Abstract
Concomitant acute myocarditis and acute coronary thrombosis is a rare presentation of acute chest pain in the emergency department, although the association between acute infections with a variety of pathogens and an increased risk of myocardial infarction has been reported. A case of acute myocardial infarction associated with acute myocarditis caused by coronavirus 229E in a middle-aged man without risk factors for coronary artery disease is described here. Coronary CT angiography with late enhancement protocol revealed areas of myocarditis and infarction, and cardiac MRI and coronary angiography were then performed.
© RSNA, 2021
Summary
In the presence of acute chest pain in a middle-aged man without risk factors for coronary artery disease, a concomitant diagnosis of acute myocarditis and acute myocardial infarction was made at coronary CT angiography with late enhancement protocol, cardiac MRI, and coronary angiography.
Key Points
■ Acute myocarditis is an inflammatory disease caused by different infectious and noninfectious triggers.
■ Infectious and inflammatory status generates instability in the atherosclerotic plaque, disrupture, and superimposed thrombosis with acute myocardial infarction.
■ Differential diagnosis includes pericarditis, myocarditis, and acute coronary syndrome.
Introduction
Acute myocarditis is an inflammatory disease of the myocardium caused by different infectious and noninfectious triggers (1,2). The clinical manifestation of acute myocarditis varies with a broad spectrum of nonspecific symptoms ranging from asymptomatic courses to manifestations with signs of myocardial infarction and cardiogenic shock. Furthermore, the diagnosis of acute myocarditis based on the clinical presentation alone is usually not possible. Chest pain, acute coronary syndrome, cardiac arrhythmias, and acute or chronic heart failure can occur during the course of the acute myocarditis (2–4). Here we report a case of chest pain caused by a concomitant acute coronary stenosis and acute myocarditis.
Case Report
A 43-year-old man visited the emergency department presenting with acute chest pain after playing soccer. He defined a “burning sensation” that ceased 30 minutes later. He also reported nasal congestion, cough, and fatigue 2 weeks before. At examination, heart and lung auscultation were normal. He did not present with any other symptoms. The patient did not have any relevant previous medical history.
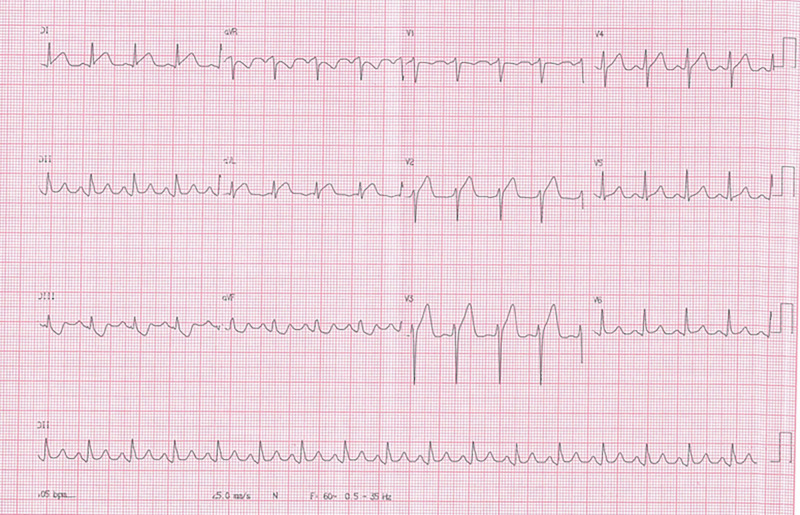
Electrocardiogram revealed diffuse ST-segment elevation (Fig 1), and the first troponin measurement was negative. The second troponin measurement 6 hours later was 14 ng/L (reference value < 14 ng/L). The following electrocardiogram, also obtained 6 hours later, revealed a reduction of the ST-segment elevation in all derivations. The other serial troponin measurements with approximately 6-hour intervals were 250, 500, and 740 ng/L.
Figure 1:
Electrocardiogram reveals diffuse ST-segment elevation.
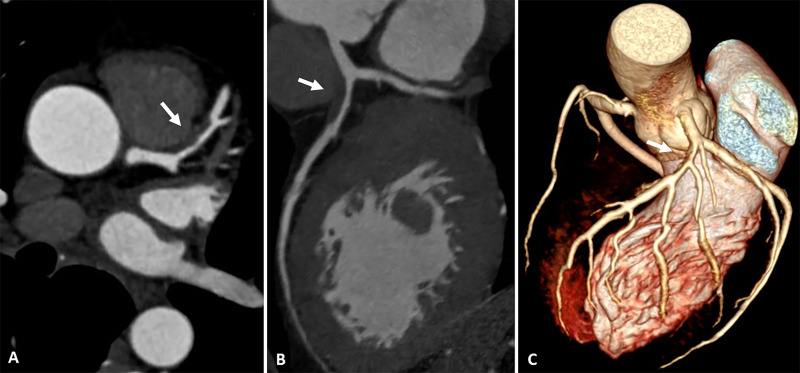
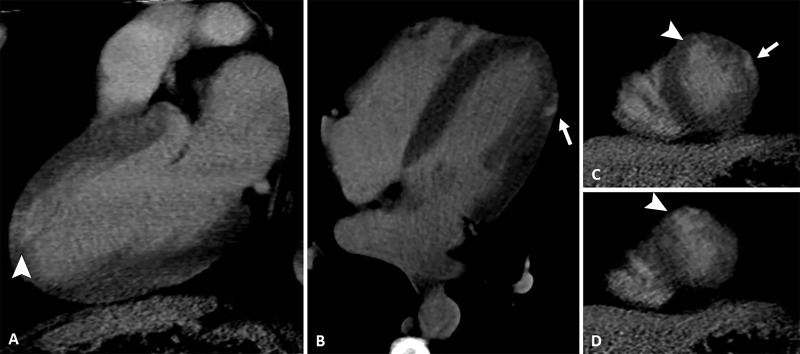
At hospital admission, it was decided to not perform thrombolysis due to the suspicion of myopericarditis in a middle-aged patient without risk factors for coronary artery disease. However, he was given 200 mg of aspirin and 300 mg of clopidogrel. Then, he was transferred to a cardiology reference hospital for further investigation. Echocardiogram did not show any alterations. Coronary CT angiography (Fig 2) revealed an important stenosis of the left anterior descending artery, and the late enhancement protocol (Fig 3) revealed areas of mesoepicardial enhancement in the lateral apical wall suggestive of myocarditis. Subendocardial enhancement in the anterior apical segment was suggestive of myocardial infarction.
Figure 2:
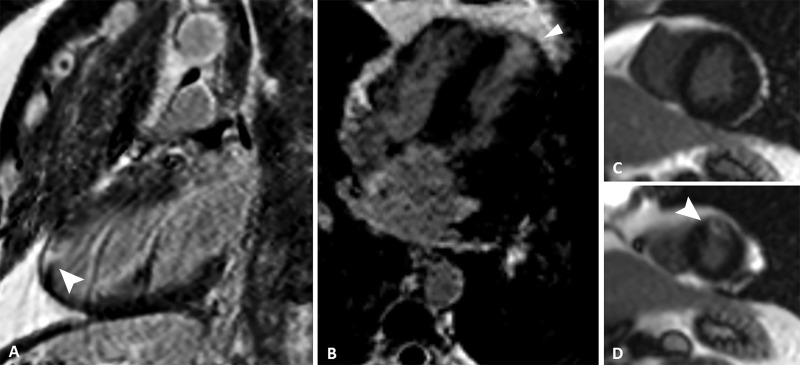
Images from coronary CT angiography. A, Axial, B, oblique multiplanar reconstruction, and, C, tridimensional reconstruction show an atherosclerotic plaque (arrow) in the proximal segment of anterior descending artery with severe luminal narrowing.
Figure 3:
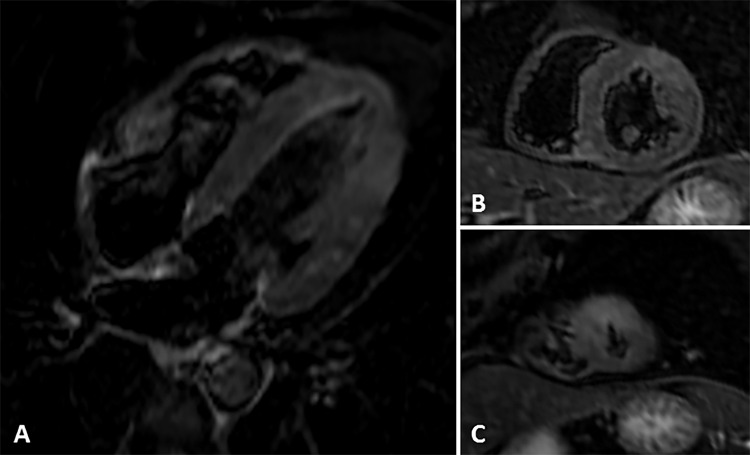
Images from coronary CT angiography with late enhancement protocol. A, Two-chamber, B, four-chamber, and, C, D, short-axis views show late-enhancing mesoepicardial area (arrow) in the lateral apical segment suggestive of myocarditis and a subendocardial area (arrowhead) in the anterior apical segment suggestive of myocardial infarction.
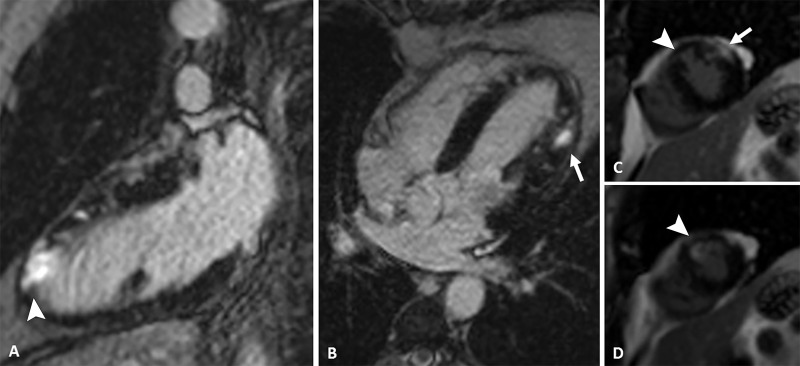
Cardiac MRI (Figs 4, 5) revealed myocardial edema and mesoepicardial late enhancement in the mid anterolateral wall and lateral apical wall suggestive of myocarditis, as well as subendocardial late enhancement of the anterior apical wall, which is suggestive of myocardial infarction.
Figure 4:
Images from phase-sensitive inversion-recovery cardiac MRI. A, Two-chamber, B, four-chamber, and, C, D, short-axis views show late-enhancing mesoepicardial area (arrow) in the lateral apical segment suggestive of myocarditis and a subendocardial area (arrowhead) in the anterior apical segment suggestive of myocardial infarction.
Figure 5:
Images from short τ inversion recovery cardiac MRI. A, Four-chamber and, B, C, short-axis views show edema in the mid anterolateral wall and lateral apical wall and in the anterior apical segment..
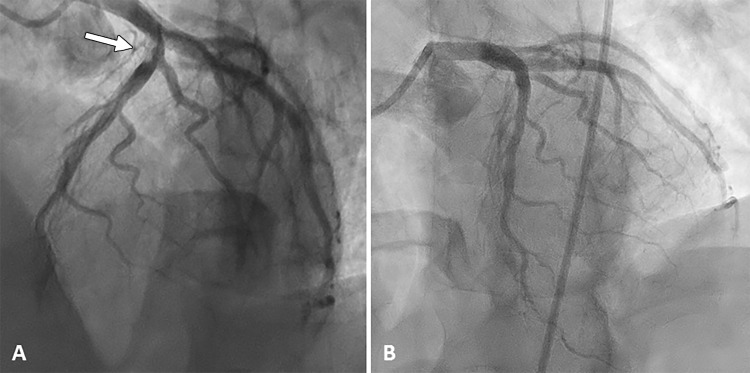
To better evaluate the anterior descending artery stenosis, the patient underwent invasive coronary angiography (Fig 6), which revealed an ulcerated lesion causing 80% luminal narrowing in the proximal segment of this artery, with a high thrombotic burden and the distal artery with Thrombolysis in Myocardial Infarction flow grade 3. He received tirofiban for 48 hours, and percutaneous coronary intervention was successfully performed with a drug-eluting stent.
Figure 6:
Images from coronary angiography. A, An ulcerated lesion causing 80% luminal narrowing in the proximal segment of the anterior descending artery, with a high thrombotic burden (arrow). B, After percutaneous coronary intervention with a drug-eluting stent.
The patient was discharged the day after the procedure, with a prescription of aspirin, clopidogrel, metoprolol, enalapril, and rosuvastatin. Respiratory virus panel test detected coronavirus subtype 229E by use of reverse-transcription polymerase chain reaction.
At 4-month follow-up, cardiac MRI results (Fig 7) showed the same area of late enhancement in the anterior apical segment suggestive of myocardial infarction. The late-enhancing mesoepicardial area in the lateral apical segment, compatible with myocarditis in the previous examination, was no longer seen.
Figure 7:
Images from phase-sensitive inversion-recovery cardiac MRI. A, Two-chamber, B, four-chamber, and, C, D, short-axis views show late-enhancing subendocardial area (arrowhead) in the anterior apical segment suggestive of myocardial infarction. The late-enhancing mesoepicardial area in the lateral apical segment, compatible with myocarditis in the previous examination, was no longer seen.
Discussion
Clinical presentations of acute myocarditis frequently mimic several causes of chest pain, and its concomitance with acute myocardial infarction is a rare condition.
Kwong et al (5) published a series of cases evidencing increased incidence of acute coronary syndrome in postviral infection. A strong correlation was observed with influenza virus types B and A, as well as respiratory syncytial virus (5–7). The main hypothesis for the development and progression of acute myocarditis is that the infectious and inflammatory status generates instability in the atherosclerotic plaque, disrupture, and superimposed thrombosis with acute myocardial infarction (5,7). Other theories are explained by the increase in metabolic demand during the inflammatory process, a phenomenon known as “demand ischemia,” or even the adverse effect on cardiac function (7).
The time after the onset of acute infection to myocardial infarction also showed a strong correlation in the 1st week, and there was a substantial decrease in the incidence in the subsequent days after the 1st week (5,7). The risk of myocardial infarction can also be evaluated with a temporal pattern in short, intermediate, and long-term risk (7).
Our patient underwent a reverse-transcription polymerase chain reaction test, which was positive for coronavirus subtype 229E. The coronavirus subtypes, including severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus, have been reported to correlate with severe manifestations of acute myocarditis (8,9). In 2016, Alhogbani published a case report of a patient with Middle East respiratory syndrome coronavirus infection associated with severe ventricular dysfunction secondary to viral infection (9).
The case presented here also highlights the importance of coronary CT angiography with late enhancement protocol in the evaluation of patients with high suspicion of myopericarditis and low cardiovascular risk factors for coronary syndrome in emergency departments with low availability of MRI. In the absence of the coronary CT angiography with late enhancement protocol, the patient would probably proceed directly to coronary angiography, and the myocarditis could be underdiagnosed.
To evaluate late iodine enhancement at CT, two steps must be followed. First, the CT angiography acquisition is performed to analyze the coronary arteries with an electrocardiographically gated protocol. After 5 minutes, a new iodine contrast media bolus (20 mL) at 4.0 mL per second is infused to enhance the definition of cardiac borders, 1 minute prior to a secondary electrocardiographically gated acquisition. Late iodine enhancement is achieved with the first injection of iodine contrast media for the assessment of coronary arteries, while the second injection is performed just to delimit the cardiac borders and better identify areas of myocardial enhancement. For the analysis of late iodine enhancement, acquired 6 minutes after the first iodine injection, diastolic phase reconstructions (long- and short-axis views) are obtained with an average section thickness of 10 mm and specific window and level settings (W250, L150). After an initial exploratory reading, radiologists could adjust the display settings to change section thickness and projections as needed. Variations of myocardial enhancement to the normal remote myocardium are assessed visually using multiplanar reformatting images and confirmed when the difference between both images is more than 20 HU.
In clinical practice, cardiac MRI with late gadolinium enhancement has a well-established diagnostic and prognostic value, mostly for the evaluation of focal myocardial fibrosis and edema in the diagnosis of myocardial infarction and nonischemic cardiomyopathies. However, late iodine enhancement at CT may represent an excellent alternative to late gadolinium enhancement MRI, because the pharmacokinetics of iodinated contrast materials are similar to those of gadolinium-containing contrast materials, including for the assessment of extracellular volume (10–12).
Gadolinium is an inert extracellular contrast agent that cannot cross cell membranes unless there are ruptures in the myocyte membrane. These ruptures allow gadolinium to diffuse into what was previously intracellular space, which increases gadolinium concentration and therefore late gadolinium enhancement, independently of the underlying acute or chronic cause (10,13). In the setting of chronic myocardial damage, myocytes have been replaced with collagenous scar, featuring an irreversible change; whereas in acute myocardial inflammatory disease, late enhancement areas seem to represent a higher activity of the inflammatory process, explained by some expansion of the interstitium by edema that may be prominent in the acute stage but disappears with healing. Furthermore, late enhancement can be reversible in acute myocarditis and perimyocarditis (13,14).
The patterns of late enhancement also help to differentiate between ischemic and nonischemic myocardial damage. The typical subendocardial or transmural late gadolinium enhancement in a coronary supply area is indicative of ischemic heart disease, while nonischemic myocardial injury can be observed mainly at the epicardium and midwall (13–15), although some other nonischemic patterns have been recognized (16). Greater attention should be given in the suspicion of embolic infarctions, which may show epicardial late enhancement, as well as microvascular obstructions, which may mimic midwall late enhancement (17).
Conclusion
We report a rare case of chest pain caused by a concomitant acute coronary stenosis and acute myocarditis in a middle-aged man without cardiovascular risk factors. This case illustrates the importance of anatomic coronary investigation in chest pain with coronary CT angiography with late enhancement protocol to enable other diagnosis. Coronary CT angiography with late enhancement protocol for suspicion of acute myocarditis should be considered by physicians, avoiding invasive angiography and MRI, as well as providing an earlier discharge from the emergency department. In this case, myocardial infarction that developed after viral myocarditis was diagnosed.
Footnotes
Disclosures of Conflicts of Interest: M.J.L.M. disclosed no relevant relationships. L.d.P.G.d.F. disclosed no relevant relationships. L.P.S.B. disclosed no relevant relationships. M.C.S. disclosed no relevant relationships. P.O.G. disclosed no relevant relationships. A.C.d.A.B. disclosed no relevant relationships. P.G.M.d.B.e.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed grants/grants pending to author’s institution from Bayer, Pfizer, and Roche Diagnostics. Other relationships: disclosed no relevant relationships. V.F. disclosed no relevant relationships.
References
- 1.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113(14):1807–1816. [DOI] [PubMed] [Google Scholar]
- 2.Luetkens JA, Faron A, Isaak A, et al. Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: results of a validation cohort. Radiol Cardiothorac Imaging 2019;1(3):e190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kindermann I, Kindermann M, Kandolf R, et al. Predictors of outcome in patients with suspected myocarditis. Circulation 2008;118(6):639–648 [Published correction appears in Circulation 2008;118(12): e493.]. [DOI] [PubMed] [Google Scholar]
- 4.Baudry G, Bouleti C, Iung B, et al. Diagnosis of acute myocarditis with dual source cardiac tomography. Int J Cardiol 2015;179:256–257. [DOI] [PubMed] [Google Scholar]
- 5.Kwong JC, Schwartz KL, Campitelli MA, et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N Engl J Med 2018;378(4):345–353. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL. Seasonal Influenza Infections and Cardiovascular Disease Mortality. JAMA Cardiol 2016;1(3):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musher DM, Abers MS, Corrales-Medina VF. Acute Infection and Myocardial Infarction. N Engl J Med 2019;380(2):171–176. [DOI] [PubMed] [Google Scholar]
- 8.Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020;17(9):1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alhogbani T. Acute myocarditis associated with novel Middle east respiratory syndrome coronavirus. Ann Saudi Med 2016;36(1):78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmisano A, Vignale D, Benedetti G, Del Maschio A, De Cobelli F, Esposito A. Late iodine enhancement cardiac computed tomography for detection of myocardial scars: impact of experience in the clinical practice. Radiol Med (Torino) 2020;125(2):128–136. [DOI] [PubMed] [Google Scholar]
- 11.Oda S, Emoto T, Nakaura T, et al. Myocardial Late Iodine Enhancement and Extracellular Volume Quantification with Dual-Layer Spectral Detector Dual-Energy Cardiac CT. Radiol Cardiothorac Imaging 2019;1(1):e180003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohta Y, Kitao S, Yunaga H, et al. Myocardial Delayed Enhancement CT for the Evaluation of Heart Failure: Comparison to MRI. Radiology 2018;288(3):682–691. [DOI] [PubMed] [Google Scholar]
- 13.Vöhringer M, Mahrholdt H, Yilmaz A, Sechtem U. Significance of late gadolinium enhancement in cardiovascular magnetic resonance imaging (CMR). Herz 2007;32(2):129–137. [DOI] [PubMed] [Google Scholar]
- 14.Hunold P, Schlosser T, Vogt FM, et al. Myocardial late enhancement in contrast-enhanced cardiac MRI: distinction between infarction scar and non-infarction-related disease. AJR Am J Roentgenol 2005;184(5):1420–1426. [DOI] [PubMed] [Google Scholar]
- 15.Vogel-Claussen J, Rochitte CE, Wu KC, et al. Delayed enhancement MR imaging: utility in myocardial assessment. RadioGraphics 2006;26(3):795–810. [DOI] [PubMed] [Google Scholar]
- 16.Wassmuth R, Schulz-Menger J. Embolic myocardial infarctions look different: a comparison of experimental and fateful embolic lesions. J Cardiovasc Magn Reson 2011;13(Supplement 1):P152. [Google Scholar]
- 17.Vermes E, Carbone I, Friedrich MG, Merchant N. Patterns of myocardial late enhancement: typical and atypical features. Arch Cardiovasc Dis 2012;105(5):300–308. [DOI] [PubMed] [Google Scholar]