Abstract
BACKGROUND AND PURPOSE: Although the cerebellum has not attracted the same degree of attention as cortical areas and the hippocampus in traumatic brain injury (TBI) literature, there is limited structural and functional imaging evidence that the cerebellum is also vulnerable to insult. The cerebellum is emerging as part of a frontocerebellar system that, when disrupted, results in significant cognitive and behavioral consequences. We hypothesized that cerebellar volume would be reduced in children following TBI and wished to examine the relation between the cerebellum and known sites of projection, including the prefrontal cortex, thalamus, and pons.
MATERIALS AND METHODS: Quantitative MR imaging was used to measure cerebellar white and gray matter and lesion volumes 1–10 years following TBI in 16 children 9–16 years of age and 16 demographically matched typically developing children 9–16 yeas of age. Cerebellar volumes were also compared with volumetric data from other brain regions to which the cerebellum projects.
RESULTS: A significant group difference was found in cerebellar white and gray matter volume, with children in the TBI group consistently exhibiting smaller volumes. Repeating the analysis after excluding children with focal cerebellar lesions revealed that significant group differences still remained for cerebellar white matter (WM). We also found a relation between the cerebellum and projection areas, including the dorsolateral prefrontal cortex, thalamus, and pons in 1 or both groups.
CONCLUSION: Our finding of reduced cerebellar WM volume in children with TBI is consistent with evidence from experimental studies suggesting that the cerebellum and its related projection areas are highly vulnerable to fiber degeneration following traumatic insult.
Experimental and clinical imaging studies of traumatic brain injury (TBI) have traditionally focused on cortical and hippocampal regions of the brain because these are common sites of focal injury. However, areas of focal injury within the cerebellum have been reported in lesion-analysis literature by using fluid-attenuated inversion recovery (FLAIR) and fast-field echo (FFE)1 and T2*-weighted gradient-echo fast low-angle shot2 techniques. More recent imaging techniques such as susceptibility-weighted imaging in TBI have also revealed small focal hemorrhagic abnormalities in the cerebellum.3 In addition to evidence of focal injury, a single-photon emission tomography study in TBI subjects revealed cerebellar hypoperfusion in patients with vertigo or balance problems, despite having morphologically normal-appearing cerebellums on CT and MR imaging.4 Finally, qualitative rating of atrophy in the cerebellum following TBI in children has also been reported in a single article,5 in which the authors described late MR imaging findings in 13 children following TBI and found unexpected cerebellar atrophy in 6 of them. However, these authors did not perform formal quantitative analysis, and no distinction was made as to whether the atrophy was primarily related to gray or white matter (WM).
Imaging studies using quantitative MR imaging measures to evaluate the extent of generalized cerebellar atrophy in patients with TBI have also been limited. One study examined a small sample of adult patients (N = 3) with pre- versus postinjury morphometric analysis and reported extensive degenerative changes in the cerebellum as well as in other structures following moderate-to-severe TBI.6 Voxel-based morphometry also demonstrated significant cerebellar gray matter (GM) volume reduction in a small sample of adult patients with TBI (N = 9) compared with control subjects.7
In this study, we characterized changes in both cerebellar WM and GM in children following TBI by using morphometric analysis. To further explore the contribution of focal-versus-diffuse injury processes, we also examined group differences that remained after excluding all children with focal cerebellar injury (ie, lesions) apparent on conventional imaging. Finally, although the cerebellum itself has not attracted much attention in clinical TBI literature, it is part of a system of frontocerebellar pathways known to result in significant cognitive and behavior consequences when disrupted. Recent functional imaging studies now suggest that most higher order cognitive tasks that require the dorsolateral prefrontal cortex also involve aspects of the cerebellum because activation in these regions is correlated and closely coupled.8 The pons and the thalamus have also been implicated as important intermediary structures in this pathway between the frontal cortex and cerebellum.9,10 Therefore, we examined the relation between cerebellar volume and the volume of known sites of projection to and from the cerebellum, including the pons, the thalamus, and areas of the prefrontal cortex such as dorsolateral, ventrolateral, and superior medial areas.
Materials and Methods
Subjects
The TBI group consisted of 16 children (8 boys, 8 girls) who had sustained moderate-to-severe TBI (Glasgow Coma Scale [GCS]; mean, 5.7 ± 2.8; range, 3–11) and were injured as a result of vehicle, bicycle, or pedestrian-versus-vehicle crashes. The mean postinjury interval was 3.1 ± 2.4 years (range, 1.0–10.1 years), and the mean age at the time of scanning was 12.9 ± 2.5 years (range, 9.0–16.8 years). Sixteen typically developing (TD) children (8 boys, 8 girls) were selected to individually match the patients with TBI in age (within 6 months; mean, 12.8 ± 2.4 years; range, 9.0–16.4 years), sex, ethnicity, handedness, and maternal education (an index of socioeconomic status) (mean, 13.2 ± 1.6 years; range, 12.0–16.0 years). No child had a pre-existing history of head injury, neurologic disorder, psychiatric disorder, or child abuse. Additional selection criteria included an Abbreviated Injury Scale score of less than 4 for areas of the body other than the head and no history of postresuscitation hypoxia or hypotension exceeding 30 minutes in duration. Institutional review board approval was obtained as well as informed consent by each subject's parent or guardian and assent by each child participant before participation in this study.
MR Imaging Acquisition
MR imaging was performed by using 1.5T Intera scanners (Philips Medical Systems, Best, the Netherlands) with identical protocols at 2 Texas medical centers. Both T1-weighted (T1WI) (15-ms TR, 4.6-ms TE, 1.0-mm sections) and T2-weighted (T2WI) (3500-ms TR, 114-ms TE, 1.5-mm sections) 3D sagittal acquisition series with a 256-mm FOV were used. Reconstructed voxel size was 1 × 1 × 1 mm for the T1WI and 1 × 1 × 1.5 mm for the T2WI series. A dual-echo acquisition proton-attenuation/T2WI (2200-ms TR, 20/120-ms TE, 5.0-mm sections) axial sequence was used for whole-brain quantification to derive an estimate of total intracranial volume (TICV). A 240-mm FOV was used with a reconstructed voxel size of 0.47 × 0.47 × 5.0 mm. A coronal T2WI FLAIR sequence was also used for identification of lesions (1100-ms TR, 140-ms TE, 5.0-mm sections). In addition, an axial FFE sequence was used (755-ms TR, 23-ms TE, 5.0-mm sections with a 1.0-mm gap). For both sequences, a 220-mm FOV was used with a reconstructed voxel size of 0.86 × 0.86 × 5.0 mm.
Volumetric Analysis
Analyze 6.0 (Biomedical Imaging Resource, Mayo Foundation, Rochester, Minn) software was used to convert each dataset into a 3D volume and to create 1-mm isovoxels. T1WI and T2WI image sets were coregistered after realignment to the anterior-to-posterior commissure line and the interhemispheric fissure. Interactive manual segmentation of GM, WM, and CSF and manual tracing of the cerebellum were performed by using Analyze 6.0 software tools. Finally, volumes (cubic centimeters) of GM, WM, and CSF were calculated by summing pixels designated as GM, WM, or CSF across the sections.
Whole-cerebellum quantitative measurement11 and an estimate of total intracranial volume12 were performed by using previously established protocols. Briefly, cerebellar measurement commenced with the most midsagittal section, determined by the clear separation of the lingual from the medullary velum. Using the midsagittal section as a starting point, we then measured every sixth section bilaterally to include all aspects of the cerebellum, and a correction factor was applied.
To further explore the relation of the cerebellar volumes and areas of focal injury to other brain areas connected by known pathways with the cerebellum, we used previously obtained volumetric measures of frontal areas, thalamus, and pons from this patient sample. Measurement of these areas was performed by using methods previously described elsewhere (M.A. Fearing et al, unpublished data, 2006).13
Lesion areas in the cerebellar hemispheres, cerebellar vermis, and other brain regions (ie, frontal areas, thalamus, and brain stem) were manually traced on each section by using software tools accompanying the PACS software (Agfa Healthcare, Mortsel, Belgium). Areas were reported in square centimeters, summed, and converted to volume measures (area multiplied by section thickness). Areas of signal-intensity abnormality were identified and traced by a board-certified neuroradiologist by using FLAIR and FFE images, which facilitate lesion conspicuity and measurement.1
Interoperator Reliability
Two raters performed segmentation and tracing under the supervision of both a neuroradiologist and an expert in volumetric imaging. Inter- and intrarater reliabilities were computed on each measure used in the cerebellar protocol to ensure consistency in both tracing and segmentation. In determining reliability, the same rater traced 6 cases (18.75% of the sample) to establish intrarater reliability, and the same 6 cases were also retraced by the second rater to establish inter-rater reliability. Intraclass correlational coefficients (ICCs) were calculated by using a Shrout-Fleiss fixed-set model to test reliability. All measures exceeded an ICC of 0.90 (mean, 0.97; range, 0.92–0.99).
Design and Statistical Analysis
A general linear model approach was used to examine group differences in cerebellar WM and GM between TBI and TD children. TICV was included as a covariate to correct for differences in head size. Then, to further examine the impact of focal injury versus diffuse injury on WM and GM, we excluded all children with TBI with lesions in the cerebellar vermis or cerebellar hemispheres (n = 4) and their matched TD children (n = 4) and performed a second analysis of variance and covariance (ANCOVA) by using the same model.
The relation of the cerebellar volumes to other brain areas connected by known pathways with the cerebellum was examined with additional ANCOVAs, again by using TICV as a covariate to correct for differences in head size. These models examined the relation between 1) cerebellar WM and dorsolateral, ventromedial, and superior medial frontal WM volumes, 2) total cerebellar and thalamic volume, 3) total thalamus and total dorsolateral frontal volume, 4) total cerebellar volume and pons volume, and 5) pontine and dorsolateral frontal volumes. Assumptions of each statistical model were verified.
Results
The TBI and TD groups of children did not significantly differ in sex, age, maternal education, or socioeconomic status. However, the groups did differ in race/ethnicity (Fisher exact test, P = .02), with the TBI group including more African-American children and the TD group including more Hispanic and Asian children.
Differences in Cerebellar Volume in TBI and TD Children
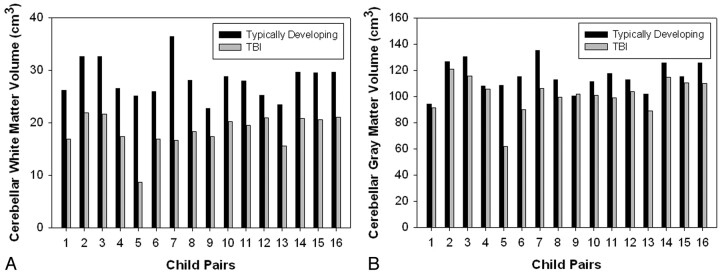
Analysis of simple group differences in the overall sample revealed a significant group difference in WM (F(1,29) = 51.91, P < .0001) and GM (F(1,29) = 5.61, P = .025) volume in the cerebellum, with children in the TBI group consistently exhibiting smaller tissue volumes (Fig 1A, -B). Specifically, least squared mean (LSM) WM volume for the TD group was 28.14 cm3 as opposed to 19.49 cm3 in the TBI group. LSM GM for the TD group was 112.49 cm3 versus 107.18 cm3 in the TBI group. Repeating the analysis after excluding all children with focal lesions in the cerebellum and their matched controls revealed that significant group differences still remained for cerebellar WM (F(1,21) = 47.97, P < .0001) despite the smaller sample size, though group differences for GM were no longer statistically significant (F(1,21) = 1.64, P = .215). Areas of focal abnormality (ie, lesions) in the cerebellar hemispheres (range, 0.125–1.524 cm3 in 3 children) and vermi (range, 0.257–0.347 cm3 in 1 child) were generally small. One child had bilateral areas of focal abnormality in both the cerebellar hemispheres and vermi, 1 child had bilateral lesions in the cerebellar hemispheres only, 1 child had a lesion in the right cerebellar hemisphere only, and 1 child had a focal abnormality in the left cerebellar hemisphere only.
Fig 1.
Bar graphs representing group differences in white matter volume (A) and gray matter volume (B) between typically developing children and those with TBI.
Cerebellum and the Prefrontal Cortex
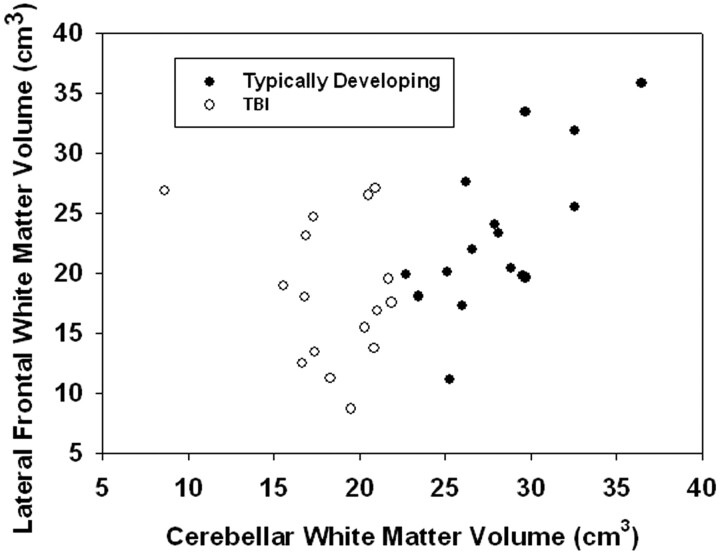
Further analyses of the relation between cerebellar and prefrontal WM revealed a significant group by area interaction for total dorsolateral frontal WM volume (F(1,27) = 9.98, P = .004). In this analysis, there was a significant positive relation between WM volume in the dorsolateral frontal and cerebellar regions for children in the TD group (t(27) = 2.84, P = .008), but not the TBI group (t(27) = −1.50, P = .144) (Fig 2). No significant relation existed between WM in the cerebellum and either the superior medial and ventromedial frontal areas for both groups.
Fig 2.
Scatterplot illustrating relation between total cerebellar white matter volume and lateral frontal white matter volume.
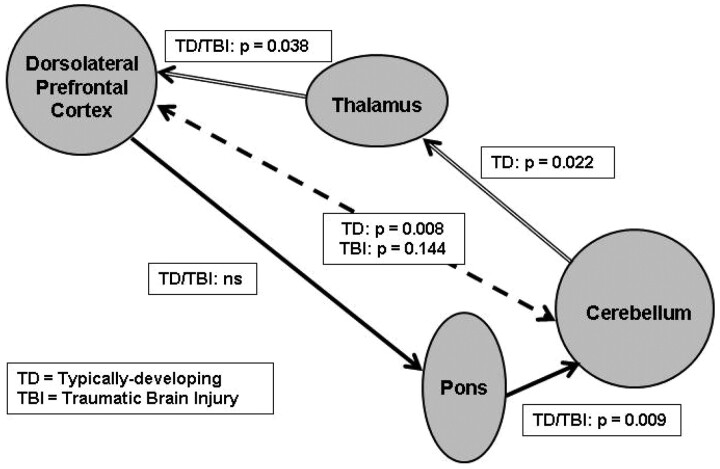
Relation Between Cerebellothalamocortical Structures
In the first part of the afferent pathway between the cerebellum and the thalamus (Fig 3), we found a significant group interaction effect (F(1,27) = 5.17, P = .0312), in which TD children demonstrated the expected positive relation in volumes between the 2 structures (t(27) = 2.43, P = .022), but children with TBI did not. In the second part of the pathway between the thalamus and cortical projections ending in the dorsolateral frontal areas, we found a significant relation between the volumes in these structures (F(1,28) = 4.77, P = .038) in both groups (eg, no group interaction effect), in which greater cerebellar WM was related to greater dorsolateral frontal WM volume.
Fig 3.
Cerebrocerebellar connections illustrating the relation between volumes in structures within the afferent pathway (cerebellothalamocortical structures connected by arrows with double lines) and the efferent pathway (corticopontocerebellar structures connected by arrows with solid lines). The relation between dorsolateral prefrontal cortex and the cerebellum is indicated by arrows with dotted lines. ns indicates statistically not significant (P > 0.05).
Relationship Between Corticopontocerebellar Structures
In the first part of the efferent pathway between the cortical dorsolateral frontal areas and their presumed projection target in the pons (Fig 3), no significant relation was found between the volumes of these structures. However, in the second part of the pathway from the pons to the cerebellum, both groups exhibited the expected positive relation in volume between these 2 structures (F(1,28) = 8.02, P = .009), with greater pons volume being associated with greater cerebellar volume.
Discussion
GM and WM Volume Differences
We found significant differences between TD children and those with TBI in total cerebellar WM and GM. Figure 4 illustrates visible atrophy in the cerebellum of a child with both focal and diffuse cerebellar insult following TBI, compared with a TD child of comparable age and sex. However, even after excluding children with focal abnormalities evident on conventional imaging, decreased cerebellar WM volume was observed in the TBI group. Although we hypothesized that group differences would exist, the magnitude of WM differences in particular was unexpected, given the relatively low frequency of focal abnormalities on imaging and the number of patients in our sample. Our findings are consistent with previous pathology reports of extensive axonal degeneration observed by using staining techniques in the cerebellum of mature and immature rodents exposed to experimental models of focal TBI.14,15 Matthews et al14 reported that axonal degeneration was noted in the cerebellum of every injured animal even though the cortex had been the target of focal injury. This is consistent with our finding of prominent cerebellar WM volume loss in every child with TBI compared with his or her matched control, regardless of the extent or location of focal injury (Fig 1A). Additionally, strong group differences in WM volume were apparent in our study even after children with focal cerebellar abnormalities were excluded from the analysis. We postulate that this finding reflects a diffuse injury mechanism such as progressive axonal pathology16 or myelin degeneration17 rather than just a focal lesion effect.
Fig 4.
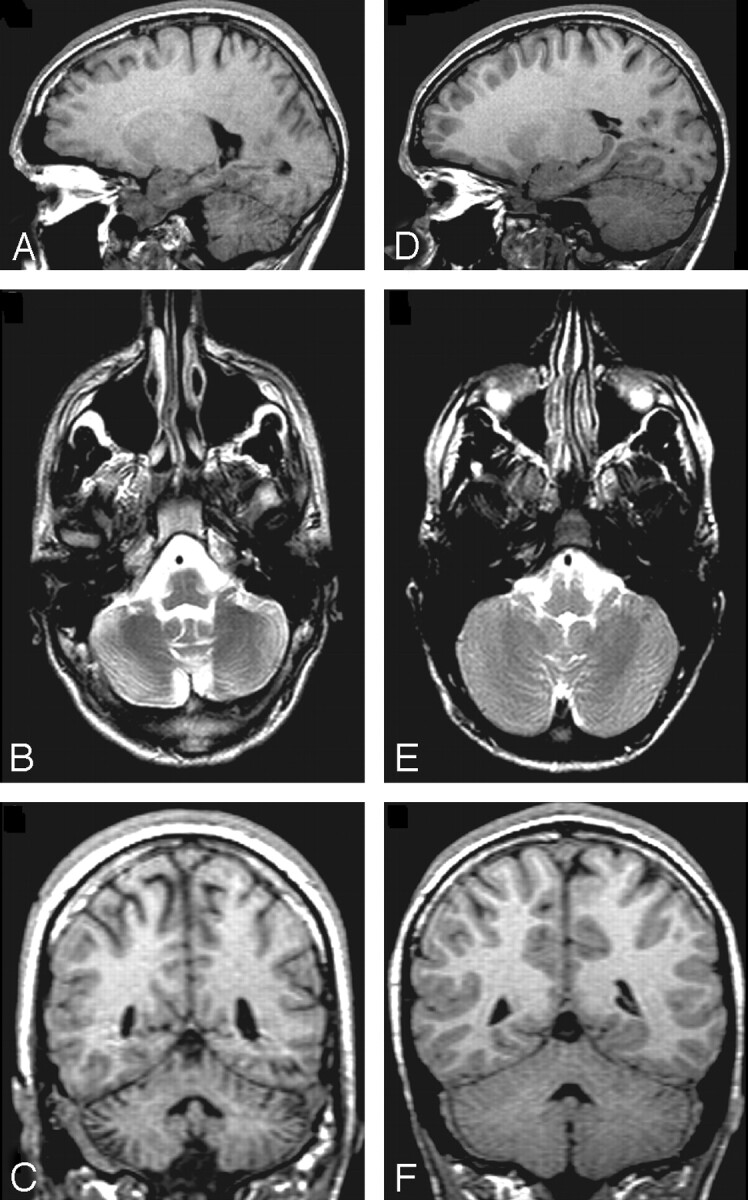
T1WI sagittal (A), T2WI axial (B), and T1WI coronal (C) images of the cerebellum from a 16-year-old boy with TBI (initial GCS = 3) and a TD child of comparable age and sex (D–F). The child with TBI has diffuse cerebellar atrophy and increased CSF within the cerebellar folia that can be noted in all planes.
Despite striking axonal degeneration observed in the cerebellum of the animals in Matthews et al,14 the authors indicated a relative absence of cerebellar cellular damage. Our findings of WM being disproportionately affected in relation to GM are again consistent with this report, though nearly every patient with TBI also had less GM volume than his or her age- and sex-matched control child (Fig 1B). In addition, other studies using experimental TBI models such as fluid-percussion impact to the forebrain have noted activation of microglia and significant loss of Purkinje cells in the vermis of the cerebellum as early as 7 days postinjury18,19 as well as positive fluoro-Jade staining in neurons (indicating degeneration)20 and evidence of high susceptibility of Purkinje neurons to chemical and mechanical damage.21 In addition, a cryogenic cortical injury model, which is considered to represent vasogenic brain edema, has also demonstrated a significant increment in c-Jun expression, a marker of stress or injury, in Purkinje cells of the cerebellar hemispheres at a week and longer postinjury in response to distant focal cortical insult.22 Finally, a previous imaging study in patients with TBI also revealed significant GM volume loss7 in the cerebellum, so it is likely that GM is also impacted as a result of TBI, albeit to a lesser degree than WM.
Developmental Factors in Cerebellar Injury
Using a similar injury mechanism, Finnie et al23 noted that the cerebellar damage observed in immature animals was not present in adult sheep.24 Cerebellar damage to the immature human brain may be of particular concern given the protracted course of development of the neocerebellum (eg, posterior lobe of the lateral hemispheres, lobules VI and VII of the vermis, and the dentate nucleus), which parallels that of the prefrontal cortex.8 In addition to the cognitive sequelae of cerebellar damage, there may be disruption in the trajectory of some motor development skills, including fine motor control, bimanual coordination, and visuomotor skills, which are not fully developed until adolescence.
Corticocerebellar Circuits
In our study, we found a significant relation between dorsolateral prefrontal WM volume and cerebellar WM volume in TD children. Recent histologic studies in primates have confirmed pathways between frontal cortical areas and the cerebellum via the thalamus by using retrograde transneuronal virus transport tracings.25 These studies used prefrontal subregions, including the medial, dorsal, ventral, and lateral areas, but found that medial and dorsolateral frontal areas specifically were targets of cerebellar output. Our study is generally consistent with these results in that the strongest relation was found between the cerebellar WM and the dorsolateral frontal WM. Additionally, recent imaging studies by using functional connectivity MR imaging, a technique that allows examination of coherence in MR signal intensity between functionally related brain regions, demonstrated coherence between the dentate nucleus of the cerebellum and the dorsolateral frontal cortex in younger healthy individuals.26 Common impairments following TBI include frontally mediated abilities such as working memory, verbal fluency, set-shifting, and attention as well as behavioral difficulties such as personality change with impaired regulation of affect, irritability, disinhibition, and inappropriate behavior. However, because the cerebellum may play a central role in networks underlying these abilities,8,27 this may be an important structure to study further in TBI. Interestingly, although we did find a relationship between dorsolateral frontal and cerebellar WM in the TD children, we did not find this relation in the TBI group. This dissociation may be due to either disproportionate damage in either the frontal or cerebellar structures in the TBI group or the disruption of normal development following TBI.
Examination of the cerebellothalamocortical system revealed the expected significant relation between the first half of the pathway (ie, cerebellum to thalamus) in the TD children, but not in the TBI group. This may indicate that the cerebellum is disproportionately affected in relation to the thalamus in children with TBI. For the second half of the pathway (ie, thalamus to dorsolateral frontal), there was a significant relation between the volumes of the 2 structures in both groups, perhaps indicating either a more direct relationship between these structures or a more proportional degree of damage in these structures.
In the corticopontocerebellar pathway, examination of the first part of the pathway (dorsolateral frontal matter to pons) revealed no relation between the volumes in these areas, consistent with a previous report examining the relation of volumetric measures in these 2 structures in alcoholic subjects.28 It is possible that this relation may not be prominent given the decreased physical proximity of these structures and multiple sources of input from the noncortical structures to the pons. However, in the second part of the circuit (pons to cerebellum), we did observe a significant relation between pontine and cerebellar volumes in both groups, in which decreased volume in the cerebellum was associated with decreased volume in the pons, even after correction for head size. This is again consistent with results indicating a significant relation between the volumes of these 2 structures in alcoholic subjects.28 The significant relation between the pons and the cerebellum may be attributed to the increased specificity of neural pathways between the cerebellum and other subcortical structures (eg, midbrain and spinal cord) that converge at the level of the pons. Alternatively, the pons and cerebellum are both significantly impacted by rotational force injury in experimental TBI models,23,29 perhaps to a greater degree than the prefrontal areas.
Mechanism of Injury
Some evidence suggests that cerebellar atrophy may be structure-specific rather than simply a consequence of retrograde or anterograde degeneration from other cortical or subcortical structures. Using histologic staining techniques in a rodent model of TBI, Matthews et al14 reported that the intracerebellar fiber degeneration did not appear to be caused by retrograde trans-synaptic extension from the thalamus or by anterograde trans-synaptic mechanisms from pontine nuclei. Rather, these authors proposed that the cerebellar degeneration they observed was more likely a direct consequence of cerebellar cortical impact against the occipital bone and not trans-synaptic degeneration from areas outside the cerebellum. However, these authors suggested that cerebellar injury via mechanical impact could initiate axonal degeneration within the cerebellum either by direct axonal shearing or by axolemmal injury and axoplasmic flow disruption within axons. The lack of relation between the thalamus and cerebellum volumes in the children with TBI in our study supports the conclusion that cerebellar damage may not be simply the result of injury-induced retrograde fiber degeneration via the thalamus.
In contrast, however, we found a significant relation between the cerebellar and the pontine volumes in children with TBI, though the relationship was again more striking in the control children. It is possible that the proximity and location of these 2 structures render them susceptible to forces more similar in nature or magnitude or that the higher concentration of WM in the pons creates an increased vulnerability to axonal injury. Alternatively, it is possible that axonal degeneration initiated within the cerebellum itself may in turn lead to anterograde fiber degeneration manifesting as decreased pontine volume. In addition to impact against the occipital bone, the cerebellum may have impact against the tentorium cerebelli or petrous temporal ridge, though this did not appear to result in the expected focal injury in our sample. An experimental ovine TBI model evaluating the effect of impact location revealed that in every animal with temporal impact, there was multifocal necrosis of the internal granular layer of the cerebellum, often with change in Purkinje cells.23 Temporal impact has been considered more likely to produce the angular acceleration of the brain responsible for diffuse injury to WM in man, and most children in our TBI sample were involved in acceleration-type injuries (eg, motor vehicle crashes). It is possible that force mechanics may prove an important factor in damage to the cerebellum. It is also possible that the striking cerebellar volume loss observed in this study may also be attributable to or exacerbated by pressure changes in the enclosed cerebellar space in combination with other factors. Clearly further studies are necessary to confirm these preliminary results and clarify the mechanism of cerebellar injury.
Limitations and Future Directions
Moderate-to-severe TBI frequently results in multiple areas of focal injury in addition to diffuse injury, and parenchymal volume loss was certainly not restricted to the cerebellum in our sample. Therefore, it is difficult to disentangle the unique relation of cerebellar injury to volume loss observed in other areas of the brain, which may have also been impacted by shear-strain forces or excitotoxic injury, which is not discernible on conventional neuroimaging. Additionally, the heterogeneity in injury severity, differences in location and extent of focal injury, and the frequent involvement of the frontal and temporal areas in our sample preclude analyses elucidating the degree to which cognitive abilities specific to the cerebellum were impacted. However, our study is the first to examine the impact of TBI on the cerebellum in children, a structure that has recently been recognized as potentially playing an important role in various cognitive capacities traditionally ascribed only to the frontal lobes. In addition, our study highlights the relative vulnerability of this often-overlooked structure in TBI.
Conclusion
In accordance with our hypothesis, this study suggests that both WM and GM volume loss occurs in the cerebellum following TBI, with decreased WM even when subjects with focal lesions were excluded from analysis. Our finding of reduced cerebellar WM volume in children with TBI is consistent with evidence from experimental studies of infrahuman primates suggesting that the cerebellum is indeed a structure highly vulnerable to fiber degeneration following traumatic insult. Examination of volumetric relationships between primary structures implicated in pathways projecting to and from the cerebellum indicates that damage in the cerebellum may be structure-specific rather than simply the result of retrograde degeneration. Future imaging studies using techniques such as diffusion tensor imaging may further elucidate the nature and degree of WM axonal disruption in the cerebellum and the degree to which this degeneration represents generalized-versus-structure-specific injury. In addition, evolving functional neuroimaging techniques may further reveal the involvement of the cerebellum in various cognitive processes frequently compromised in patients with TBI.
Acknowledgments
We gratefully acknowledge the assistance of Stacey K. Martin in manuscript preparation.
Footnotes
This work was supported by NIH grant NS-21889 and the Baylor College of Medicine, General Clinical Research Center grant U19 HD35476.
References
- 1.Pierallini A, Pantano P, Fantozzi LM, et al. Correlation between MRI findings and long-term outcome in patients with severe brain trauma. Neuroradiology 2000;42:860–67 [DOI] [PubMed] [Google Scholar]
- 2.Parizel PM, Ozsarlak, Van Goethem JW, et al. Imaging findings in diffuse axonal injury after closed head trauma. Eur Radiol 1998;8:960–65 [DOI] [PubMed] [Google Scholar]
- 3.Tong KA, Ashwal S, Holshouser BA, et al. Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann Neurol 2004;56:36–50 [DOI] [PubMed] [Google Scholar]
- 4.Kinuya K, Kakuda K, Nobata K, et al. Role of brain perfusion single-photon emission tomography in traumatic head injury. Nucl Med Commun 2004;25:333–37 [DOI] [PubMed] [Google Scholar]
- 5.Soto-Ares G, Vinchon M, Delmaire C, et al. Cerebellar atrophy after severe traumatic head injury in children. Childs Nerv Syst 2001;17:263–69 [DOI] [PubMed] [Google Scholar]
- 6.Gale SD, Johnson SC, Bigler ED, et al. Trauma-induced degenerative changes in brain injury: a morphometric analysis of three patients with preinjury and postinjury MR scans. J Neurotrauma 1995;12:151–58 [DOI] [PubMed] [Google Scholar]
- 7.Gale SD, Baxter L, Roundy N, et al. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J Neurol Neurosurg Psychiatry 2005;76:984–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev 2000;71:44–56 [DOI] [PubMed] [Google Scholar]
- 9.Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist 2001;7:315–24 [DOI] [PubMed] [Google Scholar]
- 10.Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci 1997;17:438–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardan AY, Minshew NJ, Harenski K, et al. Posterior fossa magnetic resonance imaging in autism. J Am Acad Child Adolesc Psychiatry 2001;40:666–72 [DOI] [PubMed] [Google Scholar]
- 12.Blatter DD, Bigler ED, Gale SD, et al. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR Am J Neuroradiol 1995;16:241–51 [PMC free article] [PubMed] [Google Scholar]
- 13.Wilde EA, Hunter JV, Newsome MR, et al. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J Neurotrauma 2005;22:333–44 [DOI] [PubMed] [Google Scholar]
- 14.Matthews MA, Carey ME, Soblosky JS, et al. Focal brain injury and its effects on cerebral mantle, neurons, and fiber tracks. Brain Res 1998;794:1–18 [DOI] [PubMed] [Google Scholar]
- 15.Tong W, Igarashi T, Ferriero DM, et al. Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Exp Neurol 2002;176:105–16 [DOI] [PubMed] [Google Scholar]
- 16.Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol 1992;2:1–12 [PubMed] [Google Scholar]
- 17.Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol (Berl) 2002;103:607–14 [DOI] [PubMed] [Google Scholar]
- 18.Fukuda K, Aihara N, Sagar SM, et al. Purkinje cell vulnerability to mild traumatic brain injury. J Neurotrauma 1996;13:255–66 [DOI] [PubMed] [Google Scholar]
- 19.Mautes AE, Fukuda K, Noble LJ. Cellular response in the cerebellum after midline traumatic brain injury in the rat. Neurosci Lett 1996;214:95–98 [DOI] [PubMed] [Google Scholar]
- 20.Hallam TM, Floyd CL, Folkerts MM, et al. Comparison of behavioral deficits and acute neuronal degeneration in rat lateral fluid percussion and weight-drop brain injury models. J Neurotrauma 2004;21:521–39 [DOI] [PubMed] [Google Scholar]
- 21.Slemmer JE, Weber JT, De Zeeuw CI. Cell death, glial protein alterations and elevated S-100 beta release in cerebellar cell cultures following mechanically induced trauma. Neurobiol Dis 2004;15:563–72 [DOI] [PubMed] [Google Scholar]
- 22.Liu JS, Chang YY, Wu HS, et al. Transtentorial cerebellar c-jun expression after focal cerebral cortical injury in mice. Neurosci Lett 2000;282:85–88 [DOI] [PubMed] [Google Scholar]
- 23.Finnie JW, Van den Heuvel C, Gebski V, et al. Effect of impact on different regions of the head of lambs. J Comp Pathol 2001;124:159–64 [DOI] [PubMed] [Google Scholar]
- 24.Lewis SB, Finnie JW, Blumbergs PC, et al. A head impact model of early axonal injury in the sheep. J Neurotrauma 1996;13:505–14 [DOI] [PubMed] [Google Scholar]
- 25.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci 2001;21:700–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen G, McColl R, Barnard H, et al. Magnetic resonance imaging of cerebellar-prefrontal and cerebellar-parietal functional connectivity. Neuroimage 2005;28:39–48 [DOI] [PubMed] [Google Scholar]
- 27.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain 1998;121(Pt 4):561–79 [DOI] [PubMed] [Google Scholar]
- 28.Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol Clin Exp Res 2003;27:1409–19 [DOI] [PubMed] [Google Scholar]
- 29.Hamberger A, Huang YL, Zhu H, et al. Redistribution of neurofilaments and accumulation of beta-amyloid protein after brain injury by rotational acceleration of the head. J Neurotrauma 2003;20:169–78 [DOI] [PubMed] [Google Scholar]