Abstract
Purpose
To investigate the MRI characteristics, prevalence, and outcomes of hypertrophic cardiomyopathy (HCM) with restrictive phenotype.
Materials and Methods
A total of 2592 consecutive patients with HCM were evaluated to identify individuals who fulfilled the diagnostic criteria of restrictive phenotype. Thirty-four patients with HCM (mean age, 41 years ± 16 [standard deviation]; range, 21–62 years, 16 men) with restrictive phenotype were retrospectively identified. Thirty-four patients with HCM with the same age and sex distributions were randomly selected as a control group. Kaplan-Meier survival curves were compared using log-rank statistics for survival analysis.
Results
The anteroposterior diameters of the left and right atria were 55 mm ± 5 and 61 mm ± 9, respectively, which were larger than those of the control group (P < .001). The maximum wall thickness in the restrictive group was lower than that in the control group (16 mm ± 2 vs 19 mm ± 3, P < .001). No significant difference was found in late gadolinium enhancement fraction between the restricted phenotype and the control group (15% ± 8 vs 13% ± 7, P = .376). The 5-year event-free survival from any cause of death and cardiac transplantation was 81% in the restrictive group, compared with 94% in the control group (log-rank P = .018).
Conclusion
Restrictive phenotype is a rare subtype of HCM and is associated with severe clinical symptoms and poor prognosis. The MRI features of this phenotype include mild to moderate left ventricular hypertrophy, markedly enlarged atria, moderate myocardial fibrosis, and pericardial effusion.
© RSNA, 2020
Summary
Hypertrophic cardiomyopathy with restrictive phenotype is a rare variant (1.3% in a large cohort of patients with hypertrophic cardiomyopathy) with mild to moderate left ventricular hypertrophy without obstruction of the left ventricular outflow tract, markedly enlarged atria, normal or small size of ventricles with moderate myocardial fibrosis, and poor outcomes.
Key Points
■ Hypertrophic cardiomyopathy (HCM) with restrictive phenotype is a rare variant of HCM; the prevalence of this phenotype was 1.3% in a large cohort of patients with HCM.
■ The typical MRI characteristics of HCM with restrictive phenotype are markedly enlarged atria with mild to moderate left ventricular hypertrophy, moderate myocardial fibrosis, and usually with mild to moderate pericardial and/or pleural effusion.
■ HCM with restrictive phenotype usually has severe clinical manifestations, and exercise tolerance is impaired; the prognosis of this phenotype is poor, and the 5-year event-free survival rate from any cause of death and cardiac transplantation was 81%.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiac disorder with a highly heterogeneous background, phenotypic expression, and clinical presentation (1). The estimated prevalence in the general adult population with phenotypic evidence of HCM is one in 500 persons (1). Although HCM is considered as the most common cause of sudden cardiac death in the young population (2,3), most patients have an indolent course and even normal life expectancy (4). However, some patients with HCM without obstruction of the left ventricular (LV) outflow tract have severe diastolic abnormalities with rapid early filling and restrictive physiology accompanying pericardial effusion, pleural effusion, and edema of the lower extremities. It has been reported that in a family with a specific HCM gene mutation, a small number of patients present with restrictive cardiac physiology, such as apparent lack of LV hypertrophy, restricted LV filling, and markedly enlarged atria, very similar to idiopathic restrictive cardiomyopathy (5). However, the diagnosis of restrictive HCM may be difficult because some of these patients may be misdiagnosed with restrictive cardiomyopathy of uncertain origin (6). More recently, the restrictive phenotype of HCM has been recognized as a subtype of the HCM disease spectrum (7). To date, the cardiac MRI characteristics of this rare phenotype of HCM have not been reported. The aim of our study was to evaluate MRI characteristics of patients with HCM of restrictive phenotype and to assess its prevalence, clinical manifestations, and prognosis.
Materials and Methods
Patients
The investigation conformed with the principles outlined in the Declaration of Helsinki. The institutional ethics committee of our hospital approved our study, which was a retrospective review of acquired data. We systematically evaluated the database of 2592 patients with HCM who all underwent contrast material–enhanced cardiac MRI at Fuwai Hospital encompassing the period of 2011 to 2015. There were 1623 male patients (mean age, 47 years ± 15 [standard deviation]; range, 11–79 years) and 969 female patients (mean age, 49 years ± 14; range, 12–78 years). No statistical difference in age existed between male and female patients. Patients were classified as “restrictive phenotype” of HCM according to the diagnostic criteria of the study by Kubo et al (7): (a) one or more LV myocardial segments with a wall thickness of 15 mm or greater at two-dimensional echocardiography or MRI or unexplained increased LV wall thickness of 13 mm or greater with a first-degree relative with confirmed HCM (8); (b) an instantaneous peak Doppler LV outflow tract pressure gradient at rest of 30 mm Hg or less; (c) normal systolic function, LV ejection fraction (LVEF) of 50% or greater; (d) Doppler indexes of restrictive filling (peak E wave/A wave velocity [E/A] ratio ≥ 2 and deceleration time ≤ 150 msec); and (e) reduced or normal ventricular cavity size (LV end-diastolic diameter [LVEDD] ≤ normal for age and body surface area) (9). Other causes that can lead to LV hypertrophy were excluded, such as hypertension, aortic valve disease, athletic heart, infiltrative cardiomyopathy, and primary metabolic disorders (eg, Fabry disease, amyloidosis, Danon disease) (10). Renal impairment (estimated glomerular filtration rate of 60 mL/min) and other MRI contraindications, such as claustrophobia, were also excluded. Thirty-four patients with HCM with restrictive phenotype were retrospectively identified. Thirty-four patients with HCM without LV outflow obstruction, whose LVEF was at least 50% or larger with same age and sex distributions, were selected from the remaining patients with HCM as the control group. Clinical data and family history were collected, and patients were evaluated with the New York Heart Association (NYHA) functional classification. Follow-up was conducted by telephone interviews for cardiovascular events, including cardiac death, heart transplantation, atrial and ventricular arrhythmia, and syncope.
MRI Protocols
Cardiac MRI was performed with a 1.5-T imager (Magnetom Avanto; Siemens, Erlangen, Germany) with a maximum gradient field of 45 mT/m and maximum gradient slew rate of 200 mT·m-1. For cardiac morphologic and functional analysis, steady-state free precession breath-hold cines in three long-axis planes and sequential short-axis slices from the atrioventricular ring to the LV apex were performed. The following imaging parameters were used: repetition time msec/echo time msec, 3.0/1.1; flip angle, 85° to 65°; bandwidth, 800 Hz/pixel; matrix size, 192 × 256; pixel size, 2.2 × 1.6 mm2; integrated parallel imaging technique acceleration factor of two; and temporal resolution of 38–45 msec per frame depending on R-R interval. Late gadolinium enhancement (LGE) was performed using turbo fast low-angle shot T1-weighted imaging with the phase-sensitive inversion-recovery technique. LGE MRI was performed 10 to 15 minutes after injection of 0.2 mmol per kilogram of body weight of gadodiamide (Magnevist; Bayer and Schering) using an automated injector (Spectris; Medrad, Pittsburgh, Pa). LGE images were acquired in the LV short-axis orientation as well as in two-, three-, and four-chamber views corresponding to the slice positions of MRI cines.
MRI Analysis
All MR images were transferred to an offline workstation installed with commercial postprocessing software (Argus; Siemens and CVI42; Circle Cardiovascular Imaging, Calgary, Canada) for blinded analysis (S.L., with 4 years of cardiovascular MRI experience). The dimensions of all four chambers (left atrial dimension, right atrial dimension, LVEDD, right ventricular end-diastolic diameter [RVEDD]), LV volumes (LV end-diastolic volume [LVEDV], LV end-systolic volume [LVESV] and cardiac output [CO]), LV mass, and LVEF were measured by standard volumetric techniques and analyzed by commercially available software (Argus) (Fig 1) (11,12). LV endocardial and epicardial borders on cine images were drawn manually to define the myocardium. Maximal LV wall thickness was defined as the greatest dimension at any site within the LV myocardium at the end-diastolic phase of the short axis. The LV chamber was assessed according to the American Heart Association 17-segment model (13). Regions with LGE were measured semiautomatically using a commercial software (CVI42). LGE was defined quantitatively by myocardial postcontrast signal intensity 6 standard deviations above the reference region of remote myocardium within the same slice and by visual assessment (14,15). Total LGE mass was calculated by summing LGE areas, and LGE fraction was expressed as a proportion of LV myocardium (percentage of LGE).
Figure 1a:

(a) Measurement of left atrial anteroposterior diameter and right atrial anteroposterior diameter. (b) Measurement of left and right ventricular end-diastolic diameter. LAD = left atrial diameter, LVEDD = left ventricular end-diastolic diameter, RAD = right atrial diameter, RVEDD = right ventricular end-diastolic diameter.
Figure 1b:

(a) Measurement of left atrial anteroposterior diameter and right atrial anteroposterior diameter. (b) Measurement of left and right ventricular end-diastolic diameter. LAD = left atrial diameter, LVEDD = left ventricular end-diastolic diameter, RAD = right atrial diameter, RVEDD = right ventricular end-diastolic diameter.
Inter- and Intraobserver Variability
Inter- and intraobserver variability were assessed in 20 randomly selected individuals (10 individuals from each group) in which one observer (M.L., with 16 years of cardiovascular MRI experience) measured once, and a second observer (S.L., with 4 years of cardiovascular MRI experience) who was blinded to the results of the first observer, measured at two time points at least 1 week apart.
Statistical Analysis
Statistical analysis was performed using SPSS 20.0 software. All data were expressed as mean ± standard deviation with range or as percentage with frequency. Univariate comparisons were performed by using Student t test, Mann-Whitney U test, and χ2 test for normally distributed, nonnormally distributed, and categorical variables, respectively. Noncontinuous variables, such as the incidence of atrial fibrillation and family history, were shown as rate and compared with the Fisher exact test. For survival analysis, Kaplan-Meier survival curves were compared using log-rank statistics. The primary end points were death from any cause (including HCM-related death) and heart transplantation. The HCM-related death included sudden cardiac death (unexpected within 1 hour of witnessed collapse or nocturnal), heart failure death (in the context of progressive cardiac decompensation), and stroke-related death (16). The secondary end points were cardiovascular events, including hospitalization for heart failure and implantable cardioverter defibrillators. All suspected outcome events were reviewed by two independent investigators (S.L., with 4 years of cardiovascular MRI experience and M.L., with 16 years of cardiovascular MRI experience), using previously described criteria (17). Survival values were expressed together with their 95% confidence intervals (CIs) defined as survival ± 1.96 times standard error. Results were considered significant if P < .05. The Cox proportional hazard model was used to calculate the influence of clinical and imaging parameters on adverse cardiovascular events.
Results
Population Characteristics
Thirty-four patients (1.3%) from the whole cohort of 2592 patients fulfilled the diagnostic criteria of restrictive phenotype. No differences in family history existed regarding HCM and sudden cardiac death between the two groups (Table 1). However, patients with restrictive phenotype had more severe clinical symptoms including paroxysmal atrial fibrillation (65% vs 15%, P < .001), pericardial effusion (71% vs 27%, P < .001), pleural effusion (29% vs 3%, P = .003), and lower NYHA functional classification (44% of patients were in NYHA III vs 62% of patients were in NYHA II, P < .001) compared with those of the control group. Atrial fibrillation occurred in 25 patients in the restricted group, and six patients were newly diagnosed during follow-up compared with six patients in the control group from which two patients had newly developed atrial fibrillation during follow-up. More than half of the patients with restrictive phenotype (56%) were in NYHA class III and IV, and their quality of life was markedly decreased. In contrast, most patients in the control group were NYHA class I and II (88%), only four patients were in NYHA class III, and none were NYHA class IV (P < .001). The basic clinical characteristics of the two groups of patients are summarized in Table 1.
Table 1:
Basic Characteristics of Two HCM Groups with Different Phenotypes
MRI Findings
Both left and right atria were significantly larger in patients with restrictive phenotype (55 mm ± 5 and 61 mm ± 9, all P < .001, Fig 2), compared with the control group (Fig 3). However, there was no statistical difference both in the left and right ventricular end-diastolic diameter (LVEDD: 46 mm ± 5 vs 47 mm ± 4, P = .131 and RVEDD: 31 mm ± 4 vs 30 mm ± 3, P = .263; Table 2). LV functional parameters, including LVEF and CO in the restrictive phenotype group, were all lower than those in the control group (LVEF: 61% ± 4 vs 64% ± 5, P = .008; CO: 4.3 L/min ± 0.6 vs 5.0 L/min ± 0.9, P = .001). The maximum wall thickness in the restrictive phenotype group was significantly lower than the control group (16 mm ± 2 vs 19 mm ± 3, P < .001). However, there was no significant difference in LV mass between the two groups (91 g ± 18 vs 98 g ± 18, P = .151). In addition, there were no significant differences both in LGE mass (15 g ± 10 vs 13 g ± 9, P = .340) and LGE percentage (15% ± 8 vs 13% ± 7, P = .376).
Figure 2:
Representative case of HCM with restrictive phenotype in a 44-year-old woman with cardiac MRI and pathologic findings after heart transplantation. A–C, Four-chamber, D–F, two-chamber, and, G–I, LV midshort axis views of bSSFP cine and LGE (C, F, I) show moderate ventricular septal hypertrophy with both left and right atria significantly enlarged and moderate pericardial effusion. The LGE images (C, four-chamber view, F, two-chamber view, I, midshort axis view) show severe myocardial fibrosis in hypertrophic myocardium and adjacent LV myocardium. J, Hematoxylin-eosin staining and, K, Masson staining indicate hypertrophy and disorderly arrangement of cardiomyocytes with severe interstitial myocardial fibrosis. bSSFP = balanced steady-state free precession, HCM = hypertrophic cardiomyopathy, LGE = late gadolinium enhancement, LV = left ventricular.
Figure 3a:

Representative case of typical HCM in a 52-year-old man. (a) Two-chamber, (b) four-chamber, and (c) three-chamber views of bSSFP cine images show asymmetric ventricular septal and LV apical hypertrophy without LV outlet obstruction. Both left and right atria are normal size without pericardial effusion. (d) Two-chamber, (e) four-chamber, and (f) midshort axis view LGE images show only patchy myocardial fibrosis in the interventricular septum. bSSFP = balanced steady-state free precession, HCM = hypertrophic cardiomyopathy, LGE = late gadolinium enhancement, LV = left ventricular.
Table 2:
Comparison of the Major Left Ventricular Function Parameters in Two HCM Groups
Figure 3b:

Representative case of typical HCM in a 52-year-old man. (a) Two-chamber, (b) four-chamber, and (c) three-chamber views of bSSFP cine images show asymmetric ventricular septal and LV apical hypertrophy without LV outlet obstruction. Both left and right atria are normal size without pericardial effusion. (d) Two-chamber, (e) four-chamber, and (f) midshort axis view LGE images show only patchy myocardial fibrosis in the interventricular septum. bSSFP = balanced steady-state free precession, HCM = hypertrophic cardiomyopathy, LGE = late gadolinium enhancement, LV = left ventricular.
Figure 3c:

Representative case of typical HCM in a 52-year-old man. (a) Two-chamber, (b) four-chamber, and (c) three-chamber views of bSSFP cine images show asymmetric ventricular septal and LV apical hypertrophy without LV outlet obstruction. Both left and right atria are normal size without pericardial effusion. (d) Two-chamber, (e) four-chamber, and (f) midshort axis view LGE images show only patchy myocardial fibrosis in the interventricular septum. bSSFP = balanced steady-state free precession, HCM = hypertrophic cardiomyopathy, LGE = late gadolinium enhancement, LV = left ventricular.
Figure 3d:
Representative case of typical HCM in a 52-year-old man. (a) Two-chamber, (b) four-chamber, and (c) three-chamber views of bSSFP cine images show asymmetric ventricular septal and LV apical hypertrophy without LV outlet obstruction. Both left and right atria are normal size without pericardial effusion. (d) Two-chamber, (e) four-chamber, and (f) midshort axis view LGE images show only patchy myocardial fibrosis in the interventricular septum. bSSFP = balanced steady-state free precession, HCM = hypertrophic cardiomyopathy, LGE = late gadolinium enhancement, LV = left ventricular.
Figure 3e:

Representative case of typical HCM in a 52-year-old man. (a) Two-chamber, (b) four-chamber, and (c) three-chamber views of bSSFP cine images show asymmetric ventricular septal and LV apical hypertrophy without LV outlet obstruction. Both left and right atria are normal size without pericardial effusion. (d) Two-chamber, (e) four-chamber, and (f) midshort axis view LGE images show only patchy myocardial fibrosis in the interventricular septum. bSSFP = balanced steady-state free precession, HCM = hypertrophic cardiomyopathy, LGE = late gadolinium enhancement, LV = left ventricular.
Figure 3f:

Representative case of typical HCM in a 52-year-old man. (a) Two-chamber, (b) four-chamber, and (c) three-chamber views of bSSFP cine images show asymmetric ventricular septal and LV apical hypertrophy without LV outlet obstruction. Both left and right atria are normal size without pericardial effusion. (d) Two-chamber, (e) four-chamber, and (f) midshort axis view LGE images show only patchy myocardial fibrosis in the interventricular septum. bSSFP = balanced steady-state free precession, HCM = hypertrophic cardiomyopathy, LGE = late gadolinium enhancement, LV = left ventricular.
Outcomes
The mean follow-up period was 72 months (range, 4–85 months) for HCM with the restrictive group and 91 months (range, 9–93 months) for the control group. During the follow-up period, five patients in the restrictive phenotype group (sudden death in one patient, heart failure–related death in one patient, two patients underwent cardiac transplantation, and one patient died of cerebral thrombosis) and one patient in the control group (heart failure–related death in one patient) reached the primary end points. Figure 4 shows Kaplan-Meier survival curves for all-cause mortality and cardiac transplantation-free survival in the two groups, which showed patients with HCM with restrictive phenotype had substantially shorter survival (log-rank test: P = .018). The 5-year event-free survival from any cause of death and cardiac transplantation was 81% (95% CI: 62%, 99%) in patients with HCM with restrictive phenotype, compared with 94% (95% CI: 84%, 100%) in the control group. For all cardiovascular event-free survival analysis, 11 patients (32%) with restrictive phenotype reached secondary end points, and eight patients (24%) in the control group reached secondary end points (log-rank test: P = .014). Event-free survival (freedom from death, heart failure, and implantable cardioverter defibrillator implantation) is shown in Figure 5. The 5-year event-free survival was only 49% (95% CI: 30%, 68%) compared with 75% (95% CI: 57%, 91%) in the control group. In univariate Cox regression analyses (Table 3), LGE, atrial fibrillation, and left atrial anteroposterior diameter showed significantly predictive associations with HCM-related adverse event end points.
Figure 4:
Kaplan-Meier estimates of the proportion of patients with all-cause mortality and heart transplantation-free survival in the 34 patients with restrictive phenotype of HCM and 34 patients in the control group. HCM = hypertrophic cardiomyopathy.
Figure 5:
Kaplan-Meier estimates of the proportion of patients with cardiovascular event-free survival in the 34 patients with restrictive phenotype of HCM and 34 patients in the control group. HCM = hypertrophic cardiomyopathy.
Table 3:
Results of Univariate and Multivariate Analyses in Prediction of the Outcome End Points for HCM-related Adverse Events

Intra- and Interobserver Variability
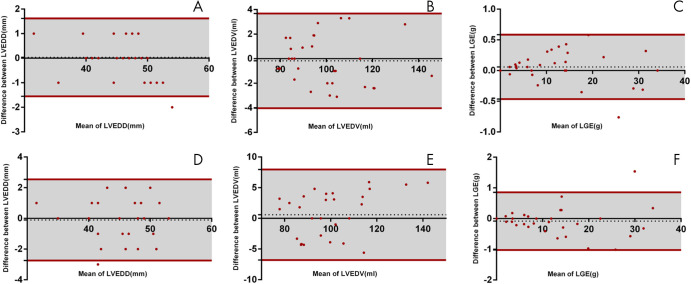
LVEDD, LVEDV, and LGE had an intraobserver variability of 0.03 mm ± 0.81, 0.17 mL ± 1.97, and 0.06 g ± 0.27, respectively, and an interobserver variability of 0.10 mm ± 1.35, 0.58 mL ± 3.77, and 0.08 g ± 0.48, respectively (Fig 6). Maximum wall thickness, left atrial anteroposterior diameter, and right atrial anteroposterior diameter had an intraobserver variability of 0.05 mm ± 0.22, 0.07 mm ± 0.12, and 0.15 mm ± 0.24, respectively, and an interobserver variability of 0.07 mm ± 0.22, 0.12 mm ± 0.23, and 0.19 mm ± 0.66, respectively.
Figure 6:
Bland-Altman plots display bias in intraobserver (top) and interobserver (bottom) agreement in, A, D, LVEDD, B, E, LVEDV, and C, F, LGE for 20 randomly selected patients (10 from restrictive group and 10 from control group), respectively. Green dashed line = bias; dark red solid line = 95% limits of agreement between intra- and interobserver variability. LGE = late gadolinium enhancement, LVEDD = left ventricular end-diastolic diameter, LVEDV = left ventricular end-diastolic volume.
Discussion
The main findings of our study revealed that HCM with restrictive phenotype is a rare variant (1.3% in a large cohort of patients with HCM) with MRI characteristics compatible with typical HCM, but in particular showing enlarged atria with mild to moderate LV hypertrophy, moderate myocardial fibrosis, and usually with mild to moderate pericardial and/or pleural effusion. The patients with restrictive phenotype have not only severe clinical symptoms but also a poor prognosis. Patients with restrictive phenotype are characterized by severe diastolic dysfunction conforming the restrictive physiology, which is one of the most obvious characteristics in these patients. Diastolic dysfunction is considered as one of the most important pathophysiologic consequences of HCM because of disturbed calcium kinetics, ischemia, LV hypertrophy, and fibrosis (18). In the current study, the diameters of both atria in restrictive phenotype were markedly larger than those in typical patients with HCM, which may reflect the manifestation of heart failure with preserved ejection fraction. In our study, the patients with restrictive physiology had more severe clinical symptoms, such as syncope, more frequent pericardial effusions, and pleural effusions. More than half of the patients with restrictive phenotype were in NYHA class III–IV, severely impairing their quality of life. In contrast, most patients in the control group were in NYHA class I (only four patients in NYHA class III and none in NYHA class IV). The genetic background of HCM with restrictive physiology is largely unknown (7,19). This particular HCM with restrictive phenotype accounts for about 1.1% of all HCM reported by Kubo et al (7), and 1.3% in our HCM cohort. The differentiation between the restrictive phenotype of HCM and specific restrictive cardiomyopathies is often challenging in a routine clinical setting. The following points may be helpful in identifying them: (a) asymmetric myocardial hypertrophy is more suggestive of HCM, (b) some specific locations of LGE, such as the anterior and posterior right ventricular insertion points are more likely to be HCM (28), and (c) patients with HCM frequently have an increase in the number of papillary muscles and other morphologic features (29).
LGE is currently widely used in vivo to identify scar or myocardial fibrosis in ischemic and nonischemic cardiomyopathy, which is highly correlated with malignant arrhythmia and sudden death (20–22). It has been reported that the incidence of ventricular tachycardia in patients with LGE-positive HCM is seven times higher than that of patients who are LGE-negative (23). The 5-year event-free survival from any cause of death and cardiac transplantation was 81%. The 5-year event-free survival from death, heart failure, and implantable cardioverter defibrillator implantation was 49%. Although the LVEF of the patients did not decrease, the patients with HCM with restrictive phenotype showed moderate myocardial fibrosis (LGE percentage: 15% ± 8), and the outcomes in our study were compatible with previous reports (7).
HCM is generally associated with relatively benign outcomes (4). However, patients with restrictive phenotype had a poor prognosis; the 5-year event-free survival rate from any cause of death and cardiac transplantation was 81%. In this study, univariate Cox regression analysis showed that atrial fibrillation and left atrial anteroposterior diameter showed significantly predictive associations with HCM-related adverse event end points. It was suggested that arrhythmias, such as atrial fibrillation, are the main cause of adverse events in patients with HCM with restrictive phenotype. Left atrial diameter and volume were the most strongly associated with atrial fibrillation (24). An increased ventricular pressure and the secondary enlargement of the atria may lead to paroxysmal or even sustained atrial fibrillation (25). Atrial fibrillation results in loss of atrial contribution to ventricular filling, which may be one of the reasons for worse NYHA function in these patients (26,27). In addition to increasing the risk of progression of heart failure, atrial fibrillation affects patients with hypertrophic cardiomyopathy by increasing the risk of embolic stroke (prevalence 6%, incidence 0.8% per year) (25). This finding is similar to our results; one patient in our restrictive group died of cerebral thrombosis.
Our study had some limitations. It is important to perform T1 mapping in both restrictive phenotype and control groups because interstitial fibrosis may differ between them and is helpful to explain the restrictive physiology. Phase contrast flow velocity-encoded cine was not performed in this study to quantify ventricular diastolic function. Moreover, we did not perform genetic analysis of the patients. Most patients with HCM included in this study were considered to be sporadic cases and gene screening of families was lacking. Although MRI results can diagnose HCM with restrictive phenotype alone, only patients with LV hypertrophy could be identified (30). Thus, some patients with HCM without LV hypertrophy may have been misdiagnosed as restrictive cardiomyopathy.
In conclusion, the restrictive phenotype may be a special phenotype or subgroup of HCM. The incidence of this phenotype is relatively low. The MRI features include mild to moderate LV hypertrophy, nonobstruction of LV outflow tract with enlarged atria, and ventricles of normal or small size with moderate myocardial fibrosis. These patients often have severe clinical symptoms of impaired cardiac function and a higher incidence of atrial fibrillation, which may result in a poor prognosis.
Supported in part by the Clinical and Translational Fund of Chinese Academy of Medical Sciences (2019XK320063), Capital’s Funds for Health Improvement and Research (CFH 2020-2-4034), Construction Research Project of Key Laboratory (Cultivation) of Chinese Academy of Medical Sciences (2019PT310025), Research Grant of National Natural Science Foundation of China (81771811 and 81971588), and Capital Clinically Characteristic Applied Research Fund (Z191100006619021).
Disclosures of Conflicts of Interest: S.L. Activities related to the present article: institution received grant from National Natural Science Foundation of China (81571647, 81620108015 and 81771811). Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. B.W. disclosed no relevant relationships. G.Y. disclosed no relevant relationships. L.S. disclosed no relevant relationships. Y.J. disclosed no relevant relationships. J.H. disclosed no relevant relationships. S.Z. disclosed no relevant relationships. M.L. disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- CO
- cardiac output
- HCM
- hypertrophic cardiomyopathy
- LGE
- late gadolinium enhancement
- LV
- left ventricle
- LVEDD
- left ventricular end-diastolic diameter
- LVEDV
- left ventricular end-diastolic volume
- LVEF
- left ventricular ejection fraction
- LVESV
- left ventricular end-systolic volume
- NYHA
- New York Heart Association
- RVEDD
- right ventricular end-diastolic diameter
References
- 1.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet 2013;381(9862):242–255. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ. Sudden death in young athletes. N Engl J Med 2003;349(11):1064–1075. [DOI] [PubMed] [Google Scholar]
- 3.Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011;58(25):e212–e260. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Rowin EJ, Casey SA, et al. Hypertrophic Cardiomyopathy in Adulthood Associated With Low Cardiovascular Mortality With Contemporary Management Strategies. J Am Coll Cardiol 2015;65(18):1915–1928. [DOI] [PubMed] [Google Scholar]
- 5.Mogensen J, Kubo T, Duque M, et al. Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J Clin Invest 2003;111(2):209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piotrowska J, Bilińska ZT, Michalak E, Ruzyłło W, Rydlewska-Sadowska W. Atypical forms of hypertrophic cardiomyopathy [in Polish]. Kardiol Pol 1991;34(4):207–217. [PubMed] [Google Scholar]
- 7.Kubo T, Gimeno JR, Bahl A, et al. Prevalence, clinical significance, and genetic basis of hypertrophic cardiomyopathy with restrictive phenotype. J Am Coll Cardiol 2007;49(25):2419–2426. [DOI] [PubMed] [Google Scholar]
- 8.Authors/Task Force members , Elliot PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35(39):2733–2779. [DOI] [PubMed] [Google Scholar]
- 9.Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA; Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 2002;15(2):167–184. [DOI] [PubMed] [Google Scholar]
- 10.Patel AR, Kramer CM. Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Nonischemic Cardiomyopathy. JACC Cardiovasc Imaging 2017;10(10 Pt A):1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu M, Du H, Gao Z, et al. Predictors of Outcome After Alcohol Septal Ablation for Hypertrophic Obstructive Cardiomyopathy: An Echocardiography and Cardiovascular Magnetic Resonance Imaging Study. Circ Cardiovasc Interv 2016;9(3):e002675. [DOI] [PubMed] [Google Scholar]
- 12.Lu M, Zhao S, Jiang S, et al. Fat deposition in dilated cardiomyopathy assessed by CMR. JACC Cardiovasc Imaging 2013;6(8):889–898. [DOI] [PubMed] [Google Scholar]
- 13.Fattori R, Biagini E, Lorenzini M, Buttazzi K, Lovato L, Rapezzi C. Significance of magnetic resonance imaging in apical hypertrophic cardiomyopathy. Am J Cardiol 2010;105(11):1592–1596. [DOI] [PubMed] [Google Scholar]
- 14.Harrigan CJ, Peters DC, Gibson CM, et al. Hypertrophic cardiomyopathy: quantification of late gadolinium enhancement with contrast-enhanced cardiovascular MR imaging. Radiology 2011;258(1):128–133. [DOI] [PubMed] [Google Scholar]
- 15.Maron MS. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2012;14(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maron BJ, Olivotto I, Spirito P, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 2000;102(8):858–864. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, Wolf PA, Castelli WP, D’Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA 1987;258(9):1183–1186. [PubMed] [Google Scholar]
- 18.Braunwald E, Seidman CE, Sigwart U. Contemporary evaluation and management of hypertrophic cardiomyopathy. Circulation 2002;106(11):1312–1316. [DOI] [PubMed] [Google Scholar]
- 19.Arad M, Seidman JG, Seidman CE. Phenotypic diversity in hypertrophic cardiomyopathy. Hum Mol Genet 2002;11(20):2499–2506. [DOI] [PubMed] [Google Scholar]
- 20.Elliott PM, Poloniecki J, Dickie S, et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol 2000;36(7):2212–2218. [DOI] [PubMed] [Google Scholar]
- 21.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 2000;342(24):1778–1785. [DOI] [PubMed] [Google Scholar]
- 22.Geske JB, Ommen SR. Role of imaging in evaluation of sudden cardiac death risk in hypertrophic cardiomyopathy. Curr Opin Cardiol 2015;30(5):493–499. [DOI] [PubMed] [Google Scholar]
- 23.Adabag AS, Maron BJ, Appelbaum E, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol 2008;51(14):1369–1374. [DOI] [PubMed] [Google Scholar]
- 24.Vaidya K, Semsarian C, Chan KH. Atrial Fibrillation in Hypertrophic Cardiomyopathy. Heart Lung Circ 2017;26(9):975–982. [DOI] [PubMed] [Google Scholar]
- 25.Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001;104(21):2517–2524. [DOI] [PubMed] [Google Scholar]
- 26.Lele SS, Thomson HL, Seo H, Belenkie I, McKenna WJ, Frenneaux MP. Exercise capacity in hypertrophic cardiomyopathy. Role of stroke volume limitation, heart rate, and diastolic filling characteristics. Circulation 1995;92(10):2886–2894. [DOI] [PubMed] [Google Scholar]
- 27.Sharma S, Elliott P, Whyte G, et al. Utility of cardiopulmonary exercise in the assessment of clinical determinants of functional capacity in hypertrophic cardiomyopathy. Am J Cardiol 2000;86(2):162–168. [DOI] [PubMed] [Google Scholar]
- 28.Maron MS, Appelbaum E, Harrigan CJ, et al. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail 2008;1(3):184–191. [DOI] [PubMed] [Google Scholar]
- 29.Harrigan CJ, Appelbaum E, Maron B, et al. Significance of papillary muscle abnormalities identified by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol 2008;101(5):668–673. [DOI] [PubMed] [Google Scholar]
- 30.Soor GS, Chakrabarti MO, Siddiqui RF, et al. Hypertrophic cardiomyopathy presenting as restrictive cardiomyopathy: a case complicated by biventricular apical aneurysms and papillary fibroelastoma. Cardiovasc Pathol 2009;18(5):308–312. [DOI] [PubMed] [Google Scholar]