Abstract
Schwannomas are typically benign, indolent neoplasms. Primary pericardial schwannomas are extremely rare and arise from the cardiac plexus and vagus nerve innervating the heart. Few case reports have been documented to date. Pericardial schwannomas are difficult to diagnose at plain radiography or transthoracic echocardiography, often leading to further characterization with either CT or MRI. Biopsy is required for definitive diagnosis. A case of primary pericardial schwannoma of the posterior pericardium with concerns for compression of the left atrium and left ventricle is presented.
© RSNA, 2021
Introduction
Schwannomas are typically benign, indolent neoplasms arising from Schwann cells. The tumors may develop in any part of the body, although they are most commonly found in the head and neck. Primary pericardial schwannomas are extremely rare and arise from the cardiac plexus and vagus nerve innervating the heart (1), and few case reports have been documented to date (2–10). Intrathoracic schwannomas are most often found in the posterior mediastinum, although there is a case report documenting a pericardial schwannoma found in the middle mediastinum, specifically in the pretracheal space and aortopulmonary window compressing the right atrium, subclavian vein, and trachea (2,3). Most of the reported cases are benign neoplasms; however, primary malignant schwannomas have also been reported and are very rare, comprising 0.75% of all primary cardiac tumors (5,6). Pericardial schwannomas are frequently misdiagnosed at plain radiography or transthoracic echocardiography, requiring further characterization with either CT or MRI. Biopsy is required for definitive diagnosis (9,11,12). Complete resection of tumor is the standard treatment for benign mediastinal schwannoma without need for adjuvant therapy (4,13).
We present a case of primary pericardial schwannoma of the posterior pericardium with concerns for compression of the left atrium and left ventricle. To our knowledge, this constellation of findings has not previously been reported in the literature.
Case Presentation
A 70-year-old woman with a past medical history of hypothyroidism, osteoporosis, obstructive sleep apnea, and lumbar degenerative disk disease presented to the emergency department with complaint of persistent, mild, and nonradiating chest pain not associated with dyspnea or weakness following a motor vehicle accident in which she was struck from the passenger side. She was otherwise physically active at baseline, up to date on all age-appropriate cancer screenings, and did not have any constitutional symptoms. Electrocardiogram showed right axis deviation, poor R wave progression, and a possible prior septal infarct. The patient had mildly elevated troponin I level that peaked at 0.18 ng/mL (reference range, 0.0–0.03 ng/mL), likely reflective from supply-demand mismatch and the trauma from the motor vehicle accident. There were no acute ischemic changes on her admission electrocardiograms.
As part of trauma evaluation, plain radiography of the chest was performed, and images demonstrated a sternal fracture and large soft-tissue mass projecting over the left heart border (Fig 1). These findings were further characterized on CT scans of the chest with intravenous contrast material administration (Fig 2), which showed a posterior pericardial mass measuring 4.7 × 5.9 × 8.1 cm with mass effect upon the adjacent left atrium and left ventricle. The differential based on CT images included metastatic disease (statistically most common given the location) versus sarcoma, paraganglioma, fibroma, and pericardial mesothelioma. Cardiac MRI (Fig 3a, 3b) revealed a predominantly T2-hyperintense and well-circumscribed heterogeneously enhancing mass (6.9 cm in maximum dimension) adjacent to the left ventricle and atrium. Additional sequences demonstrated a fat plane between the mass and the heart with two areas where no definitive fat plane was identified. There was no gross myocardial infiltration or left atrial invasion. Intracardiac blood flow was not obstructed. The mass did not contain fat. No blood vessels directly supplying the mass were visualized. The left ventricular function was normal. The circumflex artery was seen between the mass and the left ventricular myocardium. There was no tethering of the mass to the left ventricle wall, and the myocardium contracted normally, arguing against myocardial infiltration or compression, but was rather a long surface abutment in the atrioventricular groove area. A separate 1.2-cm enhancing nodule was seen within the pericardium or epicardial fat along the inferior wall of the left ventricle, suspicious for a nodal metastasis. An appearance suggestive of a primary pericardial sarcoma was initially favored but considered unlikely in this location. To evaluate for any metastatic disease, fluorine 18 fluorodeoxyglucose (18F-FDG) PET/CT of the abdomen and pelvis was performed and was negative. The whole-body PET/CT images showed a large pericardial mass with heterogeneous mild to moderate 18F-FDG avidity and areas of relative photopenia suggestive of cystic changes versus necrosis (Fig 4); there was no avid metastatic disease. There were a few small scattered calcifications within the mass at attenuation-correction low-dose CT.
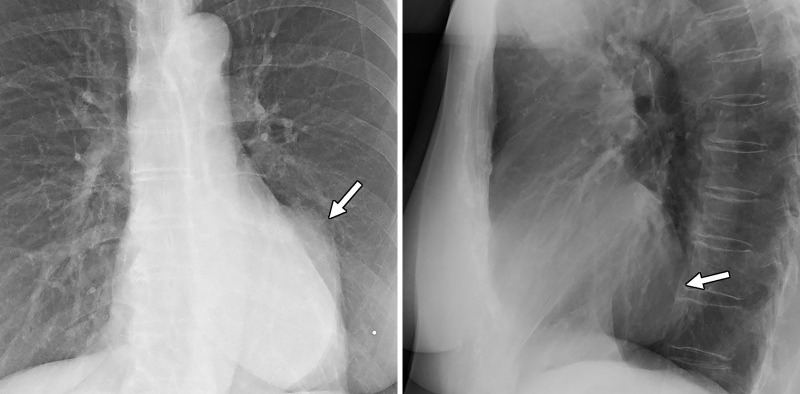
Figure 1:
Posterior-anterior and lateral chest radiographs show a large well-circumscribed middle mediastinal mass (arrow).
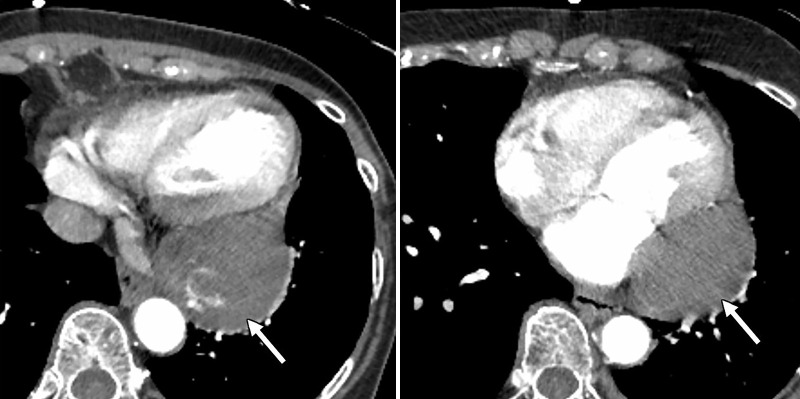
Figure 2:
Axial CT angiographic images show a large mass (arrow) associated with the posterior pericardium. Areas of high attenuation represent calcifications. There is mass effect upon the adjacent left atrium and the left ventricle.
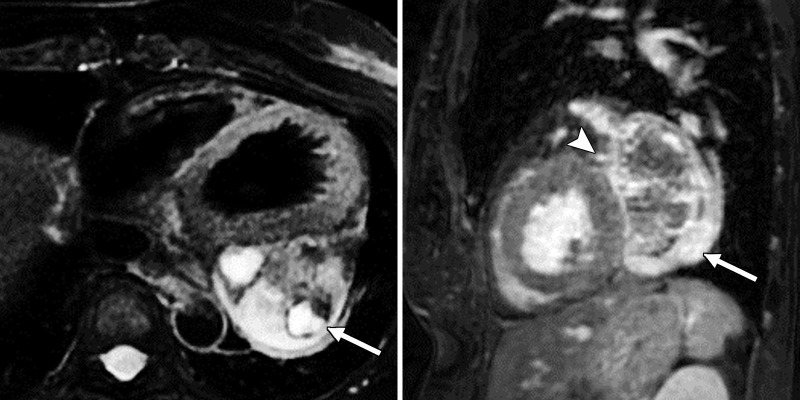
Figure 3a:
(a) Cardiac T2-weighted double inversion-recovery fat saturation (left) and sagittal three-dimensional heart fat saturation fast gradient-recalled echo sequence (right) MR images show a well-circumscribed, heterogenous, predominantly T2-hyperintense mass adjacent to the left atrium and ventricle (arrow). The arrowhead points to the left circumflex artery coursing along the mass. A 1.2-cm enhancing nodule within the pericardium or epicardial fat was noted along the inferior wall of the left ventricle, likely a lymph node. (b) Cardiac T1-weighted pre- (left) and postcontrast (right) MR images show heterogeneous enhancement (arrows).
Figure 3b:
(a) Cardiac T2-weighted double inversion-recovery fat saturation (left) and sagittal three-dimensional heart fat saturation fast gradient-recalled echo sequence (right) MR images show a well-circumscribed, heterogenous, predominantly T2-hyperintense mass adjacent to the left atrium and ventricle (arrow). The arrowhead points to the left circumflex artery coursing along the mass. A 1.2-cm enhancing nodule within the pericardium or epicardial fat was noted along the inferior wall of the left ventricle, likely a lymph node. (b) Cardiac T1-weighted pre- (left) and postcontrast (right) MR images show heterogeneous enhancement (arrows).
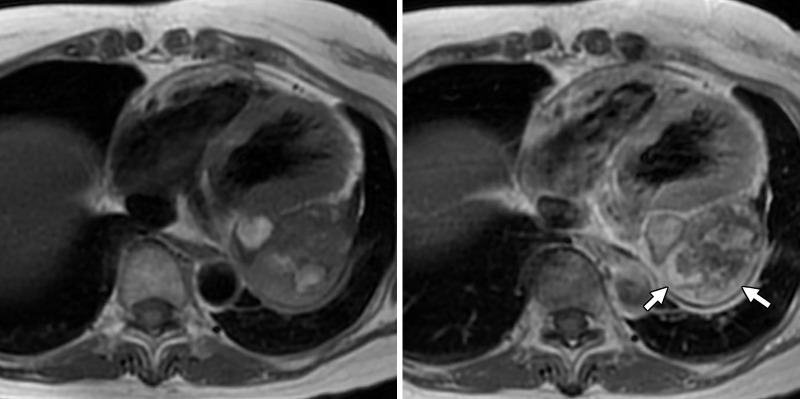
Figure 4:
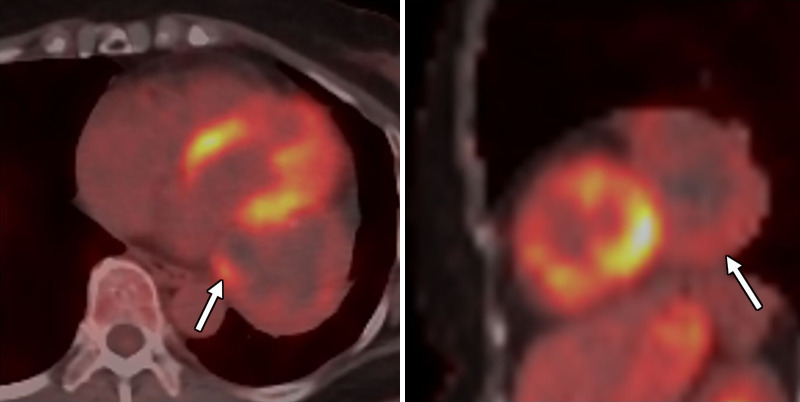
Axial and sagittal fused fluorine 18 fluorodeoxyglucose (18F-FDG) PET/CT images show again a large pericardial mass (arrow) with mild to moderate heterogeneous 18F-FDG avidity (maximum standardized uptake value of 4.8). There are photopenic regions suggestive of cystic changes or necrosis. There were scattered calcifications within that mass at attenuation-correction low-dose CT.
The pericardial mass appeared to be inseparable from the atrioventricular groove and ran along the left circumflex coronary artery to the base of the left atrial appendage, displacing the left inferior pulmonary vein, and was beginning to compress the atrium and atrioventricular groove. Given concern for eventual coronary compression or atrial irritation, resection of the lesion by surgery was recommended. Reassuringly, there was no substantial chamber compression seen at transthoracic echocardiography.
The patient underwent CT-guided core needle diagnostic biopsy. Once the final pathologic findings confirmed the diagnosis of schwannoma, the patient underwent thoracotomy with pericardial mass excision (Figs 5, 6), which remains the standard procedure for benign pericardial schwannoma. Given that there was no uptake on the PET scan, the nodal lesion was not excised. The patient was discharged home following an uneventful hospital course. At follow-up, she continues to do well and remains asymptomatic.
Figure 5a:
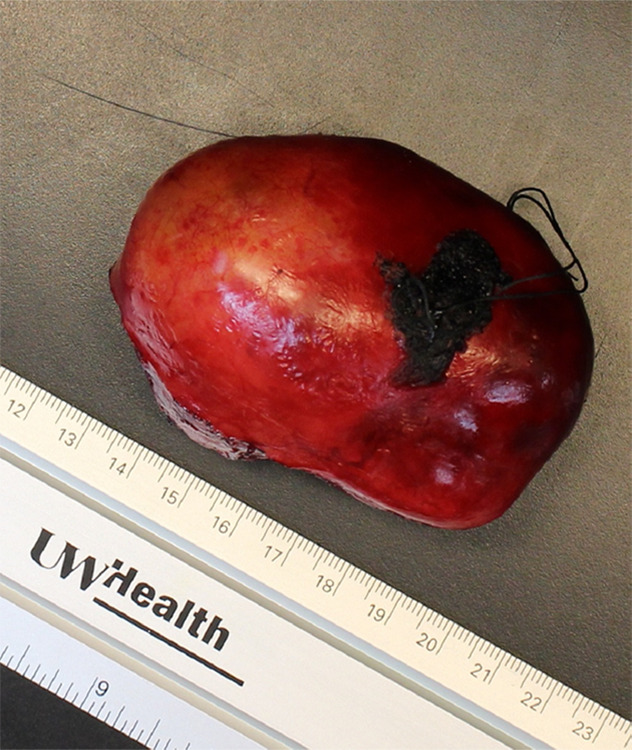
Images of resected schwannoma. (a) Left atrioventricular groove cardiac mass with intact capsule on the lateral surface and (b) thick pseudocapsule on the medial surface extending to approximately two-thirds of the specimen on the lateral side. (c) There is a thin membranous type of pseudocapsule present on the surface of the tumor. The mass appears lobulated and tan-pink to yellow-red in color.
Figure 6a:
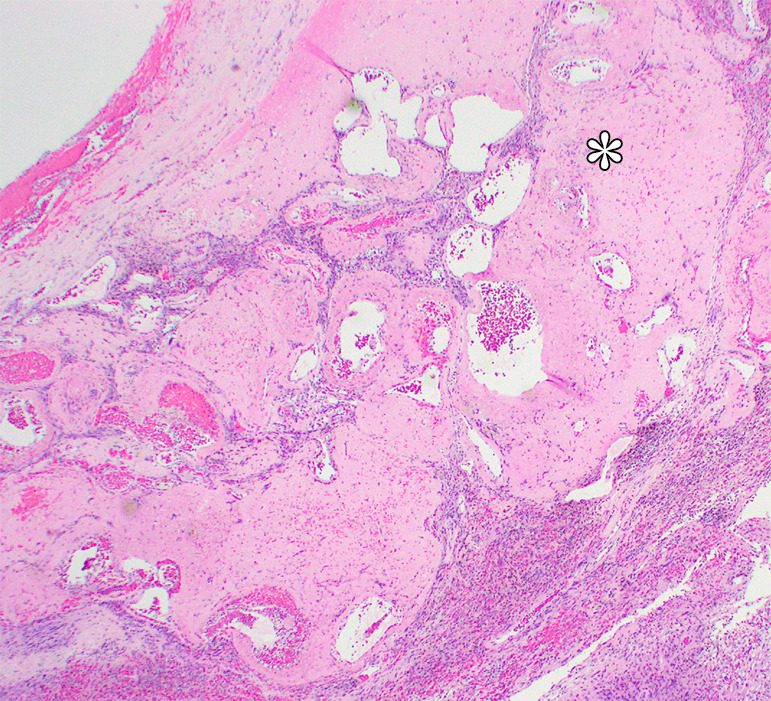
Photomicrographs show (a) cellular mass with cystic changes, hemorrhage, and hyalinized fibrin (*); (b) Antoni A area with bundles of palely eosinophilic spindle Schwannian cells with vague palisading; (c) Antoni B area with loosely arranged Schwannian cells in a myxoid stroma containing inflammatory cells; and (d) diffuse S100 positivity in Schwannian cells. (Hematoxylin-eosin stain, lower power magnification in a, high power magnification in b-d.)
Figure 5b:
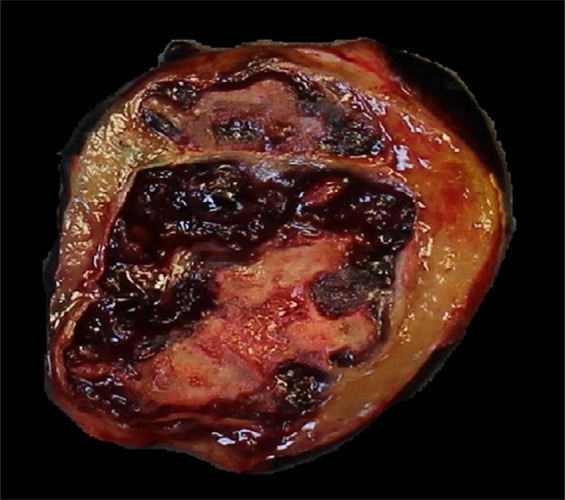
Images of resected schwannoma. (a) Left atrioventricular groove cardiac mass with intact capsule on the lateral surface and (b) thick pseudocapsule on the medial surface extending to approximately two-thirds of the specimen on the lateral side. (c) There is a thin membranous type of pseudocapsule present on the surface of the tumor. The mass appears lobulated and tan-pink to yellow-red in color.
Figure 5c:
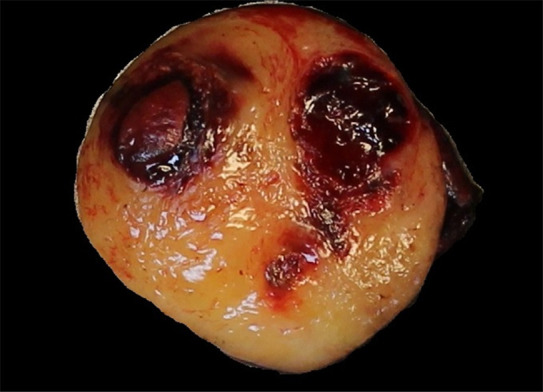
Images of resected schwannoma. (a) Left atrioventricular groove cardiac mass with intact capsule on the lateral surface and (b) thick pseudocapsule on the medial surface extending to approximately two-thirds of the specimen on the lateral side. (c) There is a thin membranous type of pseudocapsule present on the surface of the tumor. The mass appears lobulated and tan-pink to yellow-red in color.
Figure 6b:
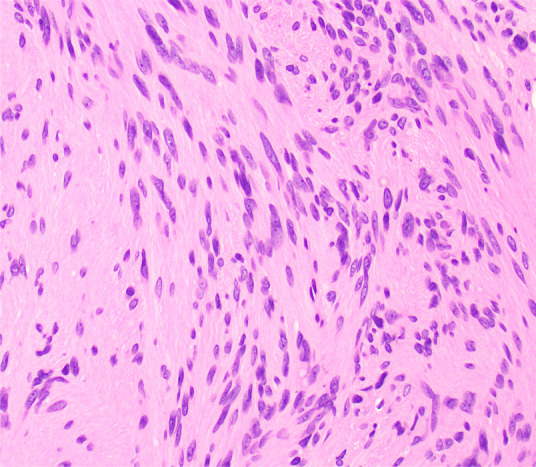
Photomicrographs show (a) cellular mass with cystic changes, hemorrhage, and hyalinized fibrin (*); (b) Antoni A area with bundles of palely eosinophilic spindle Schwannian cells with vague palisading; (c) Antoni B area with loosely arranged Schwannian cells in a myxoid stroma containing inflammatory cells; and (d) diffuse S100 positivity in Schwannian cells. (Hematoxylin-eosin stain, lower power magnification in a, high power magnification in b-d.)
Figure 6c:
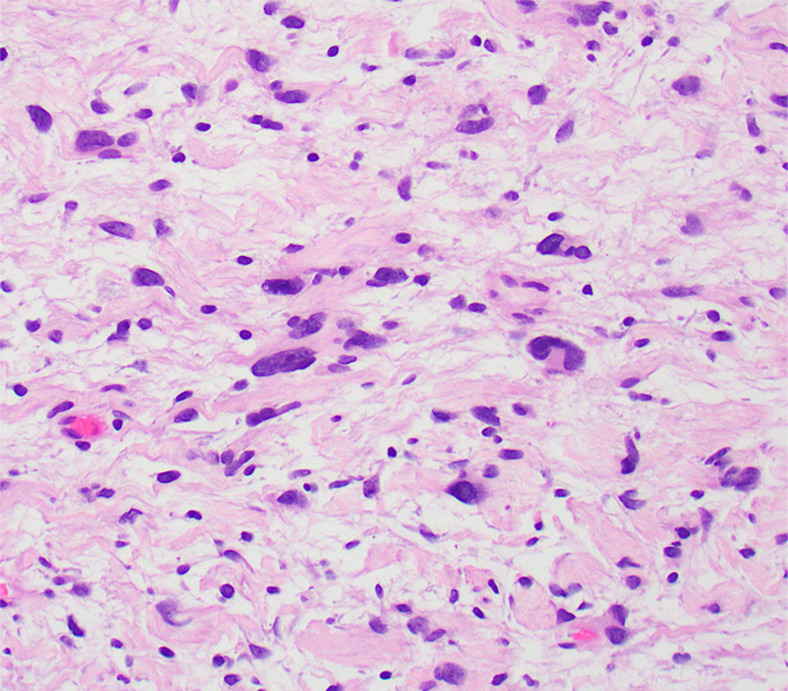
Photomicrographs show (a) cellular mass with cystic changes, hemorrhage, and hyalinized fibrin (*); (b) Antoni A area with bundles of palely eosinophilic spindle Schwannian cells with vague palisading; (c) Antoni B area with loosely arranged Schwannian cells in a myxoid stroma containing inflammatory cells; and (d) diffuse S100 positivity in Schwannian cells. (Hematoxylin-eosin stain, lower power magnification in a, high power magnification in b-d.)
Figure 6d:
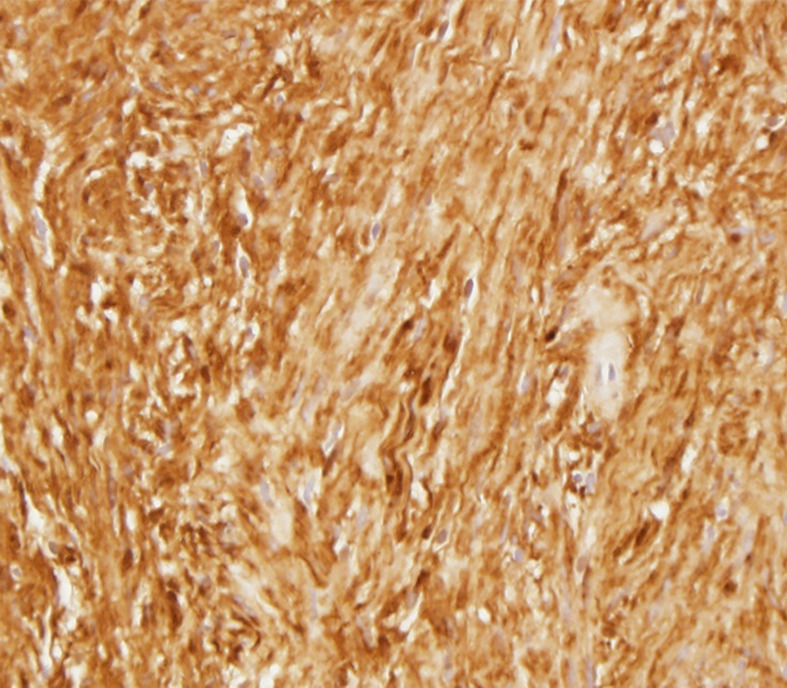
Photomicrographs show (a) cellular mass with cystic changes, hemorrhage, and hyalinized fibrin (*); (b) Antoni A area with bundles of palely eosinophilic spindle Schwannian cells with vague palisading; (c) Antoni B area with loosely arranged Schwannian cells in a myxoid stroma containing inflammatory cells; and (d) diffuse S100 positivity in Schwannian cells. (Hematoxylin-eosin stain, lower power magnification in a, high power magnification in b-d.)
Discussion
Primary cardiac tumors are rare, with a reported prevalence of 0.02%–0.056% (14). Primary pericardial tumors are even more rare when compared with primary cardiac tumors (15,16). Neurogenic tumors such as schwannomas are frequently found in the posterior mediastinum, although one case of schwannoma in the middle mediastinum has been reported (1,3,11,12). Both benign and malignant tumors may result in compression effect of the adjacent mediastinal structures, which may manifest clinically as superior vena cava syndrome, cardiac tamponade, Horner syndrome, and Pancoast syndrome.
Schwannomas belong to a subcategory of peripheral nerve sheath tumors. Schwannomas appear as well-circumscribed masses with a well-formed collagenous capsule. Histologically, degenerative changes and a variable admixture of Antoni A (compact spindled) areas and Antoni B (hypocellular and microcystic) areas are characteristic findings. At immunohistochemistry, S100 protein is often demonstrated with pericellular collagen type IV, which is consistent with pericellular basal lamina.
The clinical diagnosis of pericardial schwannoma is challenging. Patients are often asymptomatic, and when they do experience symptoms, these are often nonspecific (eg, dyspnea, cough, fatigue, and facial edema) due to variation in tumor size, location, and/or any compression effect of nearby structures such as the great vessels, mediastinal structures, or the heart chambers (2,3,10,12). As the findings at plain radiography and transthoracic echocardiography are often nonspecific, CT or MRI are often used for better characterization of the mass and its relationship with the neighboring structures, as well as any evidence of metastatic disease or myocardial invasion. Typical imaging features include well-circumscribed masses without direct invasion, often containing cystic areas of degeneration and sometimes hemorrhage; calcifications are uncommon but have been reported (3,9,11). Small lesions typically demonstrate intense homogeneous enhancement, while larger lesions enhance heterogeneously. Due to excellent soft-tissue characterization and ability to acquire multiplanar cine images, cardiac MRI was superior to CT in excluding myocardial involvement in this case. Common complications of the pericardial tumors include compression effect of the adjacent structures, regional or distant metastases, pericardial effusion, cardiac tamponade, myocardial infarction, diastolic dysfunction, and constrictive physiologic conditions (3,4,10,13).
We report an unusual case of primary pericardial schwannoma of the posterior pericardium adjacent to the left ventricle and left atrium with concern for compression. Multimodality CT and MRI were helpful in the characterization of the mass to elucidate diagnostic possibilities, with eventual definitive diagnosis provided by biopsy and resection.
Footnotes
Disclosures of Conflicts of Interest: S.L. disclosed no relevant relationships. J.E.K. disclosed no relevant relationships. D.B. disclosed no relevant relationships. A.K. disclosed no relevant relationships. R.S. disclosed no relevant relationships. C.F. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: GE Healthcare provides research support grants to department of radiology at author’s institution. Other relationships: author is editorial board member of RCTI. P.R. disclosed no relevant relationships. R.D. disclosed no relevant relationships.
References
- 1.Strollo DC, Rosado de Christenson ML, Jett JR. Primary mediastinal tumors. Part 1: tumors of the anterior mediastinum. Chest 1997;112(2):511–522. [DOI] [PubMed] [Google Scholar]
- 2.Almobarak AA, AlShammari A, Alhomoudi RI, et al. Benign pericardial schwannoma: Case report and summary of previously reported cases. Am J Case Rep 2018;19:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang XH, Wang Y, Quan XY, Liang B. Benign pericardial schwannoma in a Chinese woman: a case report. BMC Cardiovasc Disord 2013;13(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yun PJ, Huang TW, Li YF, Chang H, Lee SC, Kuo YL. Symptomatic pericardial schwannoma treated with video-assisted thoracic surgery: a case report. J Thorac Dis 2016;8(5):E349–E352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Amato N, Correale M, Ireva R, Di Biase M. A rare cause of acute heart failure: malignant schwannoma of the pericardium. Congest Heart Fail 2010;16(2):82–84. [DOI] [PubMed] [Google Scholar]
- 6.Morishita T, Yamazaki J, Ohsawa H, et al. Malignant schwannoma of the heart. Clin Cardiol 1988;11(2):126–130. [DOI] [PubMed] [Google Scholar]
- 7.Hwang SK, Jung SH. Schwannoma of the heart. Korean J Thorac Cardiovasc Surg 2014;47(2):141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung JH, Jung JS, Lee SH, Kim KT, Lee K, Lee SH. Resection of intrapericardial schwannoma co-existing with thymic follicular hyperplasia through sternotomy without cardiopulmonary bypass. Korean J Thorac Cardiovasc Surg 2014;47(3):298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taguchi S, Mori A, Suzuki R, et al. Mediastinal schwannoma diagnosed preoperatively as a cyst. Tex Heart Inst J 2014;41(1):76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajesh GN, Raju D, Haridasan V, et al. Intrapericardial schwannoma presenting as acute coronary syndrome. J Am Coll Cardiol 2013;62(25):e527. [DOI] [PubMed] [Google Scholar]
- 11.Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. RadioGraphics 2000;20(4):1073–1103; quiz 1110–1111, 1112. [DOI] [PubMed] [Google Scholar]
- 12.Miscia VF. Letter: Primary pericardial tumor. JAMA 1975;231(13):1340. [DOI] [PubMed] [Google Scholar]
- 13.Son KH, Kim KW, Ahn CB, et al. Surgical planning by 3D printing for primary cardiac schwannoma resection. Yonsei Med J 2015;56(6):1735–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol 1996;77(1):107. [DOI] [PubMed] [Google Scholar]
- 15.Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S. Echocardiographic and pathologic characteristics of primary cardiac tumors: a study of 149 cases. Int J Cardiol 2002;84(1):69–75. [DOI] [PubMed] [Google Scholar]
- 16.Lamba G, Frishman WH. Cardiac and pericardial tumors. Cardiol Rev 2012;20(5):237–252. [DOI] [PubMed] [Google Scholar]