherosclerotic burden, and quantitative plaque burden appears to be the best predictor of risk in the asymptomatic primary prevention populations (CAC should rarely lead to additional testing) (2,6). Indeed, CAC has been found to be the most robust risk prediction tool for coronary artery disease events compared with other biomarkers, such as the serum marker high-sensitivity C-reactive protein and US testing for carotid-intima media thickness, particularly when used in addition to risk scores (1,4,7,8). CAC was first recognized as a risk-classification tool in the 2006 Screening for Heart Attack Prevention and Education guidelines, where it was met with substantial controversy (9), but has gained more acceptance over the years, with the 2019 American College of Cardiology and American Heart Association (ACC/AHA) guidelines for the prevention of cardiovascular disease assigning it a class IIa recommendation for use in addition to traditional risk factor assessment for CVD risk evaluation in individuals with borderline or intermediate 10-year ASCVD risk (10).
As the literature surrounding CAC has grown, so have the different approaches to CAC measurement and reporting, leading to varying levels of detail across centers and causing occasional discrepancies in its assessment and interpretation (6,11). The landscape has become even more complex, with class I recommendations for the routine assessment of CAC at ungated chest CT—for example lung cancer screening examinations—from the Society of Cardiovascular Computed Tomography (SCCT) and the Society of Thoracic Radiology (STR) (4). This review aims to provide a summary of recent updates on CAC interpretation and seeks to unify suggestions for CAC interpretation on electrocardiographically gated CAC scans and nongated thoracic scans.
Clinical Significance of CAC
One of the major benefits of CAC is its high negative predictive value for clinically important coronary atherosclerosis, refining the threshold for initiating life-long preventive therapy in middle-aged and older individuals who had relatively increased risk estimates from conventional risk scores but were found to be very low risk by using CAC (8,12). It has also been useful in the recognition of unheralded cardiac disease in younger adults, in whom any CAC found incidentally is clinically significant, warranting preventive therapy (13,14). Furthermore, its benefit as a tool for physician-patient shared decision making is invaluable, as research has shown that patients who understand their CAC score are more likely to be adherent to their medications and stick to lifestyle modifications (15–17).
While the Pooled Cohort Equation and other risk scores are calculated based on population-derived risk, the CAC score is individualized and is thus a better reflection of individual risk (4). The incremental prognostic value of CAC over traditional risk factors has been extensively validated in several studies, and, in addition to the general middle-aged adult population, it has also been shown to be predictive of events among the young and the elderly, individuals with comorbidities including diabetes and hypertension, and among smokers (5,18–21). Higher CAC scores have been strongly associated with higher risk of incident ASCVD and CHD, with a 14% increase in ASCVD risk estimated for each doubling of CAC, and CAC scores of greater than 300 associated with 10-year event rates ranging as high as 13.1%–25.6% (22). Newer score algorithms such as the Multi-Ethnic Study of Atherosclerosis (MESA) Risk Score, which combines CAC with traditional risk factors to estimate 10-year coronary heart disease (CHD) event risk, have been shown to have better discrimination than risk factors alone (23).
Several guidelines have recognized and recommended the use of CAC testing in shared decision making between the clinician and the patient: The 2017 SCCT CAC expert consensus recommendations endorse CAC testing in asymptomatic individuals aged 40–75 years with 5%–20% 10-year ASCVD risk and in the less than 5% ASCVD risk group with family history of premature coronary artery disease (18). The 2019 ACC/AHA guidelines for the prevention of cardiovascular risk assigned a class IIa recommendation for the use of CAC in CVD risk assessment in asymptomatic individuals with intermediate 10-year ASCVD risk (10); and the 2019 European Society of Cardiology guidelines for the management of dyslipidemias also assigned a class IIa recommendation for CAC testing in asymptomatic individuals with low or intermediate CVD risk (24). The Endocrine Society and the National Lipid Association also have signaled plans to endorse CAC with IIa recommendations in their upcoming guidelines.
Traditional CAC Scoring
Absolute versus Percentile CAC Scoring
CAC is typically quantified using the Agatston score—a sum of the attenuation (in Hounsfield units) and area of all CAC lesions in the coronary arteries—which is then categorized into very low risk (CAC = 0), mildly increased risk (CAC = 1–99), moderately increased risk (CAC = 100–299), and moderate to severely increased risk (CAC ≥ 300) (4,6). The Agatston score can be reported as an absolute score (in Agatston units) or as an age-, sex-, and race-specific percentile that is derived using the MESA risk score calculator that is freely available on the MESA website (Table 1) (4,25,26).
Table 1:
Comparison of Absolute versus Percentile Coronary Artery Calcium Score Reporting
The absolute score is the best predictor of the total risk of a CHD event for an individual in the near to midterm (in the next 5 to 10 years). In contrast, the percentile score best represents relative risk of CHD event for the individual compared with other individuals of the same age, race, and sex (25). In this way, the percentile score is the better predictor of lifetime risk of developing CHD. While percentile scores are particularly useful in clinical practice for conveying relative and lifetime risk to patients, the absolute score is of more prognostic value and predicts risk better over the traditional 10-year time horizon (Table 1) (4,25).
To better portray the difference in these two values, consider the following scenarios:
Scenario 1.— Patient X is a 48-year-old White woman taking medication for diabetes and hypertension, with no history of smoking or dyslipidemia, and with a reported Agatston CAC score of 10, which places her at the 93rd percentile for women of similar age and race (26). However, her estimated 10-year risk of a CHD event is 4% (Fig 1) (26). Although the absolute risk is low, the percentile risk is above 75%, necessitating preventive therapy in patient X per guideline recommendations (10).
Figure 1:
Screenshot of output from Multi-Ethnic Study of Atherosclerosis (MESA) risk score calculator. CHD = coronary heart disease, HDL = high-density lipoprotein.
Scenario 2.— Patient Y is a 32-year-old African American man with no comorbidities and an Agatston score of 4. While there are no currently established reference range values for CAC in individuals younger than 45 years old, any evidence of CAC in this age range is considered very high, with high risk for CHD and CVD mortality (13,14). On the basis of evidence from the Coronary Artery Risk Development in Young Adults study, he is 2.6 times more likely to develop CHD than other young adults with a CAC score of 0 and can be considered to be above the 95th percentile for his age and sex (13,14). Thus, patient Y would be considered for preventive therapy.
Scenario 3.— Patient Z is a 72-year-old Hispanic man with treated prostate cancer, who recently quit smoking, has no history of diabetes, hypertension, or dyslipidemia, and has an Agatston score of 25. His 10-year risk of a CHD event is 5.5%, placing him at the 35th percentile for his age and sex (26). Given his low percentile and competing risks, preventive therapy can most likely be deferred.
Recommendation.— All CAC scores should be reported as both the absolute Agatston score and the corresponding CAC score percentile.
CAC Progression and Incidence of New CAC
The progression of CAC over time has been found in some studies to have incremental value in predicting subsequent CHD events and overall mortality (27,28). Other studies, however, have found that the most recent CAC value in addition to risk factor assessment is sufficient for risk prediction (29,30). The differing outcomes from studies could be attributed to differences in methods for assessing CAC progression (11,31). Consistently, however, the presence of any CAC at baseline, diabetes, age, systolic blood pressure, low-density lipoprotein cholesterol, and smoking have been found to be strong predictors for CAC progression and conversion (31–33), and the rate of conversion from CAC score of 0 to CAC of greater than 0 over time was found to be nonlinear and dependent on age, sex, and baseline risk profile (32).
A common clinical question is when to repeat a CAC score after an initial measurement of CAC of 0. This requires a consideration of the “warranty period” for which a CAC score of 0 remains valid for an individual (34). On the basis of new data, emerging consensus suggests repeat CAC scans at 5–7 years for individuals with low 10-year CVD risk (< 5% risk), 3–5 years for individuals with intermediate risk (5%–20% risk), and approximately 3 years for individuals with high risk (> 20% risk) or individuals with diabetes mellitus, provided a reassessment will impact clinical management (Table 2) (34,35).
Table 2:
Recommended Rescan Intervals Based on ASCVD Risk Categories
Recommendation.— In patients with a CAC score of 0 on the index scan, consider recommending repeat CAC testing in 5–7 years for low-risk individuals, 3–5 years for intermediate-risk individuals, and in approximately 3 years for high-risk individuals or those with diabetes.
New CAC Score Group: CAC of Greater than 1000
While CAC scores of greater than 300 or greater than 400 have been traditionally recognized as the highest risk classification of CAC, there are however a unique group of individuals with CAC scores of greater than 1000, many of whom are asymptomatic at the time of scanning (6,36). Arguments have been made that high CAC values are heavily influenced by CAC density, which might be associated with more favorable prognosis, as denser CAC are more indicative of stable plaques, which are less prone to rupture (6,28). Individuals with very high CAC scores of greater than 1000 have, however, been found to have greater CAC area and more extracoronary calcium, and to be at much higher risk of CVD, CHD, cancer, and all-cause mortality than those with CAC scores of 400–999 (36). These patients have been found to have as much risk as those in secondary prevention (who have had prior myocardial infarction), suggesting that even more aggressive management of modifiable risk factors might be warranted in this subgroup of individuals (36,37).
Recommendation.— CAC scores of greater than 1000 should be considered a distinct very high-risk group and should be identified as such on score reports.
CAC Distribution within the Coronary Arteries
Another consideration when scoring and reporting CAC is the CAC distribution within the coronary tree. For a given absolute CAC score, compared with single vessel CAC, CAC in multiple vessels is associated with higher risk of mortality (6,38). In addition, in limited circumstances it is important to note the vessel affected, as CAC involving the left main has been associated with increased mortality risk (6,38,39).
While the Agatston score does not factor in the distribution of CAC, other proposed scores, like the calcium coverage score, take CAC distribution into consideration but are less reproducible and require much longer reading time than the Agatston score, limiting their incremental value (40). Currently, a simple expression of the number of coronary arteries with CAC and whether there is CAC in the left main is sufficient to enhance risk discrimination.
Recommendation.— When reporting CAC scores, the number of coronary arteries with CAC should be noted within the report (0–4, including the left main). Presence of left main CAC should also be noted in the study conclusions.
CAC Interpretation
MESA Risk Score
The MESA risk score was developed using sex-balanced multiple ethnic subgroups in the MESA database for the estimation of 10-year CHD risk (23). It incorporates traditional risk factors (age, sex, high-density lipoprotein cholesterol, systolic blood pressure, antihypertensive medication use, current smoking status, and diabetes) and CAC, as well as family history of heart attack, body mass index, race and ethnicity, and lipid-lowering medication use (Fig 1) (23). It was found to provide an accurate prediction of 10-year CHD risk in the MESA database, as well as in the Heinz Nixdorf Recall Study in Germany and the Dallas Heart Study in Texas (23). Compared with a similar model without CAC, the addition of CAC improved the accuracy of the calculator significantly, increasing the area under the curve from 0.76 to 0.81 (23). In general, a MESA CHD risk score of greater than 5% (equivalent to > 7.5% total ASCVD risk) is strongly suggestive of benefit from statin therapy.
To illustrate this, consider the case of patient A, a 55-year-old Hispanic female smoker currently taking antihypertensives and lipid-lowering medication, with a CAC score of 270. Her 10-year risk of a CHD event without CAC is 4.3%, whereas with CAC the 10-year risk is 11.3% (26). Interestingly, if her CAC score was 0, the risk would be 2.5%. The more accurate risk prediction for patient A would be 11.3% (the score that factored in CAC), and discussion about further preventive therapy is warranted.
The MESA risk score calculator is currently available and easily accessible for use on the MESA website with an aim to enhance CHD risk assessment and communication between physicians and their patients (26). It is also accessible via free applications developed for mobile phone devices and can be found using the term “MESA risk score” in app stores (26). A MESA CVD risk score that separately predicts risk of CHD and stroke is due to be published in early 2021.
Recommendation.— When risk factor information is available, provide the 10-year risk of CHD from the MESA CHD risk score on CAC score reports.
CAC on Nongated Chest CT Scans
Visual Estimation of CAC on Thoracic CT Scans
More than 7 million CT scans are performed in the United States annually, with projected doubling of that number if thoracic screening with annual low-dose chest CT is performed in all patients at risk for lung cancer per the 2014 United States Preventive Services Task Force recommendation (4). CAC can be assessed visually on almost any chest CT scan but has been mostly ignored until recently (4). As lung cancer and CHD share similar risk factors, the 2016 SCCT/STR guidelines assigned a class I recommendation for routine qualitative CAC assessment on nongated thoracic scans regardless of the indication for the scan (4).
CAC scores on nongated thoracic scans can be estimated qualitatively on visual assessment as present or not present or as mild, moderate, or severe (4,41). They can also be assessed quantitatively for each of the four main coronary arteries using the following ordinal scores: 0 (no CAC), 1 (mild, calcification in less than a third of the coronary artery), 2 (moderate, calcification involving one-third to two-thirds of the artery), and 3 (severe, calcification involving > two-thirds of the artery) (42,43). One previously proposed score is calculated as the sum of the score for each of the coronary arteries and can be categorized into three categories of severity: 0, 1–3, and 4–12 (4). Although not as accurate, CAC scores assessed from nongated thoracic scans have been found to correlate well with scores obtained from electrocardiographically gated non–contrast-enhanced CT scans (4,41).
CAC Data and Reporting System
Despite the many established benefits of the CAC score, more work needs to be done to optimize and standardize its application in clinical medicine. One approach is to merge traditional and qualitative CAC scoring to yield a score that classifies individuals accurately regardless of the method of CAC assessment used. This is easily achievable, as evidence suggests that experienced readers of nongated studies can visually estimate CAC on these scans, classifying patients into general CAC score groups that correlate accurately with traditional Agatston score groups (44).
In 2018, the SCCT published the CAC Data and Reporting System (CAC-DRS), a unique approach to reporting CAC that aims to standardize the methods for reporting findings about CAC on all gated cardiac scans and nongated chest CT scans (45). The CAC-DRS categories of 0–3 were defined to correspond with the traditional Agatston score categories of 0 (very low risk), 1–99 (mild CAC, mildly increased risk), 100–299 (moderate CAC, moderately increased risk), and higher than 300 (moderately to severely increased risk) (45). Visual assessment of CAC on nongated scans is done with these same categories in mind, with scores of 0–3 corresponding to similar risk categories (45).
The CAC-DRS scoring system used is defined using the modifier “Ax” or “Vx” to represent Agatston or visually estimated CAC score, respectively, with x corresponding to the CAC score category as outlined above. Next, the number of affected arteries is outlined with the modifier “Ny,” with y corresponding to the number of affected categories (45). Both modifiers are then combined and separated by a virgule to give a composite CAC-DRS score (Ax/Ny or Vx/Ny) (45). To illustrate this, consider an individual with an Agatston CAC score of 230 affecting the left main, left anterior descending, and right coronary arteries: They would have a CAC-DRS score A2/N3, indicating an Agatston score grade 2 (100–299) affecting three vessels. If that same patient had a concomitant nongated study, this would be graded as V2/N3 (Table 3 and Fig 2). This scoring system shows substantial promise for the improvement of CVD risk prediction and communication and has been validated in a few data sets, where it showed better discrimination for risk of CHD, CVD, and all-cause death than the Agatston score alone (Figs 3 and 4) (46,47).
Table 3:
Examples of the CAC-DRS Scoring System
Figure 2:
Example of Coronary Artery Calcium Data and Reporting System (CAC-DRS). A, CT angiogram shows CAC (purple areas) within the right coronary artery (RCA), left anterior descending (LAD) artery, and left circumflex (LCX) vessels. B, Axial image of CAC in the left anterior descending artery. C, Axial image of CAC in the left circumflex vessels. D, Axial image of CAC in the right coronary artery. A2 = Agatston score of 2, N3 = three affected arteries, V2 = visually estimated CAC score of 2. (Adapted from reference 45.)
Figure 3:
Results from a validation of the Coronary Artery Calcium Data and Reporting System (CAC-DRS): risk discrimination versus CAC alone. AUC = area under the curve, CHD = coronary heart disease, CVD = cardiovascular disease. (Adapted from reference 46.)
Figure 4:
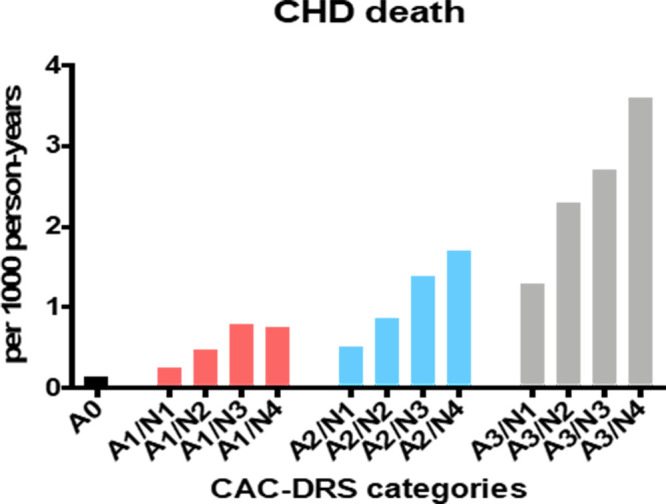
Results from a validation of the Coronary Artery Calcium Data and Reporting System (CAC-DRS): importance of both CAC score group and CAC distribution. Ax = Agatston score of x, Ny = number of affected arteries. (Adapted from reference 46.)
Work has also been done on automating CAC scoring on thoracic scans, and although the CAC scores were underestimated, initial testing showed good reliability and agreement with traditional scores (48). If further developed, this holds significant potential for further reducing the time required to assess CAC on thoracic scans, increasing the efficiency and maximizing the value from routine lung cancer screening thoracic scans.
Recommendation.— CAC should be reported on all chest CT scans, and the CAC-DRS system should be considered for use when interpreting nongated chest CT scans.
Future Directions in CAC Interpretation
CAC for the Estimation of Competing Risks for Mortality
Elevated CAC scores have been associated with increased risk of other noncardiovascular diseases including cancer, chronic kidney disease, and chronic obstructive pulmonary disease (49,50). These comorbidities raise the issue of competing risks of mortality in those with elevated CAC and should be considered when analyzing CAC as a predictor of mortality. Tools are currently being developed to further translate the CAC score into a likelihood estimator for mortality from CVD compared with cancer, which will inform and potentially impact clinical management decisions.
Calculation of Coronary and Cardiovascular Age
CAC scores, being a summation of an individual’s lifetime exposure to risk factors for both CVD and non-CVD events, have been found to be representative of “vascular arterial age,” a value that corresponds more with atherosclerotic burden than chronological age (49,51). Vascular age calculated using CAC has been validated in MESA as being more predictive of incident CHD risk than chronologic age (52). Framingham risk estimated using this CAC-derived vascular age was also more predictive of short-term incident coronary events than when chronologic age was used (52). Furthermore, vascular age has also been found to be a better representation of cardiovascular risk that is more easily understood by patients and more likely to result in compliance with treatment recommendations (53). For example, to better convey risk to a patient, a clinician might use the calculator to transform their CAC score into an arterial age and say, “You are currently 48 years old, but your arteries are more consistent with that of a 72 year old.” Several risk calculators, such as the Framingham heart age and the MESA arterial age calculator, exist for the conversion of cardiovascular risk to a vascular age (53). Widely varying definitions and methods for calculating vascular age, however, currently limit its widespread use in clinical practice (53); however, a MESA coronary age calculator has recently been developed to better harmonize vascular age estimation and will be available for use on the MESA website in late 2020 or early 2021 (54).
Improving the CAC Score
Despite its shortcomings, the Agatston score remains the standard of reporting, as many previous proposed upgrades in CAC scoring methods have had limited applicability clinically (6). Further considerations to improve the CAC score include addition of parameters for CAC distribution pattern (diffuse vs concentrated), total number of CAC lesions, consideration of mean CAC density, radiomic assessment for individual lesions, and quantification of extracoronary calcification (for example aortic calcification or aortic valve calcification; Fig 5) (6,45). Using CAC to predict new outcomes is also on the horizon, for example, using aortic valve calcification to predict future stenosis (55). To ensure applicability in clinical practice, any new CAC score needs to be reproducible, relatively easy and quick to interpret, and adaptable to automated algorithms (6).
Figure 5:
Approaches to improving the CAC score. ARC = aortic root calcification, AVC = aortic valve calcification, CAC = coronary artery calcium, MAC = mitral annulus calcification, TAC = thoracic aortic calcification.
Another consideration for improving CAC assessment is to further reduce the amount of radiation associated with CAC scans. While it is important to use the least amount of radiation possible, it is imperative that the image quality is maintained with minimal background noise (56). A typical CAC scan is associated with an exposure to approximately 1–2 mSv of radiation, and several techniques have been developed or are under investigation to further reduce this, including: recalibrating the score to accommodate scans done with reduced tube voltage (< 120 kV), high-pitch spiral acquisition on dual-source scanners, and iterative reconstruction techniques (Fig 5) (6,56). However, these techniques have been associated with some drawbacks, including increased background noise and underestimation of CAC scores (6,56). Polygenic risk scores are also being tested as a way to determine when a patient should get a first CAC score (57).
Additionally, recent developments in the application of artificial intelligence show promising prospects, with the development of CAC CT postprocessing algorithms and software to automate the estimation and reporting of CAC (Fig 5) (58,59). These methods show significant agreement with the conventional assessment of the Agatston CAC score (58,59). They have, however, been associated with an overestimation bias, and results still require double-checking by a radiologist and/or clinician (58,59).
Conclusion
CAC has been extensively shown to be invaluable in CVD risk prediction and has shown value in predicting other non-CVD conditions, as well as all-cause mortality. CAC also serves as the basis for new concepts, such as coronary and cardiovascular age, to improve risk communication between health care providers and patients. As more thoracic CT scans are routinely performed in the United States, recent guidelines have recommended CAC interpretation on all thoracic scans regardless of the original indication. In light of the expansion in utility of CAC in the clinical space, it is imperative that clinicians become comfortable with the interpretation and application of CAC in their daily practice. It is equally important for radiologists to become comfortable with reporting CAC on all scans using methods and terminology that are easily applicable to clinical practice. Recent improvements in the standardization of CAC reporting across traditional electrocardiographically gated scans and thoracic scans show promise in improving the risk prediction power of CAC, improving its clinical value and widespread use.
Disclosures of Conflicts of Interest: O.H.O. disclosed no relevant relationships. A.D.O. disclosed no relevant relationships. S.M.I.U. disclosed no relevant relationships. O.D. disclosed no relevant relationships. M.J.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received consultancy fees from Amgen, Sanofi, Regeneron, Novartis, Novo Nordisk, Bayer, Kowa, and 89Bio; author has grants/grants pending from National Institutes of Health/National Heart, Lung, and Blood Institute, Food and Drug Administration, American Heart Association, Aetna Foundation, and Amgen Foundation. Other relationships: disclosed no relevant relationships.
Abbreviations:
- ACC
- American College of Cardiology
- AHA
- American Heart Association
- ASCVD
- atherosclerotic CVD
- CAC
- coronary artery calcium
- CAC-DRS
- CAC Data and Reporting System
- CHD
- coronary heart disease
- CVD
- cardiovascular disease
- MESA
- Multi-Ethnic Study of Atherosclerosis
- SCCT
- Society of Cardiovascular Computed Tomography
- STR
- Society of Thoracic Radiology
References
- 1.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010;56(25):e50–e103. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2007;49(3):378–402. [DOI] [PubMed] [Google Scholar]
- 3.Roberts ET, Horne A, Martin SS, et al. Cost-effectiveness of coronary artery calcium testing for coronary heart and cardiovascular disease risk prediction to guide statin allocation: the Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One 2015;10(3):e0116377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hecht HS, Cronin P, Blaha MJ, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr 2017;11(1):74–84 [Published correction appears in J Cardiovasc Comput Tomogr 2017;11(2):170.]. [DOI] [PubMed] [Google Scholar]
- 5.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol 2018;72(4):434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaha MJ, Mortensen MB, Kianoush S, Tota-Maharaj R, Cainzos-Achirica M. Coronary Artery Calcium Scoring: Is It Time for a Change in Methodology? JACC Cardiovasc Imaging 2017;10(8):923–937. [DOI] [PubMed] [Google Scholar]
- 7.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S49–S73 [Published correction appears in Circulation 2014;129(25 Suppl 2):S74–S75.]. [DOI] [PubMed] [Google Scholar]
- 8.Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133(9):849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging 2015;8(5):579–596. [DOI] [PubMed] [Google Scholar]
- 10.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74(10):e177–e232 [Published corrections appear in J Am Coll Cardiol 2019;74(10):1429–1430 and J Am Coll Cardiol 2020;75(7):840.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paixao ARM, Chakravorty R, Khera A, et al. Disagreement between different definitions of coronary artery calcium progression. JACC Cardiovasc Imaging 2015;8(6):743–744. [DOI] [PubMed] [Google Scholar]
- 12.Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging 2009;2(6):692–700. [DOI] [PubMed] [Google Scholar]
- 13.Carr JJ, Jacobs DR Jr, Terry JG, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol 2017;2(4):391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miedema MD, Dardari ZA, Nasir K, et al. Association of Coronary Artery Calcium With Long-term, Cause-Specific Mortality Among Young Adults. JAMA Netw Open 2019;2(7):e197440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol 2011;57(15):1622–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orakzai RH, Nasir K, Orakzai SH, et al. Effect of patient visualization of coronary calcium by electron beam computed tomography on changes in beneficial lifestyle behaviors. Am J Cardiol 2008;101(7):999–1002. [DOI] [PubMed] [Google Scholar]
- 17.Kalia NK, Miller LG, Nasir K, Blumenthal RS, Agrawal N, Budoff MJ. Visualizing coronary calcium is associated with improvements in adherence to statin therapy. Atherosclerosis 2006;185(2):394–399. [DOI] [PubMed] [Google Scholar]
- 18.Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017;11(2):157–168. [DOI] [PubMed] [Google Scholar]
- 19.LaMonte MJ, FitzGerald SJ, Church TS, et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol 2005;162(5):421–429. [DOI] [PubMed] [Google Scholar]
- 20.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 2010;303(16):1610–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osei AD, Uddin SMI, Dzaye O, et al. Predictors of coronary artery calcium among 20-30-year-olds: The Coronary Artery Calcium Consortium. Atherosclerosis 2020;301:65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J 2018;39(25):2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClelland RL, Jorgensen NW, Budoff M, et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol 2015;66(15):1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019;290:140–205 [Published corrections appear in Atherosclerosis 2020;292:160–162 and Atherosclerosis 2020;294:80–82.]. [DOI] [PubMed] [Google Scholar]
- 25.Budoff MJ, Nasir K, McClelland RL, et al. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2009;53(4):345–352 [Published correction appears in J Am Coll Cardiol 2009;53(16):1474.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CAC Tools. https://www.mesa-nhlbi.org/CAC-Tools.aspx. Accessed July 8, 2020.
- 27.Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010;3(12):1229–1236. [DOI] [PubMed] [Google Scholar]
- 28.Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014;311(3):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehmann N, Erbel R, Mahabadi AA, et al. Value of progression of coronary artery calcification for risk prediction of coronary and cardiovascular events: Result of the hnr study (heinz nixdorf recall). Circulation 2018;137(7):665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radford NB, DeFina LF, Barlow CE, et al. Progression of CAC Score and Risk of Incident CVD. JACC Cardiovasc Imaging 2016;9(12):1420–1429. [DOI] [PubMed] [Google Scholar]
- 31.Mahabadi AA, Lehmann N, Dykun I, Müller T, Kälsch H, Erbel R. Progression of coronary artery calcification by cardiac computed tomography. Herz 2015;40(6):863–868. [DOI] [PubMed] [Google Scholar]
- 32.Min JK, Lin FY, Gidseg DS, et al. Determinants of coronary calcium conversion among patients with a normal coronary calcium scan: what is the “warranty period” for remaining normal? J Am Coll Cardiol 2010;55(11):1110–1117. [DOI] [PubMed] [Google Scholar]
- 33.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2007;115(21):2722–2730. [DOI] [PubMed] [Google Scholar]
- 34.Dzaye O, Dardari ZA, Cainzos-Achirica M, et al. Incidence of New Coronary Calcification: Time to Conversion From CAC = 0. J Am Coll Cardiol 2020;75(13):1610–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman CB, Blaha MJ, Boord JB, et al. Lipid Management in Patients with Endocrine Disorders: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2020;105(12):dgaa674. [DOI] [PubMed] [Google Scholar]
- 36.Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-Term All-Cause and Cause-Specific Mortality in Asymptomatic Patients With CAC ≥1,000: Results From the CAC Consortium. JACC Cardiovasc Imaging 2020;13(1 Pt 1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng AW, Dardari Z, Blumenthal RS, et al. Very high coronary artery calcium (CAC ≥ 1000) and association with CVD events, non-CVD outcomes, and mortality: Results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2021. doi: 10.1161/CIRCULATIONAHA.120.050545. (in press). [Google Scholar]
- 38.Tota-Maharaj R, Joshi PH, Budoff MJ, et al. Usefulness of regional distribution of coronary artery calcium to improve the prediction of all-cause mortality. Am J Cardiol 2015;115(9):1229–1234. [DOI] [PubMed] [Google Scholar]
- 39.Lahti SJ, Feldman DI, Dardari Z, et al. The association between left main coronary artery calcium and cardiovascular-specific and total mortality: The Coronary Artery Calcium Consortium. Atherosclerosis 2019;286:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown ER, Kronmal RA, Bluemke DA, et al. Coronary calcium coverage score: determination, correlates, and predictive accuracy in the Multi-Ethnic Study of Atherosclerosis. Radiology 2008;247(3):669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alluri K, Joshi PH, Henry TS, Blumenthal RS, Nasir K, Blaha MJ. Scoring of coronary artery calcium scans: history, assumptions, current limitations, and future directions. Atherosclerosis 2015;239(1):109–117. [DOI] [PubMed] [Google Scholar]
- 42.Htwe Y, Cham MD, Henschke CI, et al. Coronary artery calcification on low-dose computed tomography: comparison of Agatston and Ordinal Scores. Clin Imaging 2015;39(5):799–802. [DOI] [PubMed] [Google Scholar]
- 43.Shemesh J, Henschke CI, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology 2010;257(2):541–548. [DOI] [PubMed] [Google Scholar]
- 44.Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: A comparison of three scoring methods. Radiology 2015;276(1):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: Coronary Artery Calcium Data and Reporting System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr 2018;12(3):185–191. [DOI] [PubMed] [Google Scholar]
- 46.Dzaye O, Dudum R, Mirbolouk M, et al. Validation of the Coronary Artery Calcium Data and Reporting System (CAC-DRS): Dual importance of CAC score and CAC distribution from the Coronary Artery Calcium (CAC) consortium. J Cardiovasc Comput Tomogr 2020;14(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams MC, Moss A, Dweck M, et al. Standardized reporting systems for computed tomography coronary angiography and calcium scoring: A real-world validation of CAD-RADS and CAC-DRS in patients with stable chest pain. J Cardiovasc Comput Tomogr 2020;14(1):3–11. [DOI] [PubMed] [Google Scholar]
- 48.Takx RAP, de Jong PA, Leiner T, et al. Automated coronary artery calcification scoring in non-gated chest CT: agreement and reliability. PLoS One 2014;9(3):e91239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Handy CE, Desai CS, Dardari ZA, et al. The Association of Coronary Artery Calcium With Noncardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging 2016;9(5):568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whelton SP, Al Rifai M, Dardari Z, et al. Coronary artery calcium and the competing long-term risk of cardiovascular vs. cancer mortality: the CAC Consortium. Eur Heart J Cardiovasc Imaging 2019;20(4):389–395. [DOI] [PubMed] [Google Scholar]
- 51.Nappi C, Gaudieri V, Acampa W, et al. Coronary vascular age: An alternate means for predicting stress-induced myocardial ischemia in patients with suspected coronary artery disease. J Nucl Cardiol 2019;26(4):1348–1355. [DOI] [PubMed] [Google Scholar]
- 52.McClelland RL, Nasir K, Budoff M, Blumenthal RS, Kronmal RA. Arterial age as a function of coronary artery calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol 2009;103(1):59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groenewegen KA, den Ruijter HM, Pasterkamp G, Polak JF, Bots ML, Peters SAE. Vascular age to determine cardiovascular disease risk: A systematic review of its concepts, definitions, and clinical applications. Eur J Prev Cardiol 2016;23(3):264–274. [DOI] [PubMed] [Google Scholar]
- 54.Blaha MJ, Nazie IN, Cainzos-Achirica M, et al. Derivation of a Coronary Age Calculator Using Traditional Risk Factors and Coronary Artery Calcium: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2021;00:e019351. doi: 10.1161/JAHA.120.019351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dzaye O, Whelton SP, Blaha MJ. Aortic valve calcium scoring on cardiac computed tomography: Ready for clinical use? J Cardiovasc Comput Tomogr 2019;13(6):297–298. [DOI] [PubMed] [Google Scholar]
- 56.Baron KB, Choi AD, Chen MY. Low Radiation Dose Calcium Scoring: Evidence and Techniques. Curr Cardiovasc Imaging Rep 2016;9(4):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cainzos-Achirica M, Mortensen MB, Blaha MJ. Exploring the intersection between genetic risk scores and coronary artery calcium - Mutually exclusive or complementary? J Cardiovasc Comput Tomogr 2019;13(4):172–173. [DOI] [PubMed] [Google Scholar]
- 58.Sandstedt M, Henriksson L, Janzon M, et al. Evaluation of an AI-based, automatic coronary artery calcium scoring software. Eur Radiol 2020;30(3):1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W, Wang H, Chen Q, et al. Coronary artery calcium score quantification using a deep-learning algorithm. Clin Radiol 2020;75(3):237.e11–237.e16. [DOI] [PubMed] [Google Scholar]









