Abstract
BACKGROUND AND PURPOSE: It is essential to measure the skin dose of radiation received by patients during interventional neuroradiologic procedures performed under fluoroscopic guidance, such as embolization of cerebral aneurysms, which is regarded as a high-dose interventional radiology procedure. In this study, we report a method for evaluating maximum skin dose (MSD), an ideal marker of radiation-induced effects, based on an innovative use of radiochromic films.
MATERIALS AND METHODS: Forty-eight procedures were studied in 42 patients undergoing embolization of cerebral aneurysms. Fluoroscopic and digital dose-area product (DAP), fluoroscopy time, and total number of acquired images were recorded for all procedures. The MSD was measured using Gafchromic XR type R films.
RESULTS: The MSD was measured in one group of 21 procedures. The coefficient (κ) of the interpolation line between the skin dose and the DAP (κ = 0.0029 cm−2) was determined. An approximate value of MSD from the DAP for the remaining 27 procedures was estimated by means of an interpolation line. The mean MSD was found to be 1.16 Gy (range, 0.23–3.20 Gy).
CONCLUSION: The use of radiochromic XR type R films was shown to be an effective method for measuring MSD. These films have the advantage of supplying information on both the maximum dose and the distribution of the dose: this satisfies the most stringent interpretation of Food and Drug Administration, American College of Radiology, and international recommendations for recording skin dose.
The incidence of aneurysmal subarachnoid hemorrhage is 12 cases per 100,000 people per year in Europe and in the United States and reaches a peak of 20 cases per 100,000 per year in Japan. Aneurysms appear quite frequently during the first decade of life and become clinically evident over the years: more than 50% manifest between the ages of 40 and 60. Women are more frequently affected than men.1 The introduction of an endovascular coiling technique during the latest decade has significantly decreased morbidity and mortality in the treatment of survivors of a ruptured aneurysm with an efficacy comparable with that of neurosurgical interventions, as established by the International Subarachnoid Aneurysm Trial.2
Despite being less invasive, the endovascular procedure does expose patients to a not negligible dose of radiation, because every stage of the intervention is performed under fluoroscopic guidance. Each coil deployment is performed under fluoroscopic guidance; angiographic acquisitions from different projections, now also with 3D reformats, after each coil deployment are mandatory. Furthermore, an additional dose is usually necessary for follow-up diagnostic angiographic studies.
The Euratom 97/43 directive3 introduced the obligation to carry out a dosimetric evaluation for “high-dose practices,” including interventional radiology procedures; the US Food and Drug Administration4 requires the identification and registration of all procedures exposing patients to a maximum skin dose (MSD) of more than 1 Gy, and the International Commission on Radiological Protection (ICRP)5 has included embolization of cerebral aneurysms among high-dose procedures. The Society of Interventional Radiology (SIR) defines all these embolization procedures as potentially high-dose techniques.6
Materials and Methods
This observational study on patients was carried out in the context of a current research project approved by the Ethical Committee of Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS)-Policlinico San Matteo. Forty-eight procedures were studied in 42 patients (13 men; mean age, 68 years; range, 55–75 years; 29 women; mean age, 57 years; range, 21–81 years).
To establish the reference values for the procedures investigated, the following 3-step approach was used: 1) characterization of the x-ray system in terms of kinetic energy released in matter (KERMA) rate, including backscatter radiation, 2) characterization of the procedures in terms of dose-area product (DAP), the fluoroscopy time, and the total number of images acquired in cineradiography, and 3) measurement of the MSD using appropriately calibrated Gafchromic XR type R (International Specialty Products, Wayne, NJ) films placed in contact with the patient's occipital skin.
X-Ray System
The procedures for embolizing cerebral aneurysms were performed in the Department of Radiology of the IRCCS Policlinico San Matteo (Pavia, Italy) using 2 angiographic units: an Integris V3000 (unit 1) and an Allura 15 with a rotational program (unit 2) (Philips Medical Systems, Best, the Netherlands). The total filtration of unit 1 is 3 mm A1 in digital image acquisition mode and in fluoroscopy mode. Unit 1 has 3 fluoroscopy modes: a pulsed fluoroscopy mode and 2 different continuous modes. The total filtration of unit 2 is 3 mm A1 in digital image acquisition mode, and it varies in the 3 pulsed fluoroscopy modes: low (12.5 images/s; total filtration, 4 mm A1 +0.1 mm Copper [Cu]); normal (12.5 images/s; total filtration 3 mm A1); and high (25 images/s; total filtration, 4 mm Al +0.1 mm Cu). Unit 1 was used only as a backup when unit 2 was out of order.
To characterize these angiographic units, the dose rates (fluoroscopy) and doses per image (cine) are measured, including backscattering, at the entrance of polymethylmethacrylate phantoms of different thicknesses (16, 20, and 24 cm). The measurements were carried out using a ionization chamber (10 × 5–6 model) and radiation monitor (9015 model; Radcal, Monrovia, Calif). The measurements were repeated for different FOVs (17, 20, 25, 31, and 38 cm).
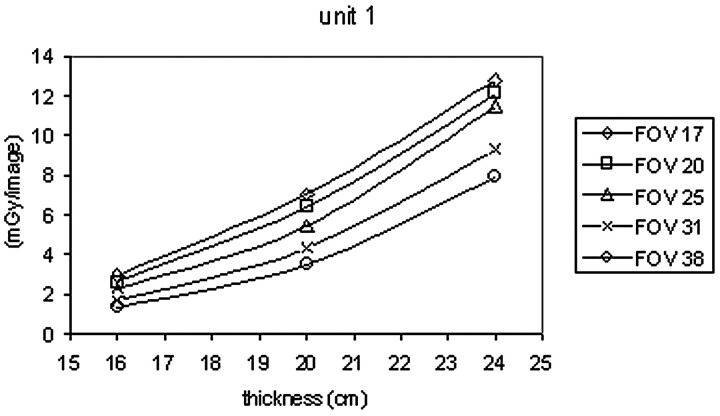
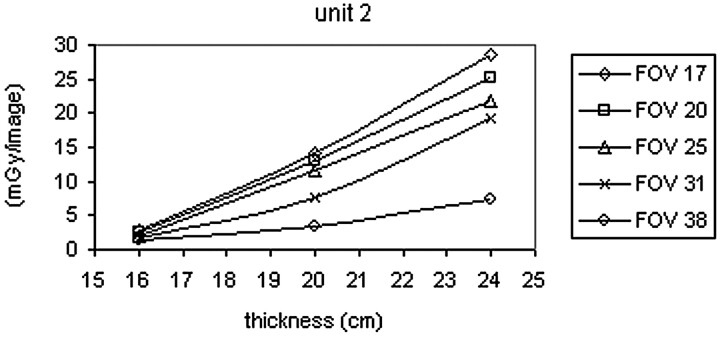
Enlargements with FOV of 17 and 20 cm are often used in cerebral embolization procedures to have a high resolution during the placement of the Guglielmi coils. Figs 1 and 2 show the trend in the dose in the cine mode as the FOV varies and the thickness of the phantom is changed. As the enlargement is increased, the dose entering the phantom rises because the amount of anodic current increases.
Fig 1.
Dose/image versus PMMA phantom thickness curves for angiographic unit 1.
Fig 2.
Dose/image versus PMMA phantom thickness curves for angiographic unit 2.
Both angiography systems included a transmission chamber (KermaX-plus-Air Kerma Product Meter; Scanditronix, Schwarzenbruck, Germany) with which it was possible to record the DAP for each angiographic procedure. The transmission chamber was calibrated by measuring KERMA free in air at 80, 90, and 110 kV, exposing the radiographic films at the same distance to evaluate the effective size of the radiation field. The measurements were repeated for FOVs of different dimensions. The DAP values were also corrected for attenuation by the bed measured at 80 kV in the region of the head. This attenuation was 10% for both angiography systems.
Characterization of the Procedures
The dose received by the patient can be measured and recorded in different ways. The parameters most commonly used are the fluoroscopy time, the number of images acquired during cineradiography, the DAP, and MSD. The use of the fluoroscopy time and the number of images acquired involves an estimation of the patient dose derived from the dose rate in fluoroscopy and the dose per image in cineradiography. This method has the advantage of being easy to use but involves measurements with a high degree of uncertainty (−70%, +130%).6 The DAP is a good indicator of stochastic risk, but it is not ideal for indicating deterministic effects at the skin, related to the threshold doses; in fact, given that the DAP is the product of the dose multiplied by the area irradiated, the same value can be obtained for a large skin area irradiated with a small dose or a small skin area irradiated with large dose. Estimating skin dose from the DAP implies an error of 30%–40% under the best conditions.7 The parameter that is best related to the deterministic effects is the MSD, which can be measured using thermoluminescent dosimeters applied to the patient, film interposed between the x-ray beam and the patient, and real-time point-measurement devices applied to the patient.
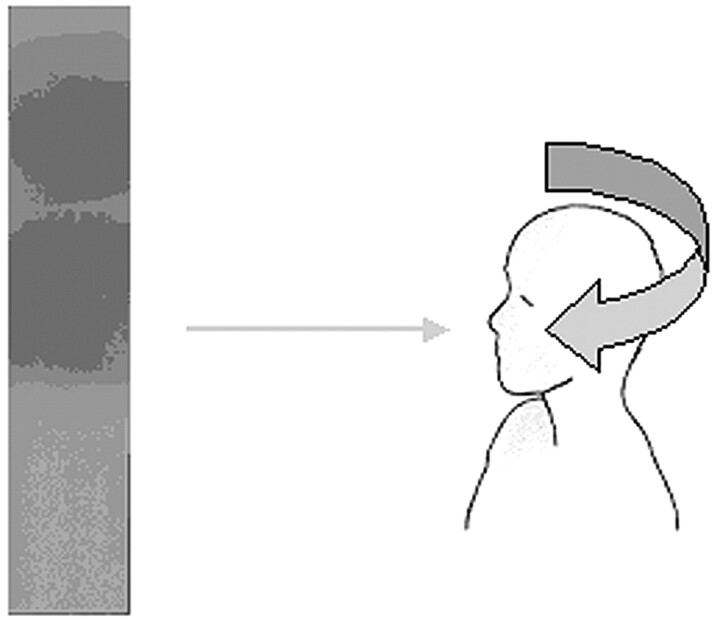
In this study, the procedures were characterized by recording the DAP, fluoroscopy time, and total number of images acquired during the cineradiography. Exposure details are presented in Table 1. The maximum skin dose was measured using Gafchromic XR type R films, 35 × 10 cm, placed in contact with the occipital skin of the patient (Fig 3).
Table 1:
Radiation exposure to 42 patients during embolization procedures (48 procedures)
| Parameter | Mean | 95% CI | Min | Max |
|---|---|---|---|---|
| DAP (Gy*cm2) | 413 | 343–482 | 81 | 1143 |
| Fluoroscopy time (min) | 29 | 23–35 | 3 | 81 |
| Number of images | 448 | 362–535 | 82 | 1326 |
Note:—CI indicates confidence interval; DAP, dose-area product.
Fig 3.
Position of the Gafchromic XR type R film and an example of a film after exposure.
Measurement of Maximum Skin Dose
The calibration of the Gafchromic films was carried out at 80 kV and for the range of doses from 0 to 600 cGy. The dose was measured by the ionization chamber before and after exposure of the film. The data from the films were acquired and processed using Picodose TA 8.0 PRO software (Tecnologie Avanzate, Torino, Italy).
Radiochromic films have the advantage of being independent of development procedures, being insensitive to light, and supplying a map of the patient's skin dose with results that are easily interpreted from visual inspection. The response of the films to 80–120-kVp x-rays is essentially energy-independent, the net density values for a given exposure dose being within approximately ± 3%. The response to 60-kVp x-rays is, on the average, approximately 7% lower than the response to 120-kVp x-rays.8 The response of the film was dose rate-independent in the range from 0.019 to 1.941 Gy/min (the variation from the mean was less than 1%) and time-independent if the reading was carried out at least 24 hours after exposure (the density changes approximately 4% between 1 and 4 days after exposure).8
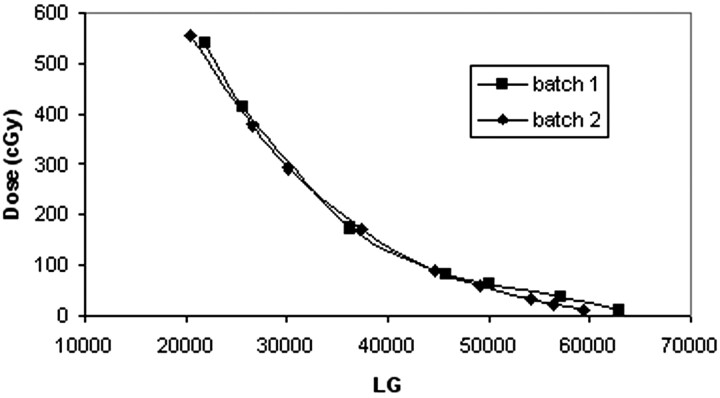
Lack of uniformity between films from the same batch was negligible (< 5%); it became significant for films from different batches (<15%).8 Two different batches were used for the procedures investigated. The calibration was repeated to reduce the uncertainty of the measurements. Figure 4 shows the calibration curves for the 2 batches used (ie, the dose [cGy] versus level of gray [LG]).
Fig 4.
Calibration curves for 2 different batches of Gafchromic XR type R films.
The method described for evaluating the MSD is usually used in research studies or on limited numbers of patients, given the amount of work required. Various studies have demonstrated that for a specific system, procedure, and protocol, there is often a reasonable correlation between MSD and the DAP; this enables identification of a warning level of DAP above which it is likely that the MSD reaches one of the threshold values that could cause skin damage. The MSD was measured with radiochromic films in a subgroup of 21 procedures to determine the MSD/DAP ratio (that is, the coefficient of the interpolation line between MSD and the dose measured by the ionizing chamber). Using the equation of the line it was possible to obtain the MSD from the value of the DAP for the remaining 27 procedures.
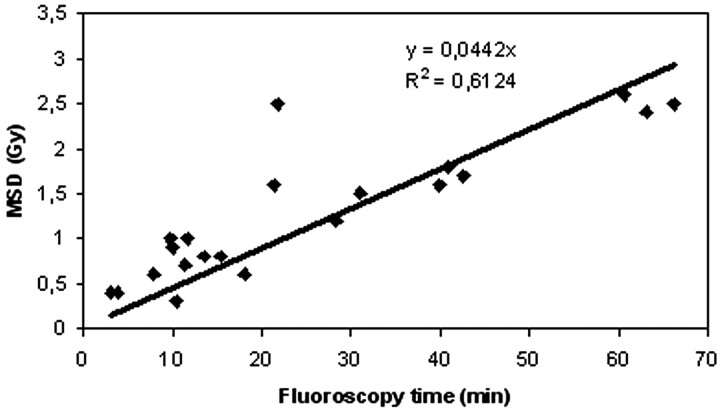
The fluoroscopy time is a poor predictor of risk because it does not correlate well with MSD; in Fig 5 the correlation (R2 = 0.6124) of the fluoroscopy time with the MSD is shown. “One cannot assume that patient injury will be prevented, or the degree of risk accurately assessed, if fluoroscopy time alone is used as a guide.”9
Fig 5.
Correlation between fluoroscopy time and maximum skin dose (MSD) measured by radiochromic films on the skull in the 21 procedures.
Results
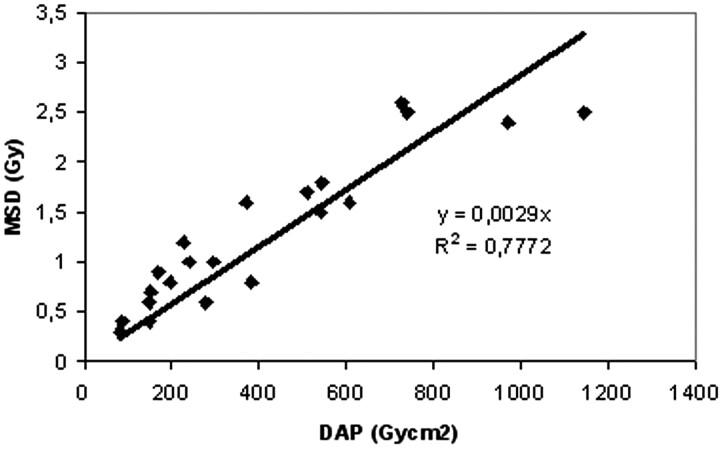
Figure 6 illustrates the correlation between DAP and MSD measured in a sample subset of 21 procedures. When measuring the dose with the radiochromic films, the error was estimated to be ≤10%, considering the dependence of the response on energy, dose rate, and reading time and the uniformity of response for films from the same batch.
Fig 6.
Correlation between total dose-area product (DAP) and maximum skin dose (MSD) measured by radiochromic films on the skull in the 21 procedures.
The calculated interpolation line between MSD and DAP has the following equation: MSD = 0.0029 DAP (R2 = 0.7772). The dose measured with the radiochromic films (21 procedures) was compared with that calculated using the interpolation line: the mean difference between the measured dose and the calculated dose was 23%. Table 2 shows the mean values of the MSD found in our study and other studies reported in the literature.
Table 2:
Published dosimetry data for MSD
| Sample Size | Mean MSD (Gy) | 95% CI (Gy) | Min (Gy) | Max (Gy) | Reference |
|---|---|---|---|---|---|
| 42 | 1.16 | 0.96–1.35 | 0.23 | 3.20 | This study |
| 143 | 1,88 | 1.723–2.036 | 0.329 | 6.414 | [10] |
| 31 | 0.869 | 0.173 | 2.332 | [11] | |
| 30* | 0.77 (PA) | [12] | |||
| 0.78 (LAT) | |||||
| 522* | 2.14 (PA) | [13] | |||
| 1.41 (LAT) | |||||
| 12 | 1.51 | [14] | |||
| 20 | 1.17 | [15] | |||
| 8 | 0.6 | 1.3 | [16] |
Note:—MSD indicates maximum skin dose; CI, confidence interval; PA, posteroanterior x-ray tube; LAT, lateral x-ray tube.
Biplane system.
The range of variability of DAP and, consequently, MSD was large for the procedures considered. This variability is the result of numerous factors, such as the complexity of the intervention, the experience of the interventionist, the size of the field, and the FOV used. However, the projections used for diagnosis are essentially standardized and the working projection for the placement of the microcatheter and the spirals within the aneurysm sac are currently decided after rotational seriography and 3D reconstruction, for which data have recently been evaluated.10
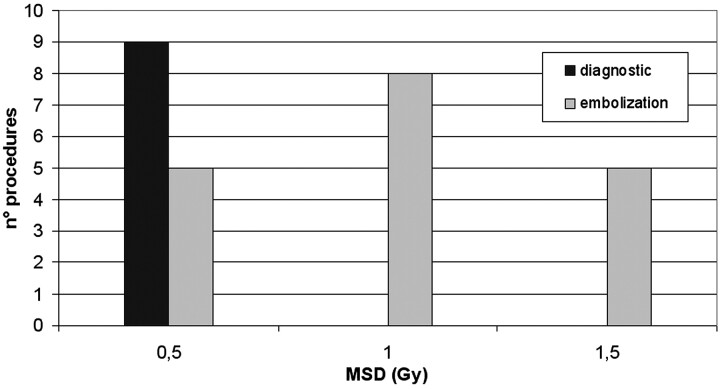
On the basis of the linear regression analysis, the MSD was calculated from the DAP values for the remaining 27 procedures of embolization (Fig 7). Diagnostic investigations usually involve doses less than 1 Gy, whereas doses exceeding 1 Gy are used in the procedures in which the embolization of the aneurysm is performed (Fig 8) or in those in which aneurysms are treated in a single session (Fig 9).
Fig 7.
Histogram of maximum skin dose calculated for 27 instances of embolization of cerebral aneurysms.
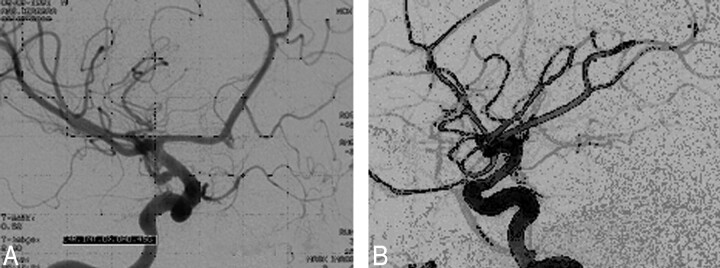
Fig 8.
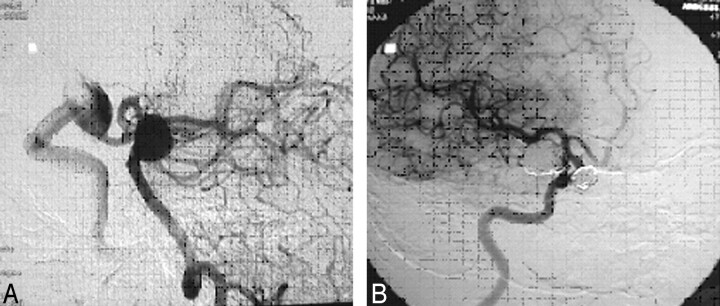
Sacciform aneurysm of the right carotid siphon (A) excluded with a spiral Guglielmi detachable coil (GDC) (B).
Fig 9.
Aneurysms of the right carotid siphon (A) and the basilar artery embolized during the same session (B).
Discussion
Unlike thermoluminescence dosimetry or semiconductor dosimetry (metal-oxide semiconductor field-effect transistor), the use of films has the advantage of supplying an image of the dose distribution on the patient and does not require prior knowledge of the maximum dose released at the skin, with the risk of underestimating the value if the dosimeter is not located correctly.
We chose to use radiochromic films because the response of this type of film, unlike radiographic ones, is not subject to saturation effects for the doses used. Furthermore, this method has the advantage of being independent of the development process, and the need for periodic quality controls is limited exclusively to the performance of the scanner and the calibration for films from different batches.
By using radiochromic XR type R film in a restricted sample of patients, we were able to determine the MSD received by the patients in the type of procedures investigated. Some measured and calculated doses exceeded the thresholds for radiation-induced effects.
Table 3 reports the threshold values of doses causing radiation-induced effects provided by the ICRP 85,5 which were associated, in our study, with a DAP value calculated from the constructed interpolation line.
Table 3:
Potential effects of fluoroscopic exposure on the reaction of skin
| Effect | Approximate Threshold Dose (Gy) | DAP (Gy*cm2) | Time of Onset |
|---|---|---|---|
| Skin | |||
| Early transient erythema | 2 | >700 | 2–24 hours |
| Main erythema reaction | 6 | >2100 | ≈1.5 weeks |
| Temporary epilation | 3 | >1100 | ≈3 weeks |
| Permanent epilation | 7 | >2400 | ≈3 weeks |
Note:—DAP indicates dose-area product.
When predicting the appearance of radiation-induced effects, the uncertainty associated with the measurements must be considered, together with the uncertainty of the threshold values. For example, threshold values higher than those indicated in Table 4 have recently been reported,11 based mainly on studies of patients exposed to radiation after the Chernobyl incident.
Regarding the frequency of skin damage, the literature provides limited information.12 These events are often not reported; furthermore, it is not always possible to relate the damage to the dose received by the patient, because data concerning exposure to radiation are not always reported. Norbash et al13 “reported that in a series of 87 patients who underwent interventional neuroradiologic procedures, nine (10%) experienced temporary or long-term epilation. Epilation after neuroradiologic procedures is probably not an uncommon finding, but it should initiate a review of radiation management practices.”12
The dose-response curve for the development of radiation-induced tumors is not clear because of the lack of published follow-up data on patients undergoing embolization of cerebral aneurysms. To optimize the exposure and therefore reduce the dose to the patient, adjustments can be made to various parameters, such as14:
Use of beam collimation: both of the angiography systems we used have beam collimation systems that reduce the area of the zone irradiated and are usually used during the insertion of the catheter guide.
Changes in the entry angle of the beam: in the procedures considered, it is possible to change the direction of the beam and, consequently, the area irradiated.
Reduction of the use of electronic enlargement: as shown by the analysis of the characteristics of the equipment, the dose increases as the electronic enlargement is increased. Electronic enlargement, therefore, is used only during the therapeutic stage.
Choice of the fluoroscopy mode: the procedures were carried out with unit 2, which employs pulsed fluoroscopy. In fact, during the analysis of the characteristics of the equipment, it was found that this reduced the dose by 20%–30% compared with that delivered using unit 1, which has continuous fluoroscopy.
Use of additional filtration: it is advisable, when possible (that is, when the image quality is diagnostically acceptable), to use the low mode, which reduces the dose by approximately 40% compared with that delivered by the normal mode. It is not always possible to use additional filtration because of its detrimental effect on the image contrast. Optimization for embolization procedures can be reached by using additional filtration during both the catheter guide insertion and the diagnostic study and by using nonadditional filtration during the therapeutic stage.
Conclusion
This study evaluated MSD in patients undergoing embolization of cerebral aneurysms. The ICRP 85 highlighted the need for maps to be drawn up of estimated entry doses received by patients during neuroradiologic interventions, indicating the entry sites of the beam for each stage of the procedure. This information should be retained in the patient's clinical records for subsequent follow-up programs and, above all, because of the possible need to repeat similar procedures. Waite and Fitzgerald15 believe that the doses of all neuroembolization procedures should be recorded. Both the ICRP and the guidelines of the SIR therefore recommend recording doses. “Published SIR guidelines for reporting and archiving of interventional radiology procedures do not specify where in the medical record radiation dose data should be recorded. Radiation dose data may be recorded in the immediate procedure note, the procedure worksheet, and/or the final report. Each institution should specify where this information is to be recorded in accordance with the needs of its own quality-improvement program and its medical record guidelines.”6 Because MSD and the DAP are both good indicators of deterministic and stochastic risks, respectively, in our case, we have chosen to record the MSD, DAP, fluoroscopy time, and total number of images in a digital archive.
The use of radiochromic XR type R films was shown to be an effective method for measuring MSD. These films have the advantage of supplying information on both the distribution of the dose (defines areas of the patient's skin where exposure should be avoided or minimized in future procedures) and the maximum dose; furthermore, they enable easy comparison between procedures carried out in different hospital structures, because they do not need development, are independent of the type of angiograph used, and are analyzed at least 2 days after exposure.
By measuring the MSD in a restricted sample of procedures it was possible to evaluate the correlation between the DAP and MSD and extrapolate the estimate of MSD to all patients for whom the DAP value was known. This method could be extended to all those procedures for which it is supposed that doses are not negligible and therefore require optimization of the radiation dose received by the patient.
References
- 1.Wiebers DO, Whisnant JP, Houston J 3rd, et al. International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of of surgical and endovascular treatment. Lancet 2003;362:103–10 [DOI] [PubMed] [Google Scholar]
- 2.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–74 [DOI] [PubMed] [Google Scholar]
- 3.European Union. Council Directive 97/43 Euratom of 30 June 1997, Official Journal of the European Communities,1997; Legislation, L. 180
- 4.Food and Drug Administration. Recording information in the patient's medical record that identifies the potential for serious x-ray induced skin injuries. Washington DC: Center for Devices and Radiological Health, US Food and Drug Administration,1995
- 5.Valentin J. Avoidance of radiation injuries from medical interventional procedures. Ann ICRP 2000;30:7–67 [DOI] [PubMed] [Google Scholar]
- 6.Miller DL, Balter S, Wagner LK, et al. Quality improvement guidelines for recording patient radiation dose in the medical record. J Vasc Interv Radiol 2004;15:423–29 [DOI] [PubMed] [Google Scholar]
- 7.McParland BJ. Entrance skin dose estimates derived from dose-area product measurements in interventional radiological procedures. Br J Radiol 1998;71:1288–95 [DOI] [PubMed] [Google Scholar]
- 8.Gafchromic XR Type R radiochromic dosimetry film. Characteristic performance data. ISP (International Specialty Products) Available at http://www.ispcorp.com/products/dosimetry/content/gafchromic/index.html
- 9.Fletcher DW, Miller DL, Balter S, et al. Comparison of four techniques to estimate radiation dose to skin during angiographic and interventional radiology procedures. J Vasc Interv Radiol 2002;13:391–97 [DOI] [PubMed] [Google Scholar]
- 10.Schueler BA, Kallmes D, Cloft HJ. 3D cerebral angiography: radiation dose comparison with digital subtraction angiography. AJNR Am J Neuroradiol 2005;26:1898–901 [PMC free article] [PubMed] [Google Scholar]
- 11.Mettler FA, Voelz GL. Major radiation exposure what to expect and how to respond. N Engl J Med 2002;346:1554–61 [DOI] [PubMed] [Google Scholar]
- 12.Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol 2001;177:13–20 [DOI] [PubMed] [Google Scholar]
- 13.Norbash AM, Busick D, Marks MP. Techniques for reducing interventional neuroradiologic skin dose: tube position rotation and supplemental beam filtration. AJNR Am J Neuroradiol 1996;17:41–49 [PMC free article] [PubMed] [Google Scholar]
- 14.Mahesh M. Fluoroscopy: patient radiation exposure issues. RadioGraphics 2001;21:1033–45 [DOI] [PubMed] [Google Scholar]
- 15.Waite JC, Fitzgerald M. An assessment of methods for monitoring entrance surface dose in fluoroscopically guided interventional procedures. Radiat Prot Dosimetry 2001;94:89–92 [DOI] [PubMed] [Google Scholar]