Abstract
SUMMARY: The ultimate methodology necessary to adopt a treatment as generally beneficial is the randomized controlled trial, a method designed by and for clinicians to maximize the care of their patients in the presence of uncertainty. Some selection is however necessary to limit trials to more promising and less risky endeavors. Experimental models are the privileged answer to the problem of finding scientific evidence while refraining from harming patients in the course of this pursuit. They allow a step by step assessment, from simple but artificial settings to more complex and realistic animal models. But the use of animal models can only be justified if the community can be convinced that alternatives have been considered but are invalid, when the project is scientifically sound and methodologically irreproachable.
As neurointerventional methods develop and gain wider clinical applications, progress should proceed in an orderly fashion, within limits set by prudence and human values, from the less risky, costly, time consuming methods, to the more definite, pragmatic, labor intensive but inescapable clinical trials. Each step is essential and the sequence cannot be violated without risks of errors that eventually translate into clinical morbidity.
All models are wrong, some models are useful.
George Edward Pelham Box1
Medicine can only progress along a path enlightened and bound by rational and ethical principles. Although some degree of tension may occur between scientific and ethical concerns during the design phase of a project, a successful research program requires harmony and synergy between these 2 perspectives. A project cannot be ethical if it does not respect rigorous scientific methods intrinsic to a good research practice. In turn, science cannot preserve the status that it enjoys and the credibility that is crucial to its survival if it strays from its goal, the advancement of the human condition. This consideration entails, by necessity, the use of methods that are respectful of moral concerns of the community.
Research plays a central role in medical progress, and only the appropriate use of clinical trials and experimental models can satisfy both rational and ethical requirements of this orderly process.
In this article, we will first review the motives for promoting independent research in neurointervention. Then, we will propose a rationale for the use of experimental animal models in a scientific ascent toward clinical progress. The moral discomfort associated with the use of healthy animals for the sake of human benefits will be discussed. A respectful response will cover the modern principles guiding the parsimonious use of this sensitive and controversial method of scientific advancement. Finally, we will propose an integrated view reconciling scientific, ethical, and pragmatic concerns such as efficiency and rationalization.
The Fundamental Role of Research in Interventional Neuroradiology
The early days of interventional neuroradiology were marked by the day-to-day management of exceptional cases, often in catastrophic clinical contexts, by using creative homemade tools. In these “glorious days,” we could experience the use of a new technique or of a new device in a patient in urgent need of care. This was acceptable because our services were often requested at last resort, perhaps after a more conventional surgical attempt had failed or on a principle of compassion. Such pioneering experiences were the foundation of a school of thought dominated by singularized decision-making processes, based on a priori opinions or intuitions, and by faith in technology championed by individual skills.
Times have changed. We are now going through the growing pains of an expert medical activity at the edge of maturity. As the specialty evolved to a more standardized expertise, caring for patients with common disorders, an increasing need to justify our decisions and to guide our actions by using a reliable and reproducible methodology emerged. Fortunately, scientific methods to do so already exist. Without science to guide our actions, we are condemned to rely on biased case series, authoritative opinions, expert guidelines, sale pitches, or even the latest fashion in cutting-edge technology. With the increasing use of endovascular techniques, industry recognized the rise of a new market. With industry, came modernity, money, marketing, and a whole new set of opinions promoted by powerful corporations. Without independent research to ensure that our knowledge is reliable, we are inclined to naively believe what we are told, to involuntarily promote the objectives and interests of third parties, at the risk of giving up physician autonomy and of losing sight of our raison d’être, the care of our patients.
Of course independent research is not immune from error. Physicians themselves are often content with case series or publish studies with flawed methodology. Industry can perform studies that are scientifically sound, but we cannot evade the fact that science is not its principal mission. A policy of respect of scientific norms in product development will more likely be adopted if it becomes a requirement for credibility and success in the clinical field.2 Research is fundamental to the development of a specialized body of knowledge, and the responsibility for pertinent research should be bestowed on those who care for patients.
The Use of Experimental Models
The randomized clinical trial (RCT) is the gold standard of evidence-based medicine. Besides the occasional resort to the use of medical devices on compassionate grounds, a practice that should always be possible because it is still within the realm of clinical care, any claim to significant progress should be subject to this rigorous test. In the face of limited resources, some trade-offs may appear necessary, but patients’ safety should not be compromised because of pressures either to publish or to bring products to market. Pilot studies are meant to provide fast results at minimal cost but may not reveal limitations that become apparent when the technique applies to a large group of unselected patients. Of course, the clinical introduction of a device has to start somewhere. A potential solution to this dilemma is to integrate the pilot study into a well-designed clinical trial, with step-wise continuation into the more scientific phase of the trial once unexpected complications have been excluded with the first cohort of patients.2
Because of a lack of verification of the utility of our actions by RCTs, we remain embarrassed today by not being able to inform patients of the risks of their condition, the efficacy of our treatments,3 or the benefits, if any, of using adjunct devices during routine procedures.4 The inventiveness of the human mind is too broad, opportunities too numerous, time and resources too scarce, and risks too high, however, to process all ideas, devices, or techniques through the heavyweight machinery of RCTs. Some selection is in order to limit RCTs to more promising and less risky endeavors.
Experimental models are the privileged answer to a fundamental rule of the scientific method: to find evidence or repeatable observations and to refrain from harming patients in the course of this pursuit.
Experimental models are invaluable tools designed for specific purposes or research activities, in a scientific context. For a hypothesis to be seriously considered, the scientific context implies the necessity to resist falsification or at least to be supported by repeatable observations that allow reliable predictions through induction.
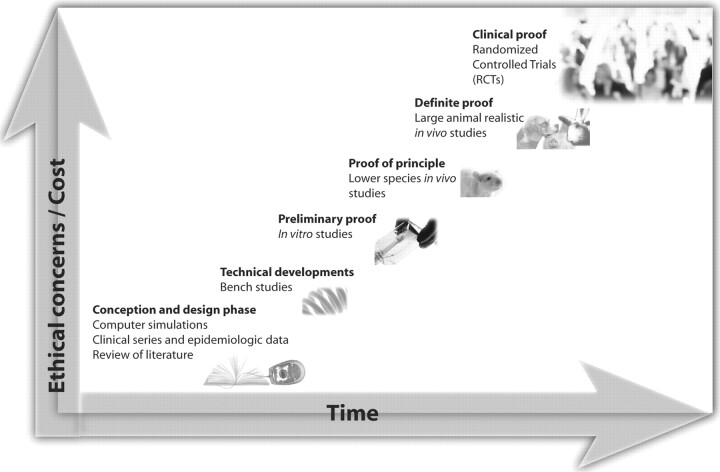
Models allow a step-by-step assessment, from simple but artificial settings (computer simulations, bench or in vitro studies) to more complex and realistic animal models. For example, technical advances should be developed by using bench studies in preference to in vivo animal experiments, and a problem should be anticipated and addressed first in animal models and not encountered a posteriori during a large-scale clinical application. There are categories of models, and perhaps hierarchies of models, within or between categories (Fig 1). These can be defined in terms of specific objectives or as a progression in levels of complexity.
Fig 1.
Rational use of experimental models in the sequence toward progress. The illustration is an artistic representation of the orderly process of resorting to models, from the early design phase, at small cost and with few ethical concerns, in a scientific ascent toward later more complex and costly methods that entail increasing moral responsibilities until the final stage of the clinical application.
The invention and optimization of devices played a crucial role in advancing the neurointerventional field. Many principles were inspired from invasive cardiology, but the cerebral vascular anatomy, and more precisely the carotid siphon, was a formidable technical challenge, a kind of new frontier difficult to surpass. Those who have fought with calibrated-leak balloons tied to microcatheters delivered through propulsion chambers still marvel at the easy and swift cerebral catheterization achievable today.
Most technical advances can be prototyped, tested, and optimized by using bench studies. The effects of varying the design can even be predicted by computer simulations (intracranial stents for aneurysms for example).5,6 However, therapy goes beyond the capacity to reach the target, and often involves the permanent implantation of a foreign object. Furthermore, devices are increasingly being empowered with biologic effects (drug-eluting stents, radioactive or coated coils).7–9 Here, knowledge of living organisms and of their reaction to the device, with all its complexity, becomes crucial. Devices are multiplying on the market, many being implanted into human cerebral vessels without any demonstration of their safety or efficacy, some even without appropriate preclinical assessment. This clinical behavior endangers the credibility and autonomy of the specialty. Besides the obvious ethical responsibilities that are evaded here, we must realize that if we do not reflect, by our actions, basic prudence in medicine, authoritative and bureaucratic rulings may take over this responsibility, and these rulings do not necessarily rely on rational principles shared by the expert community.
Although the laboratory can provide the appropriate setting for some necessary experiments (in vitro drug elution from devices, ex vivo testing on resected tissues), we cannot progress and do so while respecting human prudence and dignity without resorting to animal models.
The Necessity for Animal Models
The use of animal models presents advantages and inconveniences, but we believe it is an absolute necessity in research on endovascular devices. Certain biologic phenomena can be studied to a certain extent in vitro. There are models of neointima formation in vitro that can and should be used to study the effects of drugs on vascular smooth-muscle cell replication, for example, but to what extent they can predict in vivo restenosis and clinical outcome remains speculative. Other phenomena, such as recanalization of thrombus, have no reliable in vitro counterparts. Although the potential effects of a new coil design on packing attenuation can be addressed first in plastic or glass aneurysms,10 the more important question is whether this strategy will prevent recurrences in vivo, and before resorting to a large-scale clinical trial, which by necessity entails clinical and financial risks, only appropriate animal models can provide clues to the potential benefits and risks of this new design.7,11,12
It is important to emphasize here that different animal models can be used for various purposes, but a fundamental requirement is that the selected model reproduce the clinical phenomenon of interest. A drug-eluting stent should be assessed in porcine arteries because they have a propensity for thrombosis and exuberant neointima formation, and a drug-related decrease in neointimal thickness is demonstrable in this model. Conversely, porcine models are inappropriate to study the effects of new devices on aneurysm recurrences after treatment because the same biologic characteristics that render this species unique to the study of restenosis are responsible for the constant and permanent exclusion of aneurysms, irrespective of the embolic agent used.13,14 The inappropriate use of porcine models and its consequences are exemplified in the clinical introduction of polyglycolic/polylactic acid–covered coils.15
One important characteristic of research models is the use of relatively small sample sizes in a controlled environment. Thus, they are designed to depict effects of wide amplitude, whether we are looking at therapeutic effects or hazards; an RCT, with all the inherent variability of clinical material, would necessitate a much larger sample size to reveal the same evidence. Thus, more than 500 patients may be barely sufficient to exclude such a large increase as a doubling of thrombo-embolic complication rates associated with the use of a new coil technology.4
Another irreplaceable feature of animal models is the possibility of studying results at early, intermediate, and late time points. This is essential to understand molecular and cellular mechanisms of pertinent biologic phenomena with which one can rationalize future therapeutic strategies to improve clinical results. Again, given the limits of animal models, one must remain vigilant and modest regarding the extrapolation of results to human beings. Finally, animal models permit the discovery of adverse effects that predict potential complications, which can be anticipated in future clinical trials if the new design seems promising. Although animal studies allow some prediction of biologic effects of devices, they cannot replace RCTs in the evaluation of long-term safety and efficacy, no matter how often they are repeated, even in multiple species, and how well they are designed.
The “Unrealistic” Nature of Animal Models
Certain pathologies, such as arteriovenous malformations and intracranial stenoses cannot be realistically reproduced in animals, and important natural features of human problems may remain beyond the reach of experimental models. Like any tool, models cannot be expected to provide solutions to all questions; there are limits to what they can do and to interpretations of the results they can achieve. There is no universal problem, no universal tool, and no universal model.
Models (and tools) are human inventions; there is a built-in notion of artifact and creativity, so models necessarily step away from a certain reality. This abstraction is inescapable because problems at hand, at least in medicine, are always too complex to attack globally, at once. Analysis, decomposition, and control of confounding factors are necessary to identify meaningful relations or to evaluate potential actors in a given phenomenon. Experiments are used to create an artificially simplified environment in which a phenomenon can be studied free from the extraneous and perturbing factors that inevitably occur in real life.16 As specificity of the hypothesis and control over confounding factors in a given experiment increase, the potential to find a scientific relationship is enhanced. However, this occurs at the risk of widening the gap between the truth of the theory and the benefits that can be expected from its practical application. On the other hand, an experiment that would closely mimic the clinical scenario—biologic variability, spectrum of technical challenges, and irregularity of events of low probability—would not provide the favorable context for a scientific discovery, necessitating sample sizes of the magnitude of clinical trials. This is hardly desirable from an economic or ethical point of view. Hence, the mathematic/biologic relationship between 32P activities implanted onto radioactive coils and the recanalization rate after coil occlusion can be demonstrated in the relatively simplified single-coil arterial occlusion model setting.9 This scientific relation can be identified because the extraneous effects of various packing densities, initial angiographic occlusion rates, and multiple aneurysms of various sizes and neck widths, ineluctable in an aneurysmal embolization model, can be avoided.17
The Moral Discomforts Associated with the Use of Healthy Animals in Research
The antivivisectionist and animal rights movements of the 1970s have positively influenced regulation and practices in the use of animals in the experimental laboratory. In the minds of many activists, this change may not be enough because simple prohibition would be the only way to guarantee the end of abuse and suffering that we, as experimenters, have unjustly and unnecessarily imposed on animals. Most individuals do not espouse such extreme opinions, but many feel moral and emotional discomfort that verges on disapprobation. Such pervasive feelings should be respected like any other important value shared by a significant portion of humane societies.
It is beyond the scope of this article to argue in detail how the use of animal models could be defended morally. We will briefly mention 2 points. Some of the public disapproval concerning the use of animals may have to do with a visceral discomfort that is somewhat similar to our instinctive repulsion to autopsies or surgical procedures. If this thesis is partly correct, this feeling must be tempered by reason and, at least in some circumstances, must be overcome to reach worthwhile goals.
If we have abundant evidence to support the view that many animals can suffer pain and distress in the same way that humans do, we should not agree that considerations of sentience, sophistication, and autonomy are morally irrelevant. Perhaps inasmuch as experimenters recognize that not all human beings enjoy identical rights and duties (neonates, minors, demented or psychotic patients), they must acknowledge their speciesism in the form of a hierarchical conception of the animal kingdom. There are meaningful differences between species, and very few people would give equal consideration to snails and horses. In this perspective, there is a sliding scale of moral status; the presumption against human suffering is stronger than that concerning animals. Humans have interests that are not to be sacrificed in the name of utility, but nonhumans deserve serious but less-than-full consideration. Their interests are subject to consequentialist trade-offs for the sake of human progress.18 Experimenters admit that they prefer to test their hypotheses and discover the potential for harm in the carefully controlled environment of the laboratory rather than to test new technologies in the much-less-controlled error-prone clinical environment, in which hundreds or thousands of patients will be exposed. It is possible to deny animals some of the rights that we bestow on human beings but still treat them with considerable care, dignity, and respect, even or especially in experimental animal facilities, as we have witnessed on innumerable occasions during the past decades.
Consideration and Respect for Experimental Animals
Health professionals unfamiliar with the work done in experimental facilities are often surprised by the respect the technical staff manifests and by the loving care they provide to their subjects. One important reason they can cope with the apparent contradiction between their love of animals and the purpose of their work is their trust that everything possible is implemented to minimize distress and pain and that the lives of their protégés are not spent in vain or trivial enterprises.
To the experimenter, convinced of the importance of his own research, any procedure may seem justified. Thus, the scientific merits of research projects involving animals must be subjected to external review and procedures verified by a committee or advisory board representing animal welfare, animal care, and scientific and lay interests.18,19
We cannot emphasize too much the importance of the now-classic 3 R's as guidelines to the rational and humane use of animals in medical research: 1) replacement, the search for alternatives to animal experiments whenever possible, such as the use of computer simulations or in vitro experiments, 2) reduction, the duty to use the minimal number of animals sufficient to fulfill scientific requirements of validity, and 3) refinements, developed to enhance animal health and welfare through the use of appropriate anesthesia and husbandry methods and optimal housing and enrichment of the environment. A 4th R, respect, is increasingly being promoted: The care of animals dedicated to human welfare advancement is an outstanding privilege that should not be abused. The use of animals can only be justified if the community can be convinced that alternatives have been considered but are invalid and that the project is scientifically sound and methodologically irreproachable, worthy of sensitive methods that stir public controversy. If animals are sufficiently similar to humans to be used as scientific models in research, then they are sufficiently similar to be accorded moral status.20
The Convergence of Moral, Scientific, and Pragmatic Aspects of a Research Program
We see no contradiction in the requirements imposed by the rationality of science, the moral concerns of the community, and the economic constraints of the real world. Medical progress will be promoted by projects that are carefully planned, scientifically and economically sound, and respectful of the concerns of physicians, patients, and authorities. Progress should proceed along a carefully selected hierarchy of methods. This selection should proceed in an orderly fashion, within limits set by prudence and human values, from the less risky, costly, and time-consuming computer simulations and bench studies to the more definite, labor-intensive, but inescapable clinical trials. Each step is essential, and its place in the sequence of progress should be respected. The sequence cannot be violated without risk of errors, and errors in medical research will eventually translate into morbidity.
As physicians, our primary duty is to ensure that everything is done to minimize patient morbidity. Genuine respect for human dignity requires that the research meet scientific norms of excellence. The use of human subjects can only be justified when the objective of the research is to provide unbiased results and the golden rule to prevent bias is randomization. Hence, our privileged tool to ensure progress is the randomized clinical trial.
If a scientist relies solely on computer simulations, let him treat computers. If he relies on animal models, let him treat animals. A clinician relies on clinical trials to determine the optimal care of each individual patient.
Acknowledgments
We are indebted to Hélène Héon and Marie-Claude Gagnon, MDV; Suzanne Carioto, Chief of CHUM Animal Care Facilities; Isabelle Houle; Caroline Blais; Anick Laporte; Sophie Grenon; Suzanne Vincent; Kim Leclerc-Desaulniers; Grace Ferguson; and animal care technicians as well as the members of the Institutional Animal Care and Protection Committee for outstanding care of research subjects and enlightening discussions over the past decade.
Footnotes
This work was partially supported financially by the Canadian Institutes of Health Research (CIHR) and the Quebec Heart and Stroke Foundation.
References
- 1.Box GEP. Robustness in the strategy of scientific model building. In: Launer RL, Wilkinson GN, eds. Robustness in Statistics. New York: Academic Press;1979. :201–36
- 2.Raymond J, Guilbert F, Weill A, et al. Safety, science, and sales: a request for valid clinical trials to assess new devices for endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol 2004;25:1128–30 [PMC free article] [PubMed] [Google Scholar]
- 3.Raymond J, Guilbert F, Weill A, et al. Unruptured intracranial aneurysms: a call for randomized clinical trials. AJNR Am J Neuroradiol 2006;27:242–43 [PMC free article] [PubMed] [Google Scholar]
- 4.Raymond J, Leblanc P, Chagnon M, et al. New devices designed to improve the long-term results of endovascular treatment of intracranial aneurysms. A proposition for a randomized clinical trial to assess their safety and efficacy. Interventional Neuroradiology 2004;10:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieber BB, Stancampiano AP, Wakhloo AK. Alteration of hemodynamics in aneurysm models by stenting: influence of stent porosity. Ann Biomed Eng 1997;25:460–69 [DOI] [PubMed] [Google Scholar]
- 6.Ohta MHM, Wetzel S, Lylyk P, et al. Impact of stent design on intraaneurysmal flow: a computer simulation study. Intervenional Neuroradiology 2004;10:85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding YH, Dai D, Lewis DA, et al Angiographic and histologic analysis of experimental aneurysms embolized with platinum coils, Matrix, and HydroCoil. AJNR Am J Neuroradiol 2005;26:1757–63 [PMC free article] [PubMed] [Google Scholar]
- 8.Murayama Y, Tateshima S, Gonzalez NR, et al. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke 2003;34:2031–37 [DOI] [PubMed] [Google Scholar]
- 9.Raymond J, Leblanc P, Desfaits AC, et al. In situ beta radiation to prevent recanalization after coil embolization of cerebral aneurysms. Stroke 2002;33:421–27 [DOI] [PubMed] [Google Scholar]
- 10.Piotin M, Iijima A, Wada H, et al. Increasing the packing of small aneurysms with complex-shaped coils: an in vitro study. AJNR Am J Neuroradiol 2003;24:1446–48 [PMC free article] [PubMed] [Google Scholar]
- 11.Brisman JL, Song JK, Niimi Y, et al. Treatment options for wide-necked intracranial aneurysms using a self-expandable hydrophilic coil and a self-expandable stent combination. AJNR Am J Neuroradiol 2005;26:1237–40 [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshino Y, Niimi Y, Song JK, et al. Endovascular treatment of intracranial aneurysms: comparative evaluation in a terminal bifurcation aneurysm model in dogs. J Neurosurg 2004;101:996–1003 [DOI] [PubMed] [Google Scholar]
- 13.Desfaits AC, Raymond J, Muizelaar JP. Growth factors stimulate neointimal cells in vitro and increase the thickness of the neointima formed at the neck of porcine aneurysms treated by embolization. Stroke 2000;31:498–507 [DOI] [PubMed] [Google Scholar]
- 14.Raymond J, Metcalfe A, Desfaits AC, et al. Alginate for endovascular treatment of aneurysms and local growth factor delivery. AJNR Am J Neuroradiol 2003;24:1214–21 [PMC free article] [PubMed] [Google Scholar]
- 15.Niimi Y, Song J, Madrid M, et al. Endosaccular treatment of intracranial aneurysms using matrix coils: early experience and midterm follow-up. Stroke 2006;37:1028–32 [DOI] [PubMed] [Google Scholar]
- 16.Gillies D. Introductory survey of the interpretations. In: Philosophical Theories of Probability. London, UK: Routledge;2000. :10
- 17.Raymond J, Mounayer C, Salazkin I, et al. Safety and effectiveness of radioactive coil embolization of aneurysms: effects of radiation on recanalization, clot organization, neointima formation, and surrounding nerves in experimental models. Stroke 2006;37:2147–52 [DOI] [PubMed] [Google Scholar]
- 18.Degrazie D. On the question of personhood beyond Homo Sapiens. In: Peter Singer, ed. In Defense of Animals: The Second Wave. 2nd ed. Malden, Mass: Blackwell Publishing;2005. :40–53
- 19.Canadian Council on Animal Care Website. Available at: http://www.ccac.ca. Accessed on July 22,2006
- 20.Ryder R. Speciesism in the laboratory. In: Peter Singer, ed. In Defense of Animals: The Second Wave. 2nd ed. Malden, Mass: Blackwell Publishing;2005. :87–103