Abstract
SUMMARY: Morphometry and spectroscopy were performed in 3 patients with fragile X-associated tremor/ataxia syndrome (FXTAS). The brain stem and cerebellum were atrophic and satisfied criteria for olivopontocerebellar atrophy in 2 patients. However, the vermis was relatively spared and the basis pontis maintained its oval shape. The only spectroscopic abnormality was a decrease of the pontine N-acetylaspartate/creatine ratio in 1 patient. Atrophy and metabolic changes in FXTAS differ to some extent from those of olivopontocerebellar atrophy.
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a genetic cause of adult-onset sporadic ataxia alone1 or in combination with other extracerebellar symptoms mimicking multisystem atrophy cerebellar type (MSA-C).2 MR imaging contributes to identification of FXTAS.3 We performed MR imaging–based morphometry and proton MR spectroscopy (1H-MR spectroscopy) of the brain stem and cerebellum in 3 patients with FXTAS.
Case Reports
Case 1
A 70-year-old man presented with rest tremor of the left hand at 56 years of age. Cerebellar symptoms appeared later, and at the time of the study, he was chair-bound. MR imaging demonstrated symmetric signal intensity changes in the peridentate white matter, middle cerebellar peduncles (MCP), and cerebral periventricular white matter and moderate cerebral atrophy (Fig 1). A small hyperintense lesion was present in the basis pontis on T2-weighted images. DNA analysis demonstrated expansion in the range between 55 and 200 (premutation) of a CGG repeat in the fragile X mental retardation (FMR1) gene.
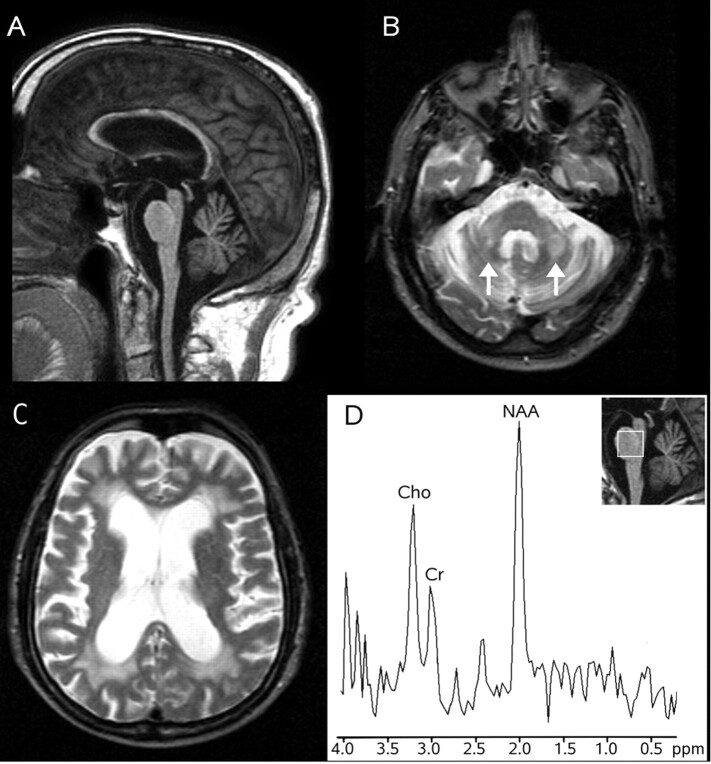
Fig 1.
MR imaging and 1H-MR spectroscopy in a patient with FXTAS (case 1). Sagittal T1-weighted gradient recalled-echo image (TR = 25 ms, TE = 4.6 ms, flip angle = 30°; 1 NEX) (A) shows diffuse brain atrophy with sparing of the oval shape of the basis pontis. Axial T2-weighted spin-echo images (TR = 2400 ms, TE = 200 ms, 1 NEX) (B and C) show symmetric hyperintensity in the middle cerebellar peduncles (arrows in B) and diffuse hyperintensity in the cerebral periventricular white matter (C). 1H-MR spectroscopy of the pons (D) shows decreased NAA/Cr ratio and a normal Cho/Cr ratio.
He was examined on a 1.5T system. Axial 3D T1-weighted gradient recalled-echo (TR = 25 ms, TE = 4.6 ms, flip angle = 30°, 1 mm thick) images were obtained for MR imaging–based morphometry. On source and reformatted sagittal images, morphometric assessment of the brain stem and cerebellum normalized to the posterior cranial fossa area was performed.4 The basis pontis (0.086; Normal Value [NV] = 0.105 ± 0.020), the medulla (0.047; NV = 0.077 ± 0.012), the MCP (0.0037; NV = 0.0049 ± 0.0005), and the cerebellar hemispheres (0.439; NV = 0.516 ± 0.027) were atrophic, and the 4th ventricle (0.057; NV = 0.033 ± 0.003) was enlarged: Namely they differed by more than 2 SDs from control values in 12 age-matched healthy subjects. The patient satisfied the morphometric criteria for olivopontocerebellar atrophy (OPCA), defined as an abnormal value of the cerebellar vermis or hemisphere and at least 2 abnormal values among the basis pontis, the medulla, and the MCP.4 However notably, the vermis (0.279; N.V. = 0.338 ± 0.043) was not abnormal, and visual evaluation showed that the oval shape of the basis pontis was preserved (Fig 1). 1H-MR spectroscopy was performed by using a point-resolved proton spectroscopy sequence (TR = 2000 ms, TE = 272 ms). Voxels of interest of 8 mL were obtained in the basis pontis and in the right dentate and peridentate white matter. The N-acetylaspartate (NAA)/creatine (Cr) ratio (2.7; NV = 4.0 ± 0.5) differed more than 2 SDs from that of control values obtained in 10 healthy subjects, whereas the choline (Cho)/Cr ratio (1.7; NV = 2.5 ± 0.7) in the pons (Fig 1) and the NAA/Cr (1.6; N.V. = 1.6 ± 0.2) and Cho/Cr (0.8; NV = 1.2 ± 0.2) ratios in the vermis were not significantly decreased.
Case 2
A 69-year-old man presented with intention tremor of the right hand at 55 years of age and subsequently developed progressive cerebellar ataxia. MR imaging showed symmetric signal-intensity changes in the peridentate white matter, MCP, and cerebral periventricular white matter and moderate cerebral atrophy. The basis pontis was diffusely hypointense on T1- and hyperintense on T2-weighted images (Fig 2). DNA analysis demonstrated premutation of the FMR1 gene. He underwent MR imaging–based morphometry and 1H-MR spectroscopy examination like patient 1. The pons (0.077), the medulla (0.049), the MCP (0.0038), and the cerebellar hemispheres (0.392) were atrophic and the 4th ventricle (0.046) was enlarged, satisfying the criteria for OPCA. However the vermis (0.261) was not abnormal, and the oval shape of the basis pontis was preserved (Fig 2).
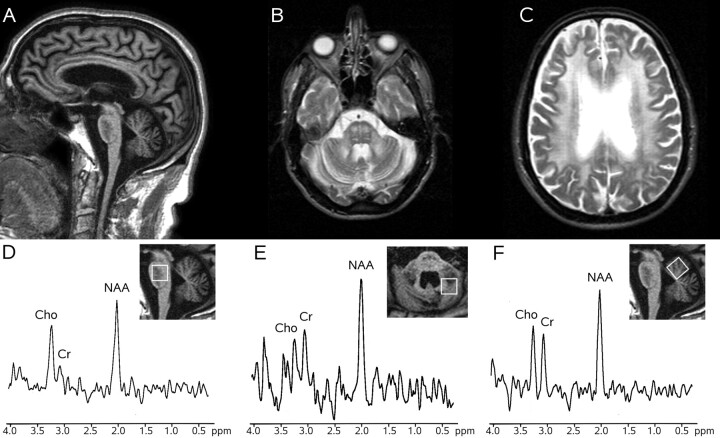
Fig 2.
MR imaging and 1H-MR spectroscopy in a patient with FXTAS (case 2). Sagittal T1-weighted gradient recalled-echo image (TR = 25 ms, TE = 4.6 ms, flip angle = 30°, 1 NEX) (A) shows diffuse brain atrophy with sparing of the oval shape of the basis pontis, which exhibits a diffuse low signal intensity. Axial T2-weighted spin-echo images (TR = 2400 ms, TE = 200 ms, 1 NEX) (B and C) show symmetric hyperintensity in the pons and middle cerebellar peduncles and diffuse hyperintensity in the cerebral periventricular white matter (C). 1H-MR spectroscopy of the pons (D), right dentate and peridentate white matter (E), and superior vermis (F) shows normal metabolite ratios.
Metabolite ratios were normal in the pons (NAA/Cr = 3.2; Cho/Cr = 2.7), the dentate and peridentate white matter (NAA/Cr = 1.6; NV = 2.1 ± 0–3; Cho/Cr = 0.8; NV = 1.3 ± 0.4), and vermis (NAA/Cr = 1.5; Cho/Cr = 1.1) (Fig 2).
Case 3
The 69-year-old grandfather of 2 children with fragile X syndrome (FXS) presented with postural and action tremor in his right hand at 62 years of age. At the time of the study, he showed cerebellar and parkinsonian findings and mild cognitive impairment. MR imaging showed symmetric signal-intensity changes in the peridentate white matter, MCP, and cerebral periventricular white matter and moderate cerebral atrophy. Two small lesions hyperintense on T2-weighted images were present in the pons. DNA analysis demonstrated premutation of the FRM1.
He underwent MR imaging–based morphometry and 1H-MR spectroscopy like patients 1 and 2. The pons (0.084) and the cerebellar hemispheres (0.399) were atrophic, and the 4th ventricle (0.066) was enlarged. The medulla (0.058), MCP (0.0041), and the vermis (0.274) were not abnormal, and the oval shape of the basis pontis was preserved.
The metabolite ratios were normal in the pons (NAA/Cr = 4.9; Cho/Cr = 3.4), dentate and peridentate white matter (NAA/Cr = 1.8; Cho/Cr = 0–9), and the vermis (NAA/Cr = 1.8; Cho/Cr = 1.1).
Discussion
FXS is the most common inherited cause of mental retardation in boys. The disorder is caused by expansion in excess of 200 repeats of a trinucleotide CGG in the 5′ untranslated regions of the FMR1 gene. Normal individuals carry between 5 and 39 FMR1 CGG repeats. Mothers of patients with FXS typically are not mentally retarded, but carry an FMR1 CGG repeat expansion between 55 and 200 repeats (premutation). The triplet expansion is unstable and tends to amplify in successive generations. Approximately 1:259 women and 1:813 men are carriers of the premutation of the FMR1 gene.5 The neuropathologic features of FXTAS include a diffuse spongy degeneration of the white matter associated with intranuclear inclusions in neurons and astrocytes throughout the brain gray and white matter.6
The extensive signal intensity change in T2-weighted images of the white matter in FXTAS was previously emphasized.3 Pontine signal-intensity abnormalities were reported in some cases.2,7 Focal or diffuse signal intensity changes in the basis pontis were present in our patients, in whom morphometry demonstrated loss of bulk in the pons, the cerebellar hemispheres, and (in 2 patients) the MCP and the medulla, consistent with OPCA. However, in all 3 patients, the vermis was relatively spared, and the oval shape of the pons was preserved.
To our knowledge, no 1H-MR spectroscopy data in FXTAS are available. The paucity of brain stem and cerebellar 1H-MR spectroscopy abnormalities in our patients, with only 1 abnormal spectrum out of 8 spectra obtained was unexpected. A similar lack of abnormality on 1H-MR spectroscopy of the brain stem and cerebellum was reported in spastic ataxia of Charlevoix–Saguenay8 and episodic ataxia type 2.9
Differential diagnosis of FXTAS with other degenerative ataxias is becoming an important clinical problem because approximately 3% of elderly men with late-onset cerebellar ataxia1 and 4% with probable MSA-C2 were found to carry the fragile X premutation. Although molecular genetic tests represent the fundamental tool for diagnosis of FXTAS, the possible contribution of MR imaging in circumscribing the number of patients to evaluate for fragile X premutation has been advocated.5
Degenerative ataxias are a complex group of sporadic or inherited diseases. Taking into account that cerebellar dysfunction due to FXTAS occurs in adult or elderly subjects, one should consider the following 2 main ataxias in the differential diagnosis: idiopathic “pure” late-onset cerebellar ataxia, whose neuropathologic and MR imaging features correspond to a pattern of cortical cerebellar atrophy (CCA), and idiopathic late-onset cerebellar ataxia with other symptoms. The latter condition is associated with an OPCA pattern and its relationship with MSA-C is debated.4,10–12 In CCA, the brain white matter signal intensity is normal, the brain stem atrophy is far less pronounced than atrophy of the cerebellum, and the bulk of the cerebral hemispheres is normal.10 In OPCA, there are no significant changes in the cerebral white matter, but white matter signal-intensity changes in the pons, MCP, and cerebellum are observed with characteristic sparing of the corticospinal tracts (creating the “cross” sign) and of the superior cerebellar peduncles.12,13 Moreover, in OPCA, the inferior part of the basis pontis becomes flattened,13 the vermis is atrophic,4,12 and some degree of atrophy of the cerebral hemispheres is common.
1H-MR spectroscopy demonstrates a variety of brain abnormalities in degenerative ataxias.8,14 In particular, a decrease of the NAA/Cr ratio in the pons was an invariable finding in patients with OPCA.14 Moreover, a decrease of the pontine NAA/Cr was also observed in patients with MSA without clinical symptoms of cerebellar dysfunction,15 suggesting that this could be an early finding.
In conclusion, extensive supratentorial white matter signal-intensity changes, preservation of the oval shape of the basis pontis, relative sparing of the vermis, and paucity of the 1H-MR spectroscopy abnormalities in the brain stem and cerebellum are features of FXTAS potentially useful for the differential diagnosis with OPCA.
Footnotes
This work was supported in part by a research grant to Nicola De Stefano from the Italian Ministry of Research and University.
References
- 1.Brussino A, Gellera C, Saluto A, et al. FRM1 gene premutation is a frequent genetic cause of late-onset sporadic cerebellar ataxia. Neurology 2005;64:145–47 [DOI] [PubMed] [Google Scholar]
- 2.Kamm C, Healy DG, Quinn NP, et al. The fragile X tremor ataxia syndrome in the differential diagnosis of MSA: data from EMSA study group. Brain 2005;128:1855–60. Epub 2005 Jun 9 [DOI] [PubMed] [Google Scholar]
- 3.Brunberg JA, Jacquemont S, Hagerman J, et al. Fragile X premutation carriers: characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. AJNR Am J Neuroradiol 2002;23:1757–66 [PMC free article] [PubMed] [Google Scholar]
- 4.Wullner U, Klockgether T, Petersen D, et al. Magnetic resonance imaging in hereditary and idiopathic ataxia. Neurology 1993;43:318–25 [DOI] [PubMed] [Google Scholar]
- 5.Hall DA, Berry-Kravis E, Jacquemont S, et al. Initial diagnosis given to persons with fragile X-associated tremor/ataxia syndrome (FXTAS). Neurology 2005;65:299–301 [DOI] [PubMed] [Google Scholar]
- 6.Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain 2006;129:243–55. Epub 2005 Dec 5 [DOI] [PubMed] [Google Scholar]
- 7.Jacquemont S, Orrico A, Galli L, et al. Spastic paraparesis, cerebellar ataxia, and intention tremor: a severe variant of FXTAS? J Med Genet 2005;42:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viau M, Marchand L, Bard C, et al. 1H magnetic resonance spectroscopy of autosomal ataxias. Brain Res 2005;1049:191–202 [DOI] [PubMed] [Google Scholar]
- 9.Sappey-Marinier D, Vighetto A, Peyron R, et al. Phosphorus and proton magnetic resonance spectroscopy in episodic ataxia type 2. Ann Neurol 1999;46:256–59 [DOI] [PubMed] [Google Scholar]
- 10.Ormerod IEC, Harding AE, Miller DH, et al. Magnetic resonance imaging in degenerative ataxic disorders. J Neurol Neurosurg Psychiatry 1994;57:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilman S, Quinn NP. The relationship of multiple system atrophy to sporadic olivopontocerebellar atrophy and other forms of idiopathic late-onset cerebellar atrophy. Neurology 1996;46:1197–99 [DOI] [PubMed] [Google Scholar]
- 12.Burk K, Buhring U, Schulz JB, et al. Clinical and magnetic resonance imaging characteristics of sporadic cerebellar ataxia. Arch Neurol 2005;62:981–85 [DOI] [PubMed] [Google Scholar]
- 13.Savoiardo M, Strada L, Girotti F, et al. Olivopontocerebellar atrophy: MR diagnosis and relationship to multi-system atrophy. Radiology 1990;174:693–96 [DOI] [PubMed] [Google Scholar]
- 14.Mascalchi M, Cosottini M, Lolli F, et al. Proton MR spectroscopy of the cerebellum and pons in patients with degenerative ataxia. Radiology 2002;223:371–78 [DOI] [PubMed] [Google Scholar]
- 15.Watanabe H, Fukatsu H, Katsuno M, et al. Multiple regional 1H-MR spectroscopy in multiple system atrophy: NAA/Cr reduction in pontine base as a valuable diagnostic marker. J Neurol Neurosurg Psychiatry 2004;75:103–09 [PMC free article] [PubMed] [Google Scholar]