Abstract
BACKGROUND: Previously, endovascular treatment of wide-necked aneurysms and stenosis involving small vessels measuring <2 mm in diameter was limited. There are no reports in the literature addressing stent placement in small distal cerebral vessels. Recent experience with the Neuroform stent has shown that this device can be safely and effectively used to treat aneurysms in vessels of this caliber.
MATERIALS AND METHODS: We report 8 cases of Neuroform stent placement into cerebral vessels measuring <2 mm in diameter (range, 1.1–1.8 mm). All stents were placed for aneurysm treatment in conjunction with coiling. Lesion locations and number were as follows: anterior communicating artery region (n = 5), pericallosal artery A2-A3 (n = 1), middle cerebral artery (MCA) M1-M2 (n = 1), and MCA M3-M4 (n = 1). Clinical follow-up ranged from 4.5 to 18 months. Imaging follow-up was performed with MR imaging/MR angiography.
RESULTS: All procedures were successfully performed without immediate or delayed device-related complications. Intraprocedural thrombus developed within the stent in 2 patients and immediately resolved with 10 mg of intra-arterial abciximab. Follow-up at 18 months demonstrated durable results. There were no clinical neurologic symptoms related to the treated vessel territory at follow-up.
CONCLUSION: Development of newer low-profile endovascular devices allows access and ability to treat lesions farther out in the smaller cerebral vessels. We have safely and successfully treated 8 vessels smaller than 2 mm in diameter with newer self-expanding stents with good short- and intermediate-term results. Further follow-up and more experience are necessary to determine long-term results.
Endovascular treatment of wide-necked aneurysms in small vessels measuring <2 mm in diameter is limited, and there are currently no reports in the literature addressing stent placement in small distal cerebral vessels. The introduction of the Neuroform stent (Boston Scientific, Natick, Mass) a self-expanding flexible nitinol stent, has greatly advanced the endovascular treatment options of cerebral aneurysms. Many aneurysms once thought untreatable due to aneurysm configuration (wide-necked) are now amenable to coiling with the use of these stent systems.1–7 We report on 8 cases with aneurysms involving vessels <2 mm treated with the Neuroform stent.
Materials and Methods
A retrospective review of all aneurysms treated with the Neuroform stent from January 2004 to October 2005 in our center was conducted. Of the 70 stents placed, 8 were in small distal vessels measuring <2 mm. Patient demographic and clinical data, indication for stent use, radiographic images, aneurysm or stenotic characteristics, procedural notes, and postprocedure hospital course were reviewed. Particular attention was given to technical- and management-related complications. CT angiography with 3D reconstruction was performed before all interventional procedures.
All procedures were performed with the patient under general anesthesia. Heparin was titrated during the procedure to achieve an activated clotting time of 2–2.5 times that of baseline. Patients were treated with 75 mg of clopidogrel and 325 mg of aspirin at least 5 days before the endovascular procedure and were maintained on the same dosage of each, daily, for at least 12 weeks. Aspirin was continued indefinitely. The Neuroform stent was either deployed before aneurysm coiling or at the termination of aneurysm coiling to treat coil herniation. The smallest and shortest Neuroform stent available at the time was chosen for all procedures.
Postprocedural MR imaging and MR angiography (MRA) were performed within 2 weeks of treatment and again at 6-month intervals up to 2 years. Clinical modified Rankin Scale (mRS) scores were obtained 24 hours posttreatment and 2 weeks, 6 months, and 1 year posttreatment. Efficacy of aneurysm coiling was assessed by using the Raymond scale. Evidence of stroke in the treated territory via MR imaging or CT, perfusion status, and stent patency was documented.
Results
Patients
Eight patients, 4 men and 4 women, underwent placement of the Neuroform stent in vessels with diameters of <2 mm. Ages ranged from 46 to 77 years with a mean of 58 years. Stent deployment was technically successful in all patients. All stents were placed as an adjunct measure for the treatment of unruptured aneurysms. Vessel diameters ranged from 1.1 to 1.8 mm with a mean of 1.5 mm as measured on CT angiography (CTA) images (Table 1). All 8 stents were placed in anterior circulation cerebral branches. Overall, follow-up ranged from 4.5 to 18 months, with a mean of 7.5 months. All patients had the same or improved clinical outcome at follow-up by mRS. There was no evidence of flow compromise in the stent or distal territory at the time of latest follow-up.
Demographics of treated lesions
| Location | Stent | Vessel size proximal (mm) | Vessel size distal (mm) | Imaging F/U | Clinical F/U | Complication - Treatment |
|---|---|---|---|---|---|---|
| AcomA | 2.5 × 15 mm | 1.6 | 1.5 | 6 months | 6 months | None |
| Pericallosal | 2.5 × 15 mm | 1.6 | 1.5 | 8 months | 8 months | Clot on stent - ReoPro; MCA branch occlusion |
| M3 | 3 × 20 mm | 1.7 | 0.9 | 4 months | 14 months | None |
| AcomA | 3 × 20 mm | 1.1 | 1.5 | 14 months | 18 months | Clot on stent - ReoPro |
| MCA | 2.5 × 15 mm | 1.7 | 1.6 | 6 months | 6 months | None |
| AcomA | 2.5 × 15 mm | 1.3 | 1.7 | 6 months | 6 months | None |
| AcomA | 2.5 × 15 mm | 1.5 | 1.4 | 6 months | 6 months | None |
| AcomA | 2.5 × 15 mm | 1.8 | 1.6 | 6 months | 6 months | None |
Note:—AcomA indicates anterior communicating artery; M3, middle cerebral artery (3rd order branch); MCA, middle cerebral artery; F/U, follow-up.
Outcomes
In the 8 aneurysms, the Neuroform stent was deployed before aneurysm coiling in 6 patients (6/8) and at the termination of aneurysm coiling (2/8) to treat coil herniation. Half of the patients (4/8) demonstrated complete aneurysm occlusion (Raymond scale, 0), whereas in the other half, near-complete aneurysm occlusion (Raymond scale, 1) was achieved. Intraprocedural thrombus occurred in 2 (25%) of the 8 patients and resolved with abciximab (ReoPro) bolus administration. No diffusion abnormalities in the treated vascular territory were found on the postoperative MR imaging in either patient.
Example case 1.
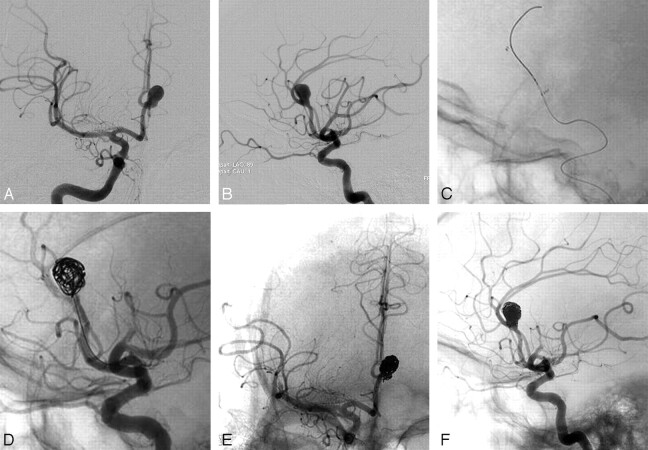
Patient 2 (Fig 1) was a 51-year-old man who presented with a large (10 × 10 × 12 mm) right pericallosal aneurysm with a wide neck. Because of the broad neck, initial placement of a 2.5 × 15 mm Neuroform stent was required in a 1.5-mm pericallosal artery to restrain the coils within the aneurysm. This allowed successful aneurysm treatment in an otherwise difficult case. Thrombus did occur along the stent interstices despite adequate intraprocedural anticoagulation. This resolved quickly with 10 mg of abciximab. At the conclusion of the case, thrombus was noted in 1 of the middle cerebral artery (MCA) branches, presumably related to thrombus from the guide catheter. The aneurysm appeared well treated without stroke in the anterior cerebral artery (ACA) territory on the postoperative MR imaging. The patient did experience mild left-handed weakness related to a small stroke from the MCA thrombus. Eight months postprocedure, the hand weakness had resolved.
Fig 1.
Large pericallosal aneurysm treated with Neuroform stent and coils. Frontal (A) and lateral (B) angiograms showing large wide-necked pericallosal aneurysm. Native lateral projection (C) image showing placement of Neuroform stent across the aneurysm neck. D, Subsequent placement of coils within the aneurysm is well constrained by the stent. Final frontal (E) and lateral (F) angiograms showing the coiled pericallosal aneurysm. At the conclusion of the case, thrombus was noted in 1 of the MCA branches, presumably related to thrombus from the guide catheter.
Example case 2.
Patient 4 (Fig 2) was a 77-year-old woman with an enlarging anterior communicating artery (AcomA) aneurysm. Because of the large neck, initial placement of a 3 × 20 mm Neuroform stent was required. The aneurysm was then treated with multiple coils. A small residual dog ear remained at the conclusion. At follow-up 18 months later, the thrombosed portion of the aneurysm had markedly diminished, and the small dog ear remained. The stent was widely patent.
Fig 2.
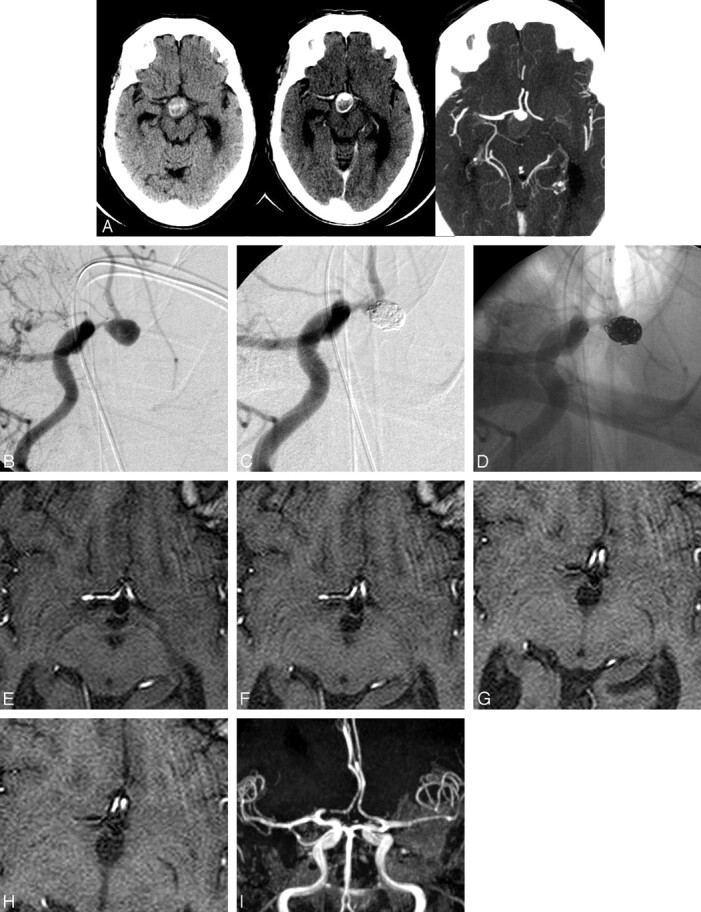
(A) Noncontrast CT, contrast-enhanced CT, and axial CTA images showing a large partially thrombosed AcomA aneurysm. Frontal oblique angiograms showing the aneurysm before (B) and after (C, D) treatment. Eighteen-month follow-up MRA sequential source images (E-H) and maximum intensity projection (I) show stent widely patent and resolution of the aneurysm thrombus. The area of signal intensity dropout in the A1 segment was stable in the immediate postoperative and other follow-up MRAs and was related to artifact from the end markers on the stent.
Complications
No major procedural or periprocedural complications, including parent artery rupture, in-stent thrombosis, or infarction in the vascular distribution of the treated lesion, occurred. In 2 aneurysms, thrombus formation occurred within the stent, which dissolved after the intra-arterial administration of 10 mg of abciximab through the treating microcatheter. No clinical or radiographic strokes were present. One patient undergoing treatment of a distal ACA aneurysm experienced a small infarction in the ipsilateral MCA territory, presumably related to thrombus from the guide catheter. The patient had mild left-handed weakness, which had resolved 8 months postprocedure (previous example).
Discussion
Previously, endovascular therapy was reserved for patients harboring aneurysms/lesions considered high risk for surgical treatment due to location and/or morphology.8,9 The results of the International Subarachnoid Aneurysm Trial (ISAT) trial10 demonstrated positive outcomes in patients undergoing endovascular therapy for ruptured cerebral aneurysms and have helped validate the role of aneurysm coiling. The confirmation of endovascular therapy has stimulated an increase in research in this field, allowing rapid development of new tools such as the Neuroform stent. The Neuroform stent is a US Food and Drug Administration–approved device indicated for use in wide-necked aneurysms.
The release of newer sophisticated devices enables treatment of lesions that were previously deemed untreatable. However, beyond the scope of the indications for use in the device manual, little is known about the limits of the device. To this end, there are no reports in the literature detailing the feasibility, use, or safety of stents in small (<2 mm) cerebral vessels. This article describes 8 patients who have safely and successfully undergone stent treatment in vessel diameters <2 mm with positive short- and intermediate-term results.
All aneurysms and stenoses were safely and effectively treated (Table 1). No untoward outcomes related to the device were observed in the short term (6 months) or intermediate term (18 months). There was increased incidence of intraprocedural thrombus formation (25%), which was successfully treated with an abciximab bolus. Although this finding suggests that there is an increased risk of thrombus formation despite adequate prophylaxis, it did not result in any untoward clinical or imaging outcomes. This outcome does reinforce the use of the Neuroform stent for electively treated aneurysms, in which additional thrombolytics can be used if required for intraprocedural thrombus formation. The 2 stents placed for aneurysm treatment related to coil herniation adequately displaced the coil loops back into the aneurysm neck. The development of sophisticated small adjunctive devices such as the Neuroform stent increases the type and number of aneurysms that can be treated by endovascular means and with improved safety.
Conclusions
The use of self-expanding stents in vessel diameters <2 mm can be performed safely and efficaciously. The development of lower profile endovascular devices allows accessibility and treatment of lesions in distal cerebral vessels. Intraprocedural thrombus formation was seen in a relatively high (25%) number of patients but resolved quickly with abciximab administration. No untoward clinical outcomes were seen. Further follow-up and experience are necessary to determine the durability of these promising results.
References
- 1.Lee YJ, Kim DJ, Suh SH, et al. Stent-assisted coil embolization of intracranial wide-necked aneurysms. Neuroradiology 2005;479:680–89. Epub 2005 Jul 19 [DOI] [PubMed] [Google Scholar]
- 2.Fiorella D, Albuquerque FC, Deshmukh VR, et al. Usefulness of the Neuroform stent for the treatment of cerebral aneurysms: results at initial (3–6-month) follow-up. Neurosurgery 2005;56:1191–201 [DOI] [PubMed] [Google Scholar]
- 3.Thorell WE, Chow MM, Woo HH, et al. Y-configured dual intracranial stent-assisted coil embolization for the treatment of wide-necked basilar tip aneurysms. Neurosurgery 2005;56:1035–40 [PubMed] [Google Scholar]
- 4.Lylyk P, Ferrario A, Pasbon B, et al. Buenos Aires experience with the Neuroform self-expanding stent for the treatment of intracranial aneurysms. J Neurosurg 2005;102:235–41 [DOI] [PubMed] [Google Scholar]
- 5.Benitez RP, Silva MT, Klem J, et al. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery 2004;54:1359–67; discussion 1368 [DOI] [PubMed] [Google Scholar]
- 6.Cohen JE, Gomori JM, Umansky F. Endovascular management of spontaneous bilateral symptomatic vertebral artery dissections. AJNR Am J Neuroradiol 2003;24:2052–56 [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta B, Burke T, Kole M, et al. Stent-within-a-stent technique for the treatment of dissecting vertebral artery aneurysms. AJNR Am J Neuroradiol 2003;24:1814–18 [PMC free article] [PubMed] [Google Scholar]
- 8.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–82 [DOI] [PubMed] [Google Scholar]
- 9.Eskridge JM, Song JK. Endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachable coils: results of the Food and Drug Administration multicenter clinical trial. J Neurosurg 1998;89:81–86 [DOI] [PubMed] [Google Scholar]
- 10.Molyneux A, Kerr R, Stratton I, et al, and the International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 2002;360:1267–74 [DOI] [PubMed] [Google Scholar]