Abstract
Fractional flow reserve derived from CT is a rapidly developing technique, with an increasing burden of literature supporting its potential role in the workup of patients suspected of having coronary artery disease.
The use of coronary CT angiography in the assessment of coronary artery disease to improve diagnosis and clinical outcomes is established (1). CT angiography is highly sensitive to the presence of both obstructive and nonobstructive coronary artery disease, with a normal CT angiography result excluding coronary atherosclerosis with low event rates out to 10 years (2). CT angiography as an anatomic test is very good, with high sensitivity for the detection of coronary artery disease and a high correlation to invasive coronary angiography. However, the specificity of CT angiography for a significant stenosis is nonetheless much lower than its sensitivity, with three false-positive findings for every false-negative finding (3). These anatomic differences are further limited when compared against a functional reference standard to detect ischemia, which remains the basis for patient management decisions (4). Fractional flow reserve derived from CT angiography (FFR CT) is one potential solution to the issue of low specificity and the lack of functional assessment at CT angiography.
FFR CT uses the anatomic three-dimensional model of the coronary arteries produced at CT angiography to perform computational flow dynamics (CFD) to derive the expected relative pressures at any point within the coronary circulation. These noninvasive estimates of flow correlate well with invasive measures of fractional flow reserve (FFR), indicating that FFR CT is highly accurate for the detection of flow limiting stenosis (5,6). In the NXT (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps) trial, the addition of FFR CT to CT angiography correctly reclassified 68% of false-positive findings to true-negative findings when compared against invasive FFR, demonstrating its potential to remedy this limitation of CT angiography (7).
Impact on Patient Management
The improved diagnostic accuracy of FFR CT affects clinical decision making. The FFR CT-RIPCORD (Does Routine Pressure Wire Assessment Influence Management Strategy at Coronary Angiography for Diagnosis of Chest Pain?) study showed the addition of FFR CT to CT angiography changed the anticipated patient management in 44% of patients. Of the cases referred to invasive coronary angiography (ICA) on the basis of CT angiographic findings, 30% had this canceled. And in the 38 patients in whom more information was felt to be required following CT angiography, all were reassigned to either ICA (26%) or optimal medical therapy (74%) (8). The ADVANCE (Assessing Diagnostic Value of Noninvasive FFR CT in Coronary Care) registry, which included 5083 patients from 38 international sites, showed a similar change in anticipated patient management in 64% of cases with a 25% reduction in planned ICA, and in those in whom more information was felt to be required following CT angiography, only 5% required further investigation following the provision of the FFR CT results, with 70% changed to optimal medical therapy and 25% to ICA (9).
In the PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trial, this potential reduction in ICA referrals would have resulted in a lower rate of nonobstructive coronary artery disease (absence of stenosis > 50%) at ICA from 28% using CT angiography alone, to 11% with CT angiography plus FFR CT (10). These benefits are more pronounced in high-risk patients where there is a higher prevalence of intermediate stenosis. In a single-center study performed in Aarhus, Denmark, FFR CT led to the cancellation of an additional 29% of ICA over CT angiography alone in high-risk patients, compared with 17% additional ICA cancellations in low-to-intermediate–risk patients (11). The impact of this increased prevalence of obstructive coronary disease at ICA following FFR CT is to increase the interventional yield of the catheterization laboratory, with high revascularization rates of 50%–77% reported (12,13). Importantly, given the high rates of ICA deferral following a negative FFR CT, such an approach has been demonstrated to be safe out to 5 years, with event rates of 0.6% in those with an FFR CT greater than 0.8 at 1 year and 3.1% at 5 years (12–15).
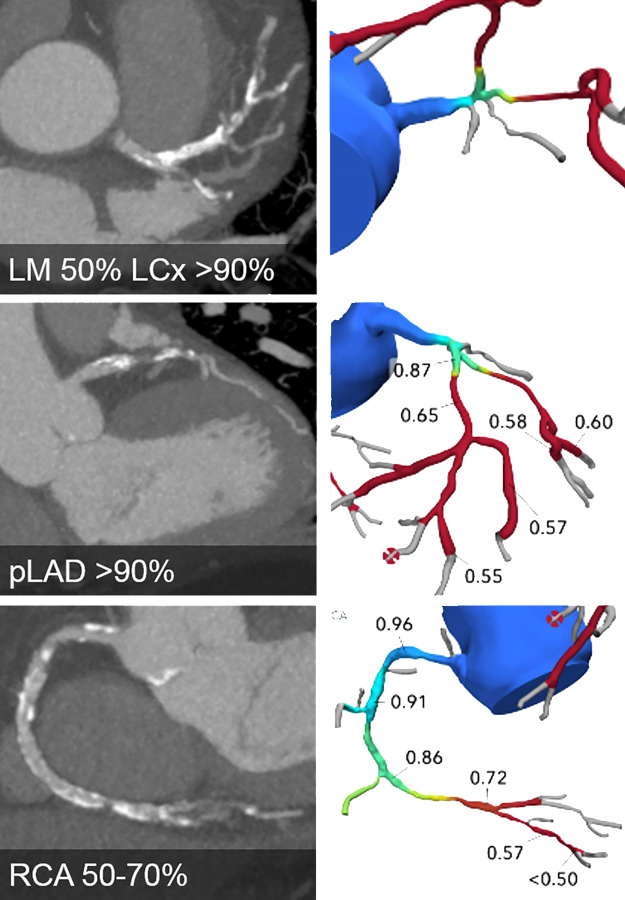
Improved clinical practice may also be achieved through the use of simulated stent placement within FFR CT models. These approaches use the vessel diameter and length of stenosis to derive the stent that will be inserted and then determine the hemodynamics of this should an optimal positioning and expansion be achieved (16). In a single study, these models correlated well with postprocedural invasive FFR measures (17). Using FFR CT to determine revascularization strategy was explored in the SYNTAX (Synergy between PCI with TAXUS and Cardiac Surgery) III Revolution trial. In this trial, heart teams (composed of an interventional cardiologist, a cardiothoracic surgeon, and a radiologist) were randomized to assess and plan management strategy based on either CT angiography or ICA in patients with left main or three-vessel coronary artery disease (18). The agreement with final management was 71% in the CT angiography and FFR CT arm compared with 78% in the ICA arm, with good agreement to the location and number of vessels to be revascularized (19). Future studies will explore the impact FFR CT can have in directing both percutaneous and surgical revascularization (Fig 1).
Figure 1:
CT angiographic images (left) and FFR CT models (right) show severe three-vessel disease with anatomically and functionally significant obstructive disease of the left main (LM), left anterior descending (LAD), left circumflex (LCx), and right coronary artery (RCA) (white arrowheads). Future developments may result in such patients being triaged straight to coronary artery bypass graft surgery without the need for further testing.
Clinical Integration and Interpretation
The clinical use of invasive FFR remains variable with only 18% of intermediate stenosis undergoing invasive FFR prior to stent placement (20,21). Additionally, when performed, invasive FFR demonstrates errors in 21.6% of the measurements (22). As CT angiography becomes the primary test for investigating angina, FFR CT has the potential to be better integrated into clinical practice and the decision-making process surrounding revascularization than its invasive counterpart. How to best incorporate FFR CT values into clinical care is an area of growing research. When one considers that the optimal invasive FFR threshold for treating symptoms is 0.76 and 0.67 for reducing myocardial infarction (23), and that the benefit-risk ratio of stent placement in a stenosis is not dichotomous, it may be reasonable to trial medical therapy before resorting to revascularization in those with less severe flow limitation. It has been proposed that those with “gray-zone” FFR CT values (0.76–0.80) can be treated medically in the first instance, with referral to ICA in the presence of persisting or worsening symptoms despite medical therapy (11). Indeed, in ADVANCE, 83% of patients with an FFR CT of 0.71–0.80 were treated medically, with only 7.1% of patients initially treated with optimal medical therapy requiring subsequent revascularization at 1 year (9,24).
While the level of evidence examining the use of FFR CT in clinical practice is currently limited to observational studies, two randomized control trials (FORECAST [Fractional Flow Reserve Derived from Computed Tomography Coronary Angiography in the Assessment and Management of Stable Chest Pain] [NCT03187639] and PRECISE [Prospective Randomized Trial of the Optimal Evaluation of Cardiac Symptoms and Revascularization] [NCT03702244]) are ongoing and will further our understanding of the comparative effectiveness of a combined CT angiography and FFR CT strategy compared with current standard of care approaches.
CFD Models
Several challenges remain for FFR CT. The diagnostic accuracy of the technique has been established using several different models, yet the comparative effectiveness of these models has not been determined. The most comprehensive three-dimensional models make the least assumptions and incorporate a greater number of pertinent variables into the simulation. Such models require substantial computing power and time often necessitating off-site analysis. Reduced-order one-dimensional and zero-dimensional models can be utilized on a standard workstation, but these involve a greater number of assumptions such as that pressure or flow are uniform across the length of the vessel (zero-dimensional) or that pressure and flow only change according to the length of the vessel (one-dimensional) (25). Preliminary assessments on small numbers of patients/vessels demonstrate good agreement between the models; however, further analysis is warranted (26,27). An alternative solution is the use of machine learning algorithms trained on simulated coronary arteries and a reduced order CFD model. One multicenter study of 351 patients showed equivalent performance of a machine learned FFR CT algorithm (28), but further work is needed to better understand the relative merits of the two approaches.
Modeling Assumptions
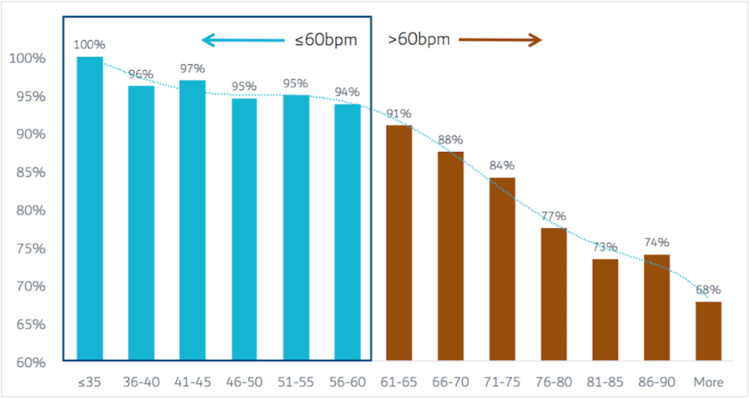
Modeling assumptions must be examined to provide confidence in the accuracy of the model. One such assumption is that of microvascular resistance, with current models assigning a standardized value to the resistance at rest and stress (29). However, microvascular dysfunction is common in those with stable coronary artery disease (30) and varies by sex and body mass index (31). Another assumption is the fidelity of the three-dimensional anatomic model of the coronary arteries, which is contingent on the quality and resolution of the CT angiographic images (32). β-blockers and nitrates, which reduce motion artifacts and increase the diameter of the coronary arteries, respectively (33,34), are essential for the accuracy of FFR CT (35). However, only 50% of cases submitted for FFR CT analysis in a multicenter study achieved the recommended heart rate of less than 60 beats per minute (36,37). While CT technology has resulted in significantly improved temporal resolution, a rate-dependent relationship remains between heart rate and image quality (Fig 2) (37). As a result, in an analysis of 10 621 cases submitted for commercial FFR CT analysis, 6.9% of scans were unable to undergo FFR CT analysis (37). The impact of technology on this is well evidenced with historic studies using predominantly 64-slice technology reporting rates of an inability to perform FFR CT of 33%, while more recent studies utilizing wide-bore or dual-source technology report rates of 2.9% (10,37). Thus, further improvements in both the accuracy and ability to perform FFR CT will likely be yielded through advances in CT technology with increased temporal resolution, higher resolution CT scanners, and spectral imaging (38,39).
Figure 2:
Bar graphs show rate of acceptance of coronary CT angiography for fractional flow analysis according to heart rate. Graphs are based on 10 621 clinical cases submitted to a central fractional flow reserve derived from CT angiography (FFR CT) core laboratory. The solid bars represent acceptance rates for FFR CT analysis while the dashed line represents the polynomial trendline of the association between heart rate and CT angiography acceptance (37).
Future Directions
The ability of FFR CT to be measured at every point along the vessel and in relation to the stenosis offers the potential to improve our understanding of lesion-specific ischemia. After stenosis, distal vessel and the change in FFR CT across the stenosis (ΔFFR CT) are all potential measures of flow limitation (40,41). Currently, it is recommended to measure FFR CT 2 cm distal to the stenosis for the determination of lesion-specific ischemia, with more distal measurements associated with a high rate of false-positive findings (41,42). However, the ΔFFR CT may provide for a more accurate measure of lesion-induced ischemia as it reflects the pressure drop attributable to a particular stenosis. Modeling of the effects of stent placement on a stenosis may yield more accurate results still (17). While the poststenosis FFR CT may provide for the best marker of lesion-induced ischemia, it may not provide the best marker of prognostic risk, which may be better reflected by the end-vessel values where the summative effect of all the upstream plaque and stenosis is captured (43). While FFR CT provides for useful lesion-specific determination of flow limitation, myocardial perfusion continues to provide useful information on prognosis and on the presence of microvascular disease and globally reduced flow secondary to diffuse atherosclerosis (44,45). There is likely a complementary role between the techniques, and further work is required to best select which patients will benefit most from these tests (46).
One of the strengths of FFR derived from CT angiography is the availability of stenosis and plaque information available in addition to the FFR CT values. The EMERALD (Exploring the Mechanism of Plaque Rupture in Acute Coronary Syndrome Using Coronary CT Angiography and Computational Fluid Dynamic) study demonstrated that stenosis, high-risk plaque features, and ΔFFR CT all provided independent incremental benefit in the identification of plaques at risk for rupture (47,48). Further work in understanding the interplay between plaque, stenosis, FFR CT, and risk may allow for better case selection for intervention in future.
Conclusion
FFR CT augments the current anatomic assessment available at CT angiography with a modeled functional assessment. Currently, this reduces the number of patients requiring additional functional diagnostic tests and reduces the number of people undergoing invasive angiography without revascularization. Better understanding of the interplay between anatomic and functional markers of disease and clinical outcomes are needed to advance our knowledge of how best to integrate FFR CT into decision-making clinical practice, guided by further high-quality clinical effectiveness studies.
Footnotes
Disclosures of Conflicts of Interest: J.R.W. disclosed no relevant relationships. T.A.F. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received $2000 from HeartFlow for lecture(s); author received $2000 from HeartFlow for travel/accommodations/meeting expenses. Other relationships: disclosed no relevant relationships.
References
- 1.SCOT-HEART Investigators , Newby DE, Adamson PD, et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med 2018;379(10):924–933. [DOI] [PubMed] [Google Scholar]
- 2.Finck T, Hardenberg J, Will A, et al. 10-Year Follow-Up After Coronary Computed Tomography Angiography in Patients With Suspected Coronary Artery Disease. JACC Cardiovasc Imaging 2019;12(7 Pt 2):1330–1338. [DOI] [PubMed] [Google Scholar]
- 3.Haase R, Schlattmann P, Gueret P, et al. Diagnosis of obstructive coronary artery disease using computed tomography angiography in patients with stable chest pain depending on clinical probability and in clinically important subgroups: meta-analysis of individual patient data. BMJ 2019;365:l1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knuuti J, Ballo H, Juarez-Orozco LE, et al. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J 2018;39(35):3322–3330. [DOI] [PubMed] [Google Scholar]
- 5.Celeng C, Leiner T, Maurovich-Horvat P, et al. Anatomical and Functional Computed Tomography for Diagnosing Hemodynamically Significant Coronary Artery Disease: A Meta-Analysis. JACC Cardiovasc Imaging 2019;12(7 Pt 2):1316–1325. [DOI] [PubMed] [Google Scholar]
- 6.Driessen RS, Danad I, Stuijfzand WJ, et al. Comparison of Coronary Computed Tomography Angiography, Fractional Flow Reserve, and Perfusion Imaging for Ischemia Diagnosis. J Am Coll Cardiol 2019;73(2):161–173. [DOI] [PubMed] [Google Scholar]
- 7.Nørgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63(12):1145–1155. [DOI] [PubMed] [Google Scholar]
- 8.Curzen NP, Nolan J, Zaman AG, Nørgaard BL, Rajani R. Does the Routine Availability of CT-Derived FFR Influence Management of Patients With Stable Chest Pain Compared to CT Angiography Alone?: The FFRCT RIPCORD Study. JACC Cardiovasc Imaging 2016;9(10):1188–1194. [DOI] [PubMed] [Google Scholar]
- 9.Fairbairn TA, Nieman K, Akasaka T, et al. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: lessons from the ADVANCE Registry. Eur Heart J 2018;39(41):3701–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu MT, Ferencik M, Roberts RS, et al. Noninvasive FFR Derived From Coronary CT Angiography: Management and Outcomes in the PROMISE Trial. JACC Cardiovasc Imaging 2017;10(11):1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen JM, Bøtker HE, Mathiassen ON, et al. Computed tomography derived fractional flow reserve testing in stable patients with typical angina pectoris: influence on downstream rate of invasive coronary angiography. Eur Heart J Cardiovasc Imaging 2018;19(4):405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel MR, Nørgaard BL, Fairbairn TA, et al. 1-Year Impact on Medical Practice and Clinical Outcomes of FFRCT: The ADVANCE Registry. JACC Cardiovasc Imaging 2019 Mar 17 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Nørgaard BL, Terkelsen CJ, Mathiassen ON, et al. Coronary CT Angiographic and Flow Reserve-Guided Management of Patients With Stable Ischemic Heart Disease. J Am Coll Cardiol 2018;72(18):2123–2134. [DOI] [PubMed] [Google Scholar]
- 14.Douglas PS, De Bruyne B, Pontone G, et al. 1-Year Outcomes of FFRCT-Guided Care in Patients With Suspected Coronary Disease: The PLATFORM Study. J Am Coll Cardiol 2016;68(5):435–445. [DOI] [PubMed] [Google Scholar]
- 15.Ihdayhid AR, Norgaard BL, Gaur S, et al. Prognostic Value and Risk Continuum of Noninvasive Fractional Flow Reserve Derived from Coronary CT Angiography. Radiology 2019;292(2):343–351. [DOI] [PubMed] [Google Scholar]
- 16.Ihdayhid AR, White A, Ko B. Assessment of Serial Coronary Stenoses With Noninvasive Computed Tomography-Derived Fractional Flow Reserve and Treatment Planning Using a Novel Virtual Stenting Application. JACC Cardiovasc Interv 2017;10(24):e223–e225. [DOI] [PubMed] [Google Scholar]
- 17.Modi BN, Sankaran S, Kim HJ, et al. Predicting the Physiological Effect of Revascularization in Serially Diseased Coronary Arteries. Circ Cardiovasc Interv 2019;12(2):e007577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collet C, Onuma Y, Andreini D, et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J 2018;39(41):3689–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonck J, Miyazaki Y, Collet C, et al. Feasibility of planning coronary artery bypass grafting based only on coronary computed tomography angiography and CT-derived fractional flow reserve: a pilot survey of the surgeons involved in the randomized SYNTAX III Revolution trial. Interact Cardiovasc Thorac Surg 2019 Mar 17 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Pothineni NV, Shah NN, Rochlani Y, et al. U.S. trends in inpatient utilization of fractional flow reserve and percutaneous coronary intervention. J Am Coll Cardiol 2016;67(6):732–733. [DOI] [PubMed] [Google Scholar]
- 21.Dattilo PB, Prasad A, Honeycutt E, Wang TY, Messenger JC. Contemporary patterns of fractional flow reserve and intravascular ultrasound use among patients undergoing percutaneous coronary intervention in the United States: insights from the National Cardiovascular Data Registry. J Am Coll Cardiol 2012;60(22):2337–2339. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura M, Johnson NP, Fearon WF, et al. Accuracy of Fractional Flow Reserve Measurements in Clinical Practice: Observations From a Core Laboratory Analysis. JACC Cardiovasc Interv 2017;10(14):1392–1401. [DOI] [PubMed] [Google Scholar]
- 23.Johnson NP, Tóth GG, Lai D, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol 2014;64(16):1641–1654. [DOI] [PubMed] [Google Scholar]
- 24.Kitabata H, Leipsic J, Patel MR, et al. Incidence and predictors of lesion-specific ischemia by FFRCT: Learnings from the international ADVANCE registry. J Cardiovasc Comput Tomogr 2018;12(2):95–100. [DOI] [PubMed] [Google Scholar]
- 25.Morris PD, Narracott A, von Tengg-Kobligk H, et al. Computational fluid dynamics modelling in cardiovascular medicine. Heart 2016;102(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boileau E, Pant S, Roobottom C, et al. Estimating the accuracy of a reduced-order model for the calculation of fractional flow reserve (FFR). Int J Numer Methods Biomed Eng 2018;34(1):e2908. [DOI] [PubMed] [Google Scholar]
- 27.Blanco PJ, Bulant CA, Müller LO, et al. Comparison of 1D and 3D Models for the Estimation of Fractional Flow Reserve. Sci Rep 2018;8(1):17275 [Published correction appears in Sci Rep 2018;8(1):17962.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coenen A, Kim YH, Kruk M, et al. Diagnostic Accuracy of a Machine-Learning Approach to Coronary Computed Tomographic Angiography-Based Fractional Flow Reserve: Result From the MACHINE Consortium. Circ Cardiovasc Imaging 2018;11(6):e007217. [DOI] [PubMed] [Google Scholar]
- 29.Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013;61(22):2233–2241. [DOI] [PubMed] [Google Scholar]
- 30.Corcoran D, Young R, Adlam D, et al. Coronary microvascular dysfunction in patients with stable coronary artery disease: The CE-MARC 2 coronary physiology sub-study. Int J Cardiol 2018;266:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JM, Layland J, Jung JH, et al. Integrated physiologic assessment of ischemic heart disease in real-world practice using index of microcirculatory resistance and fractional flow reserve: insights from the International Index of Microcirculatory Resistance Registry. Circ Cardiovasc Interv 2015;8(11):e002857. [DOI] [PubMed] [Google Scholar]
- 32.Gould KL, Johnson NP, Kirkeeide RL. Approximate Truth. J Am Coll Cardiol 2017;70(25):3097–3101. [DOI] [PubMed] [Google Scholar]
- 33.Takx RAP, Suchá D, Park J, Leiner T, Hoffmann U. Sublingual Nitroglycerin Administration in Coronary Computed Tomography Angiography: a Systematic Review. Eur Radiol 2015;25(12):3536–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Achenbach S, Manolopoulos M, Schuhbäck A, et al. Influence of heart rate and phase of the cardiac cycle on the occurrence of motion artifact in dual-source CT angiography of the coronary arteries. J Cardiovasc Comput Tomogr 2012;6(2):91–98. [DOI] [PubMed] [Google Scholar]
- 35.Leipsic J, Yang TH, Thompson A, et al. CT angiography (CTA) and diagnostic performance of noninvasive fractional flow reserve: results from the Determination of Fractional Flow Reserve by Anatomic CTA (DeFACTO) study. AJR Am J Roentgenol 2014;202(5):989–994. [DOI] [PubMed] [Google Scholar]
- 36.Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2016;10(6):435–449. [DOI] [PubMed] [Google Scholar]
- 37.Pontone G, Weir-McCall JR, Baggiano A, et al. Determinants of Rejection Rate for Coronary CT Angiography Fractional Flow Reserve Analysis. Radiology 2019;292(3):597–605. [DOI] [PubMed] [Google Scholar]
- 38.Takagi H, Tanaka R, Nagata K, et al. Diagnostic performance of coronary CT angiography with ultra-high-resolution CT: Comparison with invasive coronary angiography. Eur J Radiol 2018;101:30–37. [DOI] [PubMed] [Google Scholar]
- 39.Kalisz K, Halliburton S, Abbara S, et al. Update on Cardiovascular Applications of Multienergy CT. RadioGraphics 2017;37(7):1955–1974. [DOI] [PubMed] [Google Scholar]
- 40.Takagi H, Ishikawa Y, Orii M, et al. Optimized interpretation of fractional flow reserve derived from computed tomography: Comparison of three interpretation methods. J Cardiovasc Comput Tomogr 2019;13(2):134–141. [DOI] [PubMed] [Google Scholar]
- 41.Kueh SH, Mooney J, Ohana M, et al. Fractional flow reserve derived from coronary computed tomography angiography reclassification rate using value distal to lesion compared to lowest value. J Cardiovasc Comput Tomogr 2017;11(6):462–467. [DOI] [PubMed] [Google Scholar]
- 42.Renard BM, Cami E, Jiddou-Patros MR, et al. Optimizing the Technique for Invasive Fractional Flow Reserve to Assess Lesion-Specific Ischemia. Circ Cardiovasc Interv 2019;12(10):e007939. [DOI] [PubMed] [Google Scholar]
- 43.Lee JM, Koo BK, Shin ES, et al. Clinical implications of three-vessel fractional flow reserve measurement in patients with coronary artery disease. Eur Heart J 2018;39(11):945–951. [DOI] [PubMed] [Google Scholar]
- 44.Gould KL, Johnson NP. Coronary Physiology Beyond Coronary Flow Reserve in Microvascular Angina: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72(21):2642–2662. [DOI] [PubMed] [Google Scholar]
- 45.van Assen M, De Cecco CN, Eid M, et al. Prognostic value of CT myocardial perfusion imaging and CT-derived fractional flow reserve for major adverse cardiac events in patients with coronary artery disease. J Cardiovasc Comput Tomogr 2019;13(3):26–33. [DOI] [PubMed] [Google Scholar]
- 46.Pontone G, Baggiano A, Andreini D, et al. Stress Computed Tomography Perfusion Versus Fractional Flow Reserve CT Derived in Suspected Coronary Artery Disease: The PERFECTION Study. JACC Cardiovasc Imaging 2019;12(8 Pt 1):1487–1497. [DOI] [PubMed] [Google Scholar]
- 47.Lee JM, Choi G, Koo BK, et al. Identification of High-Risk Plaques Destined to Cause Acute Coronary Syndrome Using Coronary Computed Tomographic Angiography and Computational Fluid Dynamics. JACC Cardiovasc Imaging 2019;12(6):1032–1043. [DOI] [PubMed] [Google Scholar]
- 48.Park J, Lee JM, Koo BK, et al. Relevance of anatomical, plaque, and hemodynamic characteristics of non-obstructive coronary lesions in the prediction of risk for acute coronary syndrome. Eur Radiol 2019;29(11):6119–6128. [DOI] [PubMed] [Google Scholar]