Abstract
Biatrial drainage of the right superior vena cava (SVC) is a rare form of interatrial shunting that can have substantial clinical consequences. Cross-sectional imaging techniques (CT and MRI) are well suited for evaluation and surgical planning. This review article focuses on the embryologic development, hemodynamics, and imaging features to enable a timely diagnosis. Biatrial drainage of the right SVC has important clinical implications, and knowledge of its imaging appearance and hemodynamics is essential in diagnosis and treatment planning.
© RSNA, 2020
Summary
Biatrial (or left atrial) drainage of the right superior vena cava is a rare but important cause of intracardiac left-to-right or right-to-left shunts and accurately diagnosed at CT and cardiac MRI examinations.
Essentials
■ Biatrial drainage of the right superior vena cava (SVC) is a rare form of interatrial shunting that can lead to either right-to-left or left-to-right shunts with associated symptoms.
■ Cross-sectional imaging techniques such as CT and cardiac MRI are well suited to diagnose biatrial drainage of the right SVC and differentiate it from other similar interatrial shunts.
■ Imaging features of this defect and other associated cardiac abnormalities can help in planning for precise transcatheter or surgical interventions.
Introduction
Biatrial (or left atrial) drainage of the right superior vena cava (SVC) is a rare form of interatrial communication caused by a defect in the wall between the right SVC and the right upper and/or middle pulmonary vein (1,2). The pulmonary vein maintains its normal connection to the left atrium, which may allow a left-to-right shunt from the left atrium through the right upper pulmonary vein into the right atrium (Fig 1a). If the defect is associated with stenosis or atresia of the right SVC orifice to the right atrium, a predominantly right-to-left shunt ensues with systemic venous return from the right SVC directed through the defect into the right upper pulmonary vein and into the left atrium (Fig 1b). This type of defect, described as biatrial drainage of the right SVC (3), cavopulmonary venous defect, or venovenous bridge (1), is not a true atrial septal defect, but an interatrial communication (due to a venovenous connection [1]) from the right SVC to the right upper pulmonary vein and is closely related to the more common SVC-type sinus venosus defect (1–3). The earliest described case of this defect was published in 1914 (4); Van Praagh et al later described three cases and reviewed findings from 26 previously published cases (3). It is important to recognize this entity on cross-sectional imaging and to understand its hemodynamic implications so that it can be managed appropriately.
Figure 1a:

(a,b) Diagrams of the defect between the right superior vena cava (SVC, arrowhead) and the right upper pulmonary vein (arrows). The right upper pulmonary vein continues its normal course and connects to the left atrium. Anterior walls of the right and left atria are removed, showing the intact true atrial septum. Mechanism of left-to-right shunt is shown in (a) with pulmonary venous return from the left atrium flowing retrograde through the right upper pulmonary vein (red arrow), through the defect, and into the right atrium. Pulmonary venous return from the right upper pulmonary vein is also shown joining the red arrow going into the right atrium. (b) Stenosis of the SVC orifice to the right atrium leads to a predominantly right-to-left shunt. In this case, systemic venous return from the right SVC flows through the defect (blue arrow) and into the right upper pulmonary vein, joining pulmonary venous return from the right upper pulmonary vein (red arrow) and shunting into the left atrium (purple arrow).
Figure 1b:
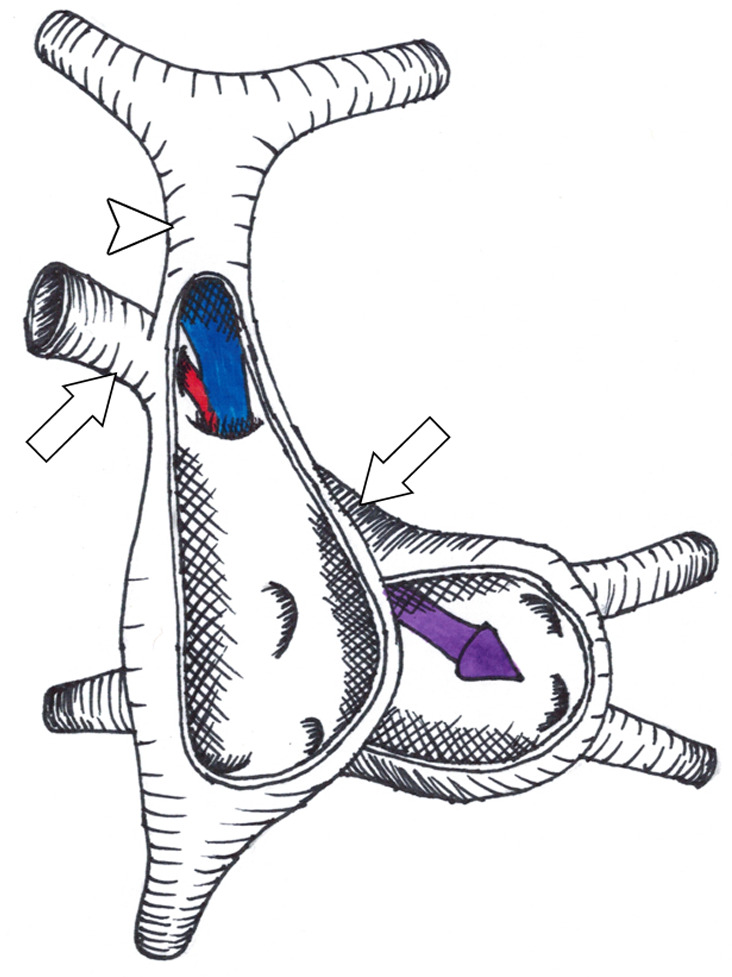
(a,b) Diagrams of the defect between the right superior vena cava (SVC, arrowhead) and the right upper pulmonary vein (arrows). The right upper pulmonary vein continues its normal course and connects to the left atrium. Anterior walls of the right and left atria are removed, showing the intact true atrial septum. Mechanism of left-to-right shunt is shown in (a) with pulmonary venous return from the left atrium flowing retrograde through the right upper pulmonary vein (red arrow), through the defect, and into the right atrium. Pulmonary venous return from the right upper pulmonary vein is also shown joining the red arrow going into the right atrium. (b) Stenosis of the SVC orifice to the right atrium leads to a predominantly right-to-left shunt. In this case, systemic venous return from the right SVC flows through the defect (blue arrow) and into the right upper pulmonary vein, joining pulmonary venous return from the right upper pulmonary vein (red arrow) and shunting into the left atrium (purple arrow).
In this article we will describe the embryologic development, hemodynamics, and imaging features that differentiate biatrial drainage of the right SVC from other more common interatrial communications and shunts.
Embryologic Development
Biatrial drainage of the right SVC does not result from a defect of the atrial septum. As discussed above, it is an interatrial communication caused by a defect between the right SVC and the right upper pulmonary vein. Thus, it is a unique subtype of an SVC-type sinus venosus defect (1,3). The embryologic basis for sinus venosus defect is not well understood. The development of the right SVC and pulmonary veins occur through separate but related embryologic processes (1,5). One theory proposes that abnormal development of the right horn of the sinus venosus, “with relative leftward and cephalic distortion” (6), aligns the SVC into the left atrium. However, this theory does not explain the defect between the right SVC and right upper pulmonary vein. Furthermore, these defects can occur with normal development of the true atrial septum. It has been proposed that involution (or lack of formation) of the wall tissue between the posterior wall of the right SVC and anterior wall of the right upper pulmonary vein leads to the defect (or “unroofing”) after both vessels have independently formed (2,3,5), and the pulmonary veins retain their normal anatomic connections to the left atrium. An alternative anatomic explanation states that these defects arise from persistent embryonic pulmonary-to-systemic venous connections (described as venovenous bridges [1]) that abnormally persist beyond fetal development. Some cases of biatrial (or left atrial) drainage of the right SVC are associated with stenosis or atresia of the right SVC orifice to the right atrium. One proposed explanation is that blood flow may predominate from the right SVC to the left atrium in these cases in fetal life (due to lower fetal left atrial pressure) (3,7), leading to decreased flow and eventually stenosis or atresia of the right SVC orifice to the right atrium (7).
Clinical Presentation
Biatrial or left atrial drainage of the right SVC is an overall rare defect, with fewer than 40 patients reported in medical literature (3,8–11). Age of presentation can vary widely, ranging from neonates to adults. While most previous case reports and series show only a rare association with other congenital heart defects (1,3), some cases may be associated with complex congenital heart disease. Some patients may have clinically evident cyanosis or arterial desaturation.
The clinical presentation can be variable and depends on multiple factors, such as the size of the right SVC orifice (which in turn determines the direction and degree of shunting), presence of bilateral SVCs (which allows a separate route of systemic venous return to the right atrium, as seen in Fig 2), and other associated congenital heart defects. If a right-to-left shunt predominates, the patient may present first with cyanosis. Previously reported cases of patients with biatrial or left atrial drainage of the right SVC describe sequelae of polycythemia, shortness of breath, dyspnea, decreased exercise tolerance, and systemic emboli (3), including cerebrovascular events and brain abscesses (such as with the case in Fig 2).
Figure 2a:

Images in a 58-year-old man with biatrial drainage of the right superior vena cava (SVC), bilateral SVCs (left SVC draining to a dilated coronary sinus), small bridging innominate vein, and pulmonary artery hypertension. (a, b) Electrocardiographically gated steady-state free precession cine MR images in axial plane show right SVC (white arrowhead) and left SVC (black arrowhead). (a) The right upper and middle pulmonary veins (arrow) course posterior to the right SVC connecting normally to the left atrium (LA). The defect between the right SVC and right pulmonary veins is shown between the thick arrowhead and arrow. (b) Axial image which is more caudal to the defect and shows the inferior right SVC (white arrowhead) coursing to the right atrium and the right lower pulmonary vein (arrow) connecting to the LA. (c) Oblique coronal reformat and (d) volume-rendered reconstruction from gadolinium-enhanced MR angiography show right and left SVCs (black arrowhead), as well as the right upper and middle pulmonary veins (arrows) joining the right SVC through the defect. The pulmonary veins connect normally to the LA. The right SVC inferior to the defect (white arrowhead) is normal size and connects to the right atrium.
Figure 2c:

Images in a 58-year-old man with biatrial drainage of the right superior vena cava (SVC), bilateral SVCs (left SVC draining to a dilated coronary sinus), small bridging innominate vein, and pulmonary artery hypertension. (a, b) Electrocardiographically gated steady-state free precession cine MR images in axial plane show right SVC (white arrowhead) and left SVC (black arrowhead). (a) The right upper and middle pulmonary veins (arrow) course posterior to the right SVC connecting normally to the left atrium (LA). The defect between the right SVC and right pulmonary veins is shown between the thick arrowhead and arrow. (b) Axial image which is more caudal to the defect and shows the inferior right SVC (white arrowhead) coursing to the right atrium and the right lower pulmonary vein (arrow) connecting to the LA. (c) Oblique coronal reformat and (d) volume-rendered reconstruction from gadolinium-enhanced MR angiography show right and left SVCs (black arrowhead), as well as the right upper and middle pulmonary veins (arrows) joining the right SVC through the defect. The pulmonary veins connect normally to the LA. The right SVC inferior to the defect (white arrowhead) is normal size and connects to the right atrium.
Figure 2d:

Images in a 58-year-old man with biatrial drainage of the right superior vena cava (SVC), bilateral SVCs (left SVC draining to a dilated coronary sinus), small bridging innominate vein, and pulmonary artery hypertension. (a, b) Electrocardiographically gated steady-state free precession cine MR images in axial plane show right SVC (white arrowhead) and left SVC (black arrowhead). (a) The right upper and middle pulmonary veins (arrow) course posterior to the right SVC connecting normally to the left atrium (LA). The defect between the right SVC and right pulmonary veins is shown between the thick arrowhead and arrow. (b) Axial image which is more caudal to the defect and shows the inferior right SVC (white arrowhead) coursing to the right atrium and the right lower pulmonary vein (arrow) connecting to the LA. (c) Oblique coronal reformat and (d) volume-rendered reconstruction from gadolinium-enhanced MR angiography show right and left SVCs (black arrowhead), as well as the right upper and middle pulmonary veins (arrows) joining the right SVC through the defect. The pulmonary veins connect normally to the LA. The right SVC inferior to the defect (white arrowhead) is normal size and connects to the right atrium.
If a left-to-right shunt predominates, then the patient may present instead with clinical symptoms typical of most interatrial communications. Patients are usually asymptomatic in young age with a soft systolic ejection murmur and possibly a widely fixed split-second heart sound (12). Older children with a large left-to-right shunt may complain of fatigue and dyspnea or remain asymptomatic. Growth failure and other signs of high-output heart failure are very uncommon. Occasionally, this defect may remain undiagnosed until adulthood and may be found incidentally on imaging studies performed for other reasons (Fig 3). Hence, it becomes important for imagers to be aware of this diagnosis and its imaging features. Adult patients can develop atrial arrhythmias (such as atrial flutter or atrial fibrillation) due to right atrial dilatation. A small proportion (6%–16%) of patients with interatrial communications, predominantly female (12–14), can develop pulmonary vascular disease (Figs 3, 4). These patients have substantially decreased long-term survival and increased perioperative mortality.
Figure 3a:

Images in a 67-year-old woman with pulmonary arterial hypertension presenting for pulmonary embolism evaluation due to dyspnea. (a–c) Axial contrast-enhanced chest CT images in a cranial-to-caudal direction. The right superior vena cava (arrowhead) is well opacified with shunting of contrast material through the defect (shown in b) into the right upper pulmonary vein (arrow) and left atrium (LA). Note the lack of enhancement of the right upper pulmonary vein just cranial to the defect (a). All pulmonary veins connected normally to the LA. (d) The defect is also shown in an oblique coronal reformat.
Figure 4a:

Images in a 64-year-old woman with biatrial drainage of the right superior vena cava (SVC) and mildly dilated right ventricle. (a) Oblique sagittal and (b) axial reformat from electrocardiogram and respiratory navigator-gated three-dimensional steady-state free precession MRI sequence show defect between right upper pulmonary vein and right SVC (between arrow and arrowhead). Note how the right SVC appears to override the atrial septum and connects to both atria. Axial image in (b) shows right SVC (arrowhead) and right upper pulmonary vein (arrow), with the defect superior to the plane of imaging. (c) Color-coded image from phase-contrast MRI sequence in similar plane as (b) shows cranially directed flow in the right upper pulmonary vein (arrow), opposite to the SVC flow direction (arrowhead). The flow pattern is consistent with a left-to-right shunt with retrograde flow from the left atrium (LA) through the right upper pulmonary vein (arrow). The calculated pulmonary-to-systemic flow ratio was 1.2:1.
Figure 3b:

Images in a 67-year-old woman with pulmonary arterial hypertension presenting for pulmonary embolism evaluation due to dyspnea. (a–c) Axial contrast-enhanced chest CT images in a cranial-to-caudal direction. The right superior vena cava (arrowhead) is well opacified with shunting of contrast material through the defect (shown in b) into the right upper pulmonary vein (arrow) and left atrium (LA). Note the lack of enhancement of the right upper pulmonary vein just cranial to the defect (a). All pulmonary veins connected normally to the LA. (d) The defect is also shown in an oblique coronal reformat.
Figure 3c:

Images in a 67-year-old woman with pulmonary arterial hypertension presenting for pulmonary embolism evaluation due to dyspnea. (a–c) Axial contrast-enhanced chest CT images in a cranial-to-caudal direction. The right superior vena cava (arrowhead) is well opacified with shunting of contrast material through the defect (shown in b) into the right upper pulmonary vein (arrow) and left atrium (LA). Note the lack of enhancement of the right upper pulmonary vein just cranial to the defect (a). All pulmonary veins connected normally to the LA. (d) The defect is also shown in an oblique coronal reformat.
Figure 3d:

Images in a 67-year-old woman with pulmonary arterial hypertension presenting for pulmonary embolism evaluation due to dyspnea. (a–c) Axial contrast-enhanced chest CT images in a cranial-to-caudal direction. The right superior vena cava (arrowhead) is well opacified with shunting of contrast material through the defect (shown in b) into the right upper pulmonary vein (arrow) and left atrium (LA). Note the lack of enhancement of the right upper pulmonary vein just cranial to the defect (a). All pulmonary veins connected normally to the LA. (d) The defect is also shown in an oblique coronal reformat.
Figure 4b:

Images in a 64-year-old woman with biatrial drainage of the right superior vena cava (SVC) and mildly dilated right ventricle. (a) Oblique sagittal and (b) axial reformat from electrocardiogram and respiratory navigator-gated three-dimensional steady-state free precession MRI sequence show defect between right upper pulmonary vein and right SVC (between arrow and arrowhead). Note how the right SVC appears to override the atrial septum and connects to both atria. Axial image in (b) shows right SVC (arrowhead) and right upper pulmonary vein (arrow), with the defect superior to the plane of imaging. (c) Color-coded image from phase-contrast MRI sequence in similar plane as (b) shows cranially directed flow in the right upper pulmonary vein (arrow), opposite to the SVC flow direction (arrowhead). The flow pattern is consistent with a left-to-right shunt with retrograde flow from the left atrium (LA) through the right upper pulmonary vein (arrow). The calculated pulmonary-to-systemic flow ratio was 1.2:1.
Figure 4c:

Images in a 64-year-old woman with biatrial drainage of the right superior vena cava (SVC) and mildly dilated right ventricle. (a) Oblique sagittal and (b) axial reformat from electrocardiogram and respiratory navigator-gated three-dimensional steady-state free precession MRI sequence show defect between right upper pulmonary vein and right SVC (between arrow and arrowhead). Note how the right SVC appears to override the atrial septum and connects to both atria. Axial image in (b) shows right SVC (arrowhead) and right upper pulmonary vein (arrow), with the defect superior to the plane of imaging. (c) Color-coded image from phase-contrast MRI sequence in similar plane as (b) shows cranially directed flow in the right upper pulmonary vein (arrow), opposite to the SVC flow direction (arrowhead). The flow pattern is consistent with a left-to-right shunt with retrograde flow from the left atrium (LA) through the right upper pulmonary vein (arrow). The calculated pulmonary-to-systemic flow ratio was 1.2:1.
Imaging Diagnosis
The earliest reported cases (4,9,10) of biatrial drainage of the right SVC were diagnosed at postmortem examination. In the early era of congenital heart disease treatment, diagnosis was made mainly by cardiac catheterization and invasive angiography. Today, echocardiography remains the primary method of diagnosing atrial septal defects and other interatrial communications (12). The combination of high-contrast US between septal tissue with blood pool and color Doppler visualization of blood flow allows rapid and precise evaluation of the atrial septum. Additionally, contrast-enhanced echocardiography using intravenous agitated saline can be used to visualize evidence of intracardiac shunts. A major limitation of echocardiography is inadequate visualization in patients with poor acoustic windows, particularly of the atrial septum, systemic veins, and pulmonary veins.
As biatrial drainage of the right SVC requires careful evaluation of the SVC (or bilateral SVCs), its relationship to the right-sided pulmonary veins, and connections of these veins to the left and right atria, noninvasive three-dimensional cross-sectional imaging techniques such as CT and cardiac MRI are well suited for definitive diagnosis. Cardiac MRI and CT allow exquisite definition of tortuous vessels and complex anatomic relationships in various multiplanar projections (15). These studies demonstrate the course of the pulmonary veins and also assess stenotic or atretic connection of the right SVC to the right atrium. Right atrial and right ventricular dilatation can be evaluated during the same examination, supporting evidence of a left-to-right interatrial communication (15–17). An advantage of cardiac MRI is the ability to assess hemodynamic data by quantifying blood flow with phase-contrast imaging and calculating the degree and direction of the shunt (16). Thus, a comprehensive assessment including detailed anatomic and physiologic data can be provided in a single cardiac MRI examination (16,17).
Imaging Features and Hemodynamics
The key imaging features of biatrial drainage of the right SVC include: (a) defect between the right SVC and the right upper (or middle) pulmonary vein (unroofing of the right pulmonary vein, or venovenous bridge) (Figs 1–5), (b) normal course and connection of the right pulmonary veins to the left atrium (Figs 1–5), and (c) in some cases, stenosis or atresia of the right SVC orifice to the right atrium leading to right SVC drainage into the left atrium (Fig 1b). The right SVC may connect to both atria or only the left atrium.
Figure 5a:

Images in a 40-year-old man with biatrial drainage of the right superior vena cava (SVC) and mildly dilated right ventricle (RV). The patient was incidentally noted to have dilated right heart chambers. Echocardiography revealed intact interatrial septum and normal pulmonary vein connections. MRI was performed to elucidate any underlying shunt. (a) Steady-state free precession cine MR image in four-chamber plane confirms dilated right heart chambers. (b, c) Sequential axial reformatted images (cranial to caudal) from gadolinium-enhanced MR angiography show right SVC (arrowhead), right upper pulmonary vein (arrow), and the defect between these structures (b). Note the normal connection of the right upper pulmonary vein (arrow) to the left atrium (LA) (c). (d) The defect between the mid SVC and adjacent right pulmonary vein (*) is also well seen on the volume-rendered reconstruction. The patient had a pulmonary-to-systemic flow ratio of 2:1 based on phase-contrast MRI.
Figure 5b:

Images in a 40-year-old man with biatrial drainage of the right superior vena cava (SVC) and mildly dilated right ventricle (RV). The patient was incidentally noted to have dilated right heart chambers. Echocardiography revealed intact interatrial septum and normal pulmonary vein connections. MRI was performed to elucidate any underlying shunt. (a) Steady-state free precession cine MR image in four-chamber plane confirms dilated right heart chambers. (b, c) Sequential axial reformatted images (cranial to caudal) from gadolinium-enhanced MR angiography show right SVC (arrowhead), right upper pulmonary vein (arrow), and the defect between these structures (b). Note the normal connection of the right upper pulmonary vein (arrow) to the left atrium (LA) (c). (d) The defect between the mid SVC and adjacent right pulmonary vein (*) is also well seen on the volume-rendered reconstruction. The patient had a pulmonary-to-systemic flow ratio of 2:1 based on phase-contrast MRI.
Figure 5c:

Images in a 40-year-old man with biatrial drainage of the right superior vena cava (SVC) and mildly dilated right ventricle (RV). The patient was incidentally noted to have dilated right heart chambers. Echocardiography revealed intact interatrial septum and normal pulmonary vein connections. MRI was performed to elucidate any underlying shunt. (a) Steady-state free precession cine MR image in four-chamber plane confirms dilated right heart chambers. (b, c) Sequential axial reformatted images (cranial to caudal) from gadolinium-enhanced MR angiography show right SVC (arrowhead), right upper pulmonary vein (arrow), and the defect between these structures (b). Note the normal connection of the right upper pulmonary vein (arrow) to the left atrium (LA) (c). (d) The defect between the mid SVC and adjacent right pulmonary vein (*) is also well seen on the volume-rendered reconstruction. The patient had a pulmonary-to-systemic flow ratio of 2:1 based on phase-contrast MRI.
Figure 5d:

Images in a 40-year-old man with biatrial drainage of the right superior vena cava (SVC) and mildly dilated right ventricle (RV). The patient was incidentally noted to have dilated right heart chambers. Echocardiography revealed intact interatrial septum and normal pulmonary vein connections. MRI was performed to elucidate any underlying shunt. (a) Steady-state free precession cine MR image in four-chamber plane confirms dilated right heart chambers. (b, c) Sequential axial reformatted images (cranial to caudal) from gadolinium-enhanced MR angiography show right SVC (arrowhead), right upper pulmonary vein (arrow), and the defect between these structures (b). Note the normal connection of the right upper pulmonary vein (arrow) to the left atrium (LA) (c). (d) The defect between the mid SVC and adjacent right pulmonary vein (*) is also well seen on the volume-rendered reconstruction. The patient had a pulmonary-to-systemic flow ratio of 2:1 based on phase-contrast MRI.
The defect between the right pulmonary veins and the right SVC creates a systemic-to-pulmonary venous connection, allowing pulmonary venous return (from the right pulmonary veins or left atrium) to flow through the defect into the right atrium (allowing a left-to-right shunt), or continuing its normal course into the left atrium. The defect between the right upper pulmonary vein and right SVC (and location of shunting) is located close to the SVC–right atrial junction in SVC-type (or superior) sinus venosus defects (1–3,7). In biatrial drainage of the right SVC, the defect is located relatively superior (or proximal) to the SVC–right atrial junction (Fig 2a, 2b) and sometimes associated with stenosis or atresia of the right SVC orifice to the right atrium (3), allowing drainage of systemic venous return from the right SVC to the left atrium (right-to-left shunt). Other major factors determining direction of shunt include (3,7): right and left atrial pressure, right and left ventricular compliance, atrioventricular valve regurgitation, and presence of other congenital heart defects (such as bilateral SVCs). The anatomic distinction between these two very similar defects can sometimes be blurred; indeed, a shared systemic-to-pulmonary venous connection (venovenous bridge) has been referred to as the forerunner of sinus venosus defects (1). Stenosis or atresia of the right SVC orifice to the right atrium should be evaluated in these cases. If a right-to-left shunt is present, this increases the risk for paradoxical embolism.
Figure 2b:

Images in a 58-year-old man with biatrial drainage of the right superior vena cava (SVC), bilateral SVCs (left SVC draining to a dilated coronary sinus), small bridging innominate vein, and pulmonary artery hypertension. (a, b) Electrocardiographically gated steady-state free precession cine MR images in axial plane show right SVC (white arrowhead) and left SVC (black arrowhead). (a) The right upper and middle pulmonary veins (arrow) course posterior to the right SVC connecting normally to the left atrium (LA). The defect between the right SVC and right pulmonary veins is shown between the thick arrowhead and arrow. (b) Axial image which is more caudal to the defect and shows the inferior right SVC (white arrowhead) coursing to the right atrium and the right lower pulmonary vein (arrow) connecting to the LA. (c) Oblique coronal reformat and (d) volume-rendered reconstruction from gadolinium-enhanced MR angiography show right and left SVCs (black arrowhead), as well as the right upper and middle pulmonary veins (arrows) joining the right SVC through the defect. The pulmonary veins connect normally to the LA. The right SVC inferior to the defect (white arrowhead) is normal size and connects to the right atrium.
Identification of the defect is straightforward on cross-sectional imaging. The differences in contrast opacification between systemic and pulmonary veins and abnormal mixing can provide a clue to the diagnosis (Fig 3), even on studies done for other reasons, and uncover this previously unrecognized abnormality. The direction of flow and shunt quantification can be assessed with cardiac MRI using phase-contrast imaging (15–17). By comparing flows (such as in the ascending aorta and main pulmonary artery), the relative pulmonary-to-systemic flow ratio (called the Qp:Qs ratio) can be calculated (16). If the SVC flow is measured, the plane of imaging should be carefully noted, as it may incorporate the left-to-right shunt (if inferior to the defect and its connection between the right upper pulmonary vein and right SVC). In a similar way, phase contrast imaging across the right upper pulmonary vein can demonstrate the shunt direction through the cardiac cycle (right-to-left, left-to-right, or bidirectional) and help quantify the net shunt flow (Fig 4).
Differential Diagnosis
As discussed above, biatrial drainage of the right SVC shares physiologic, and some anatomic, features with other interatrial communications. If suspicion for an interatrial communication arises (such as evidence of shunt on contrast-enhanced echocardiography), complete assessment of the atrial septum, pulmonary veins, and large systemic veins should reveal the source of interatrial communication or atrial level shunt.
Differential diagnosis includes SVC-type sinus venosus defects and partial anomalous pulmonary venous return (12,16). The relatively superior location of the defect to the SVC–right atrial junction differentiates this diagnosis from SVC-type sinus venosus defects (Fig 6) (3). Partial anomalous pulmonary venous connection can be diagnosed by determining the abnormal connection of the pulmonary vein to one of the systemic venous structures and the lack of the normal connection to the left atrium (Fig 7) (12,16).
Figure 6:

Image in a 40-year-old woman with superior sinus venosus defect. Note the location of the defect (*) near the cavoatrial junction on this axial cine steady-state free precession MR image.
Figure 7a:

(a, b) Contrast-enhanced axial chest CT images show partial anomalous pulmonary venous return of the right upper (white arrow, a), right lower (white arrow, b) and left lower pulmonary veins (black arrow, b) to the right atrium (RA). In contrast to biatrial drainage of the right superior vena cava, the anomalous pulmonary veins do not have any connection to the left atrium. (c) Normal connection of the left upper pulmonary vein (arrow) to the left atrium (LA).
Figure 7b:

(a, b) Contrast-enhanced axial chest CT images show partial anomalous pulmonary venous return of the right upper (white arrow, a), right lower (white arrow, b) and left lower pulmonary veins (black arrow, b) to the right atrium (RA). In contrast to biatrial drainage of the right superior vena cava, the anomalous pulmonary veins do not have any connection to the left atrium. (c) Normal connection of the left upper pulmonary vein (arrow) to the left atrium (LA).
Figure 7c:

(a, b) Contrast-enhanced axial chest CT images show partial anomalous pulmonary venous return of the right upper (white arrow, a), right lower (white arrow, b) and left lower pulmonary veins (black arrow, b) to the right atrium (RA). In contrast to biatrial drainage of the right superior vena cava, the anomalous pulmonary veins do not have any connection to the left atrium. (c) Normal connection of the left upper pulmonary vein (arrow) to the left atrium (LA).
Management
Once the exact anatomy is outlined, various interventions can be considered. These include transection of the right SVC superior to the defect and either anastomosis to the right atrial appendage (as described for repair of superior sinus venosus defects [2]) or end-to-side anastomosis to a left SVC. Depending on other associated congenital heart defects or size of the patient, the precise surgical intervention may differ. In one of our patients with bilateral SVCs and an intact bridging innominate vein (Fig 2), transcatheter device closure was attempted by occluding the right SVC orifice to the right atrium inferior to the unroofed right upper pulmonary vein, followed by device occlusion of the right SVC superior to the unroofed right upper pulmonary vein. However, device implantation was unsuccessful, and the patient instead underwent surgical ligation at these two points of the right SVC without cardiopulmonary bypass. This allowed unobstructed drainage of the right upper pulmonary vein to the left atrium and systemic venous drainage through the bridging innominate vein to the left SVC.
Conclusion
Biatrial (or left atrial) drainage of the right SVC is a rare but important form of interatrial communication and can lead to left-to-right or right-to-left shunts. Unique imaging features allow precise diagnosis with noninvasive cross-sectional imaging techniques such as CT and cardiac MRI and differentiation from other more common defects. Knowledge of the embryologic development, hemodynamics, associated defects, and imaging appearance is essential to understand the clinical effects, enable early detection, and plan interventions.
Disclosures of Conflicts of Interest: M.D.P. disclosed no relevant relationships. S.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author from UpToDate as annual payment for chapter written on tricuspid valve atresia. Other relationships: disclosed no relevant relationships. A.L.D. disclosed no relevant relationships. A.J. disclosed no relevant relationships. J.C.L. disclosed no relevant relationships. M.G.M. disclosed no relevant relationships. P.P.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed grants/grants pending to author’s institution for heart failure substudy for SPIROMICS and for Myokardia-sponsored study in hypertrophic cardiomyopathy; disclosed money paid to author from MRI Online for case review lecture done in June 2020.
Abbreviation:
- SVC
- superior vena cava
References
- 1.Butts RJ, Crean AM, Hlavacek AM, et al. Veno-venous bridges: the forerunners of the sinus venosus defect. Cardiol Young 2011;21(6):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Praagh S, Carrera ME, Sanders SP, Mayer JE, Van Praagh R. Sinus venosus defects: unroofing of the right pulmonary veins--anatomic and echocardiographic findings and surgical treatment. Am Heart J 1994;128(2):365–379. [DOI] [PubMed] [Google Scholar]
- 3.Van Praagh S, Geva T, Lock JE, Nido PJ, Vance MS, Van Praagh R. Biatrial or left atrial drainage of the right superior vena cava: anatomic, morphogenetic, and surgical considerations--report of three new cases and literature review. Pediatr Cardiol 2003;24(4):350–363. [DOI] [PubMed] [Google Scholar]
- 4.Nützel H. Beitrag zur Kenntnis der Missbildungen im Bereiche der oberen Hohlvene. Frankf Z Pathol 1914;15:1–19. [Google Scholar]
- 5.Blom NA, Gittenberger-de Groot AC, Jongeneel TH, DeRuiter MC, Poelmann RE, Ottenkamp J. Normal development of the pulmonary veins in human embryos and formulation of a morphogenetic concept for sinus venosus defects. Am J Cardiol 2001;87(3):305–309. [DOI] [PubMed] [Google Scholar]
- 6.Kirsch WM, Carlsson E, Hartmann AF Jr. A case of anomalous drainage of the superior vena cava into the left atrium. J Thorac Cardiovasc Surg 1961;41(4):550–556. [PubMed] [Google Scholar]
- 7.Shapiro EP, Al-Sadir J, Campbell NP, Thilenius OG, Anagnostopoulos CE, Hays P. Drainage of right superior vena cava into both atria. Review of the literature and description of a case presenting with polycythemia and paradoxical embolization. Circulation 1981;63(3):712–717. [DOI] [PubMed] [Google Scholar]
- 8.Alday LE, Maisuls H, De Rossi R. Right superior caval vein draining into the left atrium—diagnosis by color flow mapping. Cardiol Young 2008;5(4):345–349. [Google Scholar]
- 9.Bharati S, Lev M. Direct entry of the right superior vena cava into the left atrium with aneurysmal dilatation and stenosis at its entry into the right atrium with stenosis of the pulmonary veins: a rare case. Pediatr Cardiol 1984;5(2):123–126. [DOI] [PubMed] [Google Scholar]
- 10.Hackensellner HA. Abnormal drainage of the pulmonary vein into the superior vena cava [in German]. Virchows Arch Pathol Anat Physiol Klin Med 1955;327(5):603–606. [DOI] [PubMed] [Google Scholar]
- 11.Khoshhal S. Anomalous Connection of the Right Superior Vena Cava to the Left Atrium in a Child with Bilateral Superior Vena Cavae: An Unusual Cause of Cyanosis. Pediatr Cardiol 2019;40(1):226–229. [DOI] [PubMed] [Google Scholar]
- 12.Geva T, Martins JD, Wald RM. Atrial septal defects. Lancet 2014;383(9932):1921–1932. [DOI] [PubMed] [Google Scholar]
- 13.Steele PM, Fuster V, Cohen M, Ritter DG, McGoon DC. Isolated atrial septal defect with pulmonary vascular obstructive disease--long-term follow-up and prediction of outcome after surgical correction. Circulation 1987;76(5):1037–1042. [DOI] [PubMed] [Google Scholar]
- 14.Vogel M, Berger F, Kramer A, Alexi-Meshkishvili V, Lange PE. Incidence of secondary pulmonary hypertension in adults with atrial septal or sinus venosus defects. Heart 1999;82(1):30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crean A. Cardiovascular MR and CT in congenital heart disease. Heart 2007;93(12):1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beerbaum P, Parish V, Bell A, Gieseke J, Körperich H, Sarikouch S. Atypical atrial septal defects in children: noninvasive evaluation by cardiac MRI. Pediatr Radiol 2008;38(11):1188–1194. [DOI] [PubMed] [Google Scholar]
- 17.Weber OM, Higgins CB. MR evaluation of cardiovascular physiology in congenital heart disease: flow and function. J Cardiovasc Magn Reson 2006;8(4):607–617. [DOI] [PubMed] [Google Scholar]


