Abstract
PURPOSE: To determine the analgesic efficacy of percutaneous vertebroplasty in treating osteoblastic and mixed spinal metastases.
MATERIALS AND METHODS: Fifty-two patients underwent 59 vertebroplasty procedures for 103 painful vertebral metastases, among which 53 were pure osteoblastic and 50 were mixed (blastic and lytic). Analgesic efficacy was classified as “excellent,” “good,” “fair,” and “poor.” The patients were followed up at 1 month, 6 months, 12 months, 2 years, and 5 years. The mean follow-up period was 17 months.
RESULTS: The analgesic efficacy rate was 86% at 1 month and 92% at 6 months (among which 71% of patients had “excellent” results and 21% had with “good” results). In most cases, it was stable. It was correlated with vertebral filling quality (Fisher test, P = .0932 at 1 month follow-up) but neither with filling volume (Mann-Whitney test, P = .143 at 1 month) nor with the vertebral structure, pure blastic or mixed (Fisher test, P = .784 at 1 month). There were 5 filling failures (4.7%) whose occurrence was correlated with the pure blastic structure of the vertebra (Mann-Whitney test, P = .033). Local clinical complications were observed in 5 cases (8.5%): 1 transitory radiculalgia (1.7%), 2 durable radiculalgias (3.4%), 1 cauda equina syndrome (1.7%), and 1 hemothorax (1.7%). General clinical complications were 2 pulmonary embolisms (3.4%). No patients died. The occurrence of clinical complications was not correlated with the vertebral structure (Fisher test, P = .279).
CONCLUSION: Vertebroplasty for osteoblastic and mixed metastases allows, with a well-trained operator, a satisfactory anesthesia with acceptable clinical complication rates.
ertebroplasty is a minimally invasive, radiologically guided procedure that consists of percutaneous injection of surgical cement into a vertebra. This local treatment has already proved its analgesic efficacy for osteoporotic vertebral collapses and for lytic spinal metastases.1-4 The purpose of this study was to determine the analgesic efficacy of vertebroplasty for pure blastic or mixed spinal metastases.
Materials and Methods
Between 1996 and 2002, 52 patients (46 women, 6 men; age, 27–84 years; mean age, 54.7 years) presenting painful blastic spinal metastases underwent 59 vertebroplasty procedures in our department. The 103 treated vertebrae had CT imaging evidence of blastic metastases; among them, 53 were pure blastic and 50 were mixed (blastic with lytic zones). Pure blastic changes could correspond to nodular deposits (rounded areas), mottled deposits (irregular zones of bone sclerosis that are interspersed between areas of normal appearing bone); and diffuse deposits (larger zones of increased attenuation). Pure lytic metastases were excluded.
The origin of the metastases was a breast cancer (40 cases), a prostate cancer (3 cases), a lung cancer (3 cases), and a colon, rectum, stomach, heart, kidney, or testis cancer for the 6 other cases. The treated levels were cervical (2 cases), thoracic (59 cases), and lumbar (42 cases). The number of treated vertebrae was 1.72 per procedure.
The criterion of inclusion was a significantly painful spinal metastasis with pure blastic or mixed change, responsible for a chronic pain for at least 3 months. The indication of vertebroplasty was chosen by members of a multidisciplinary staff including oncologists, radiation therapists, orthopedists, and radiologists. In 36% of cases, vertebroplasty was realized after surgery or radiation therapy; in these cases, the analgesic effect of the first local treatment had to be considered as inefficient for at least 3 months. The goal of vertebroplasty was anesthesia in all the procedures.
The assessment of pain was systematic before the procedure; the pain had to be estimated by the patient at 7.5 or more on a verbal analogic scale going from 0 to 10 (10 corresponding to the maximal imaginable pain).
Twelve vertebrae presented a posterior wall disruption caused by the presence of a posterior lytic zone with cortical rupture: they were included because it has been proved that they did not increase the clinical complications rate even if they induced a potential risk of intracanalar leak.4 Among them, 4 vertebrae presented an epidural tumoral extension; they were also included because the epidural component was not important and was not associated with any neurologic symptoms.
Vertebroplasty was always performed under fluoroscopic guidance with a C-arm digitalized angiographic system (Angiostar; Siemens, Erlangen, Germany), which allowed anteroposterior or lateral views. All procedures were performed under neuroleptanalgesia with propofol infusion (1–2 mg/kg/h propofol [Diprivan]). At the cervical level, an anterolateral approach was used and a 12-gauge needle (Ecoffier, Thonon-les-Bains, France) was introduced toward the center of the vertebra. At the thoracic and lumbar levels, a 10-gauge needle was used, generally with a transpedicular route; the posterolateral route was chosen in case of filling failure or of pedicle lysis. The cement (Surgical Simplex-P; Stryker-Howmedica, Limerick, Ireland) was mixed with tungsten powder for opacification and then injected under fluoroscopic guidance. The aim was first to fill the lesion, and as far as possible we tried to fill the whole vertebral volume. The lesion filling began with the filling of the lytic zones in case of mixed vertebra (Fig 1) and of the microfractures in case of pure blastic vertebra (Fig 2). The injection was always stopped when the cement reached the posterior vertebral wall. For each vertebra, we evaluated the filling quality as “good” (more than 2/3 of the vertebral volume), “mild” (1/3 to 2/3), or “insufficient” (less than 1/3) and also the filling volume in milliliters. Immediately after the procedure, standard anteroposterior and lateral radiographs were obtained. Then the patients underwent transverse CT scanning (Somatom, Siemens, Erlangen, Germany) with a collimation of 2 mm, a pitch of 1, and 1.5-mm reconstruction intervals, to check the vertebral filling and to detect eventual cement leaks. Patients were allowed to stand up 6 hours after the procedure.
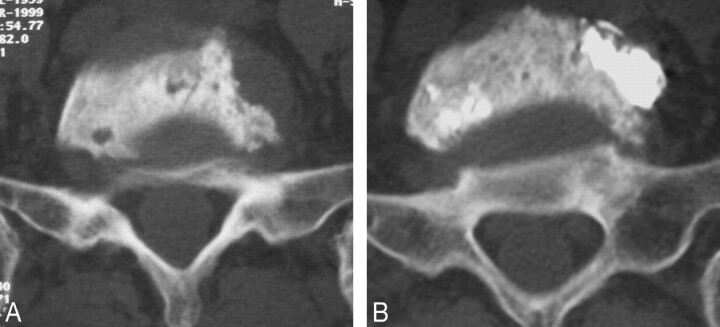
Fig 1.
Example of a mixed vertebral metastasis. Transverse CT scan reconstruction obtained through level of L5 before (A) and after (B) vertebroplasty shows good filling of the lytic portions.
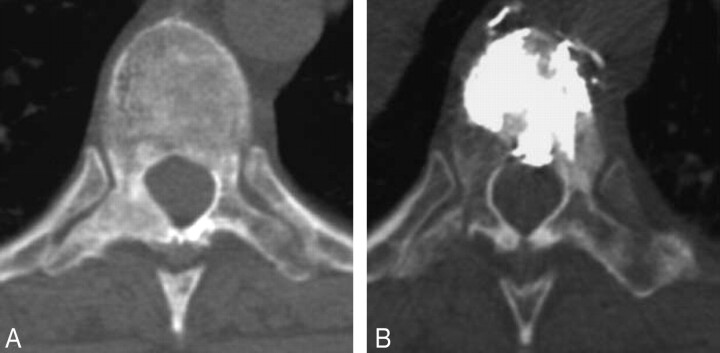
Fig 2.
Example of a diffuse and heterogeneous pure blastic metastasis. Transverse CT scan reconstruction obtained through level of T12 before (A) and after (B) vertebroplasty shows good filling of the vertebral body.
Analgesic result of vertebroplasty was evaluated at 1 and 6 months, and at 1, 2, and 5 years; the mean follow-up after procedure was 17 months per patient. Analgesic efficacy of the vertebroplasty was established for an “excellent result” or for a “good” result, which corresponded to a residual pain of 0–2 and 2.5–4.5, respectively, on the verbal analogic scale going from 0 to 10.
Results
Analgesic Efficacy
Analgesic efficacy (Table 1) was 86% at 1-month follow-up (among which 67% were “excellent” results and 19% were “good” results) and was 92% at 6-month follow-up (among which 71% were “excellent” results and 21% were “good” results). In all of them, the result was immediate (within 24 hours) and there were only few clinical changes at 1 month, 6 months, and at long-term follow-up. When present, the residual pain was occasional, occurring when the patient stood for a long time, coughed, or carried heavy loads; it was minimal in the supine position.
Table 1:
Clinical results (52 patients)
| Follow-Up |
Residual Pain(out of 10): | |||||
|---|---|---|---|---|---|---|
| 1 month | 6 months | 12 months | 2 years | 5 years | ||
| Excellent | 31 (67%) | 27 (71%) | 16 (69%) | 13 (76%) | 2 (67%) | 0–2 |
| Good | 9 (19%) | 8 (21%) | 6 (26%) | 4 (24%) | 1 (33%) | 2.5–4.5 |
| Fair | 2 | 2 | 1 | 0 | 0 | 5–7 |
| Poor | 4 | 1 | 0 | 0 | 0 | 7.5–10 |
| Missing data | 6 | 14 | 29 | 35 | 49 | |
| Efficacy (Excellent + Good) | 86% | 92% | 95% | 100% | 100% | |
Analgesic efficacy was not correlated with the age or the sex of the patients, with the intensity of the initial pain, with the type of cancer, or with the number of treated vertebrae per procedure (Table 2). Moreover, analgesic efficacy was independent of the vertebral structure (pure blastic or mixed): Fisher test, P = .784 at 1-month follow-up, P > .999 at 6 months, and P = .609 at 12 months follow-up. Thus, the proportion of successfully treated vertebrae was 80% for the 50 mixed vertebrae (among which 75% had an “excellent” result) and 64% for the 53 pure blastic vertebrae (among them 79% had an “excellent” result).
Table 2:
Correlation tests for analgesic efficacy
| Analgesic efficacy was not correlated with: |
| -Age of patient (Mann-Whitney test, P = .36 at 6 months) |
| -Sex of patient (Fisher test, P = .58 at 6 months) |
| -Intensity of the initial pain (Mann-Whitney test: P > .9999 at 6 months) |
| -Origin of the cancer (Fisher test: P > .9999 for each type of cancer) |
| -Number of vertebrae treated per procedure (Mann-Whitney test: P = .21 at 6 months) |
| -Filling volume (Mann-Whitney test, P = .368 at 1 month and P = .397 at 6 months) |
Most of the correlation tests were made at 6 months, and the analgesic results are representative (with very few changes at long-term follow-up for the available data).
Cement Leaks
We observed 52 leaks (50.5%) of the 103 treated vertebrae; they were more frequently extraspinal (22%) or in the epidural veins (9.7%). These leaks were always detected through fluoroscopic guidance and injection was immediately stopped; they were always moderate. Most of them were asymptomatic, except in 5 cases (8.5%) in which they induced clinical complications (Table 3).
Table 3:
Types of cement leaks (out of 103 vertebrae)
| Cases |
||
|---|---|---|
| Number | % | |
| 1) Venous leaks | ||
| 1a. Perivertebral | 8 | 7.7% |
| 1b. Epidural | 10 | 9.7% |
| 1c. Toward azygous vein | 2 | 1.9% |
| 2) Intradiscal leaks | 3 | 2.9% |
| 3) Soft-tissue leaks | ||
| 3a. Into the spinal canal | 4 | 3.9% |
| 3b. Posterior-mediastinal space | 24 | 23% |
| 3c. Along the puncture path | 1 | 1% |
| TOTAL | 52 | 50.5% |
A leak was systematically noticed in the 4 cases of epidural tumoral extension (Fig 3): 2 leaks in the spinal canal, 2 leaks in the posterior mediastinal space, 1 foraminal leak (1 vertebrae presented 2 leaks); no leak was noticed among the 8 cases of posterior wall disruption without any epidural tumoral extension. The leak occurrence was correlated neither with the type of vertebral structure (Fisher test, P = .551) nor with the vertebral level (Kruskal-Wallis test, P = .570).
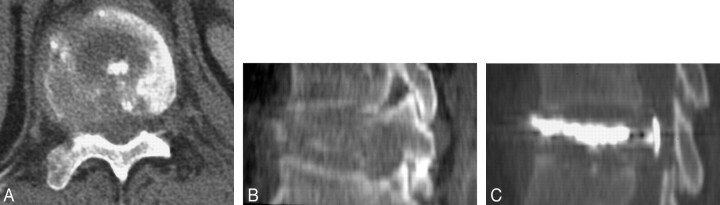
Fig 3.
Example of a mixed vertebral metastasis presenting a posterior wall disruption associated with an epidural tumoral component; the patient didn’t present any neurologic symptom. Prevertebroplasty transverse (A) and sagittal (B) CT scan reconstruction obtained through level of L4; sagittal reconstruction after cement injection (C) shows an intracanalar leak.
Clinical Complications
The occurrence of clinical complications was not correlated with the type of vertebral structure (pure blastic or mixed): Fisher test, P = .279.
General Complications.
The 2 (3.4%) symptomatic pulmonary embolisms were secondary to a cement leak toward the azygous vein, which was detected by fluoroscopic monitoring. The vertebra was pure blastic in 1 case and mixed in the other case. These pulmonary embolisms were moderate in severity, without overt clinical sign and were resolved with anticoagulant treatment.
Local Complications.
Of the 5 cases (8.5%), 1 transient radiculalgia (1.7%) was caused by a foraminal leak of a pure blastic vertebra; it disappeared a few days later under analgesic and corticosteroid treatment. The 2 chronic radiculalgias (3.4%) worsened a pre-existing sciatica in both cases. In the first case, a foraminal leak occurred in a pure blastic vertebra, which presented as a posterior wall disruption with a tumoral epidural extension; the patient was successfully treated by surgery. In the other case, the vertebral structure was mixed, and the posterior wall was not disrupted; the patient was also treated by surgery, but the analgesic result was only “good” at 6 months (with a residual pain evaluated between 5 and 7.5 of 10). However, this case was complicated by the presence of a pre-existing disk herniation and local tumoral progression.
One cauda equina syndrome (1.7%) occurred after an intracanalar leak; this patient was treated in L4 for a pure blastic lesion with an epidural tumoral extension, but the vertebra presented also a constitutional canalar stenosis worsened by an exophytic left pedicular extension of the metastasis. The patient required a surgical treatment including the removal of the epidural component, L4 vertebrectomy, and osteosynthesis, with a good analgesic result at 5-year follow-up.
One moderate sized hemothorax (1.7%) was caused by a leak along the puncture path that occurred during a posterolateral approach in a pure blastic vertebra at the thoracic level; it resolved spontaneously.
Filling Rate
The mean filling volume was 4.15 mL. Among the 103 vertebrae, the vertebral filling was considered good in 55% of cases, mild in 25%, and insufficient in 20%.
There were 5 filling failures (4.7%); all of them occurred in a pure blastic vertebra. Four of them occurred in cases of posterior wall disruption, and the relation between filling failure and posterior wall disruption was found to be significant (Fisher test, P = .044).
Analgesic efficacy of the vertebroplasty depended upon filling quality (Fisher test, P = .0932 at 1 month follow-up); thus an analgesic efficacy was obtained in 87% of cases after a “good” vertebral filling (more than two thirds of the vertebral volume) and was obtained in only 59% of cases after an “insufficient” vertebral filling (less than one third).
Analgesic efficacy was not correlated with the filling volume (Mann-Whitney test, P = .143 at 1 month and P = .205 at 6 months); thus, the minimal effective filling volume was 1 mL and the maximum was 8 mL.
Discussion
In the literature, most of the vertebroplasty series involve osteoporotic patients, with few series dealing with spinal metastatic disease. Among the latter, the most recent was published in 2006 by Barragan et al5; it evaluated the leaks and clinical complications rates after vertebroplasty for 304 treated vertebrae, among which 78.3% had pure lytic changes, 20.1% had mixed changes, and 1.6% had pure osteoblastic changes. In 1996, Weill et al4 evaluated the clinical complications and the analgesic efficacy of vertebroplasty for 52 pure lytic vertebrae. A contemporary study published by Cotten et al6 evaluated the relation between the percentage of lesion filling and the clinical follow-up after the treatment of 40 vertebrae, among which were 30 lytic metastases and 10 myeloma lesions.
Analgesic Efficacy
In the present series analgesic efficacy rate was 86% at 1 month and 92% at 6 months. The better results at 6 months could be explained by the fact that vertebroplasty induces a transitory local inflammation that can limit the short-term analgesic efficacy and that can even be responsible for transitory lumbar pain. These rates are higher than the one published by Weill et al for lytic metastases,4 which was 73% at 6 months, and that found by Cotten et al,6 which was 68% (corresponding to a “complete” or “marked” pain relief). This improvement of analgesic efficacy in vertebral metastases can be explained by a greater experience of the operators. Moreover, analgesic efficacy was statistically independent of the type of vertebral structure (mixed or pure blastic), which supports the treatment of pure blastic vertebrae as well as mixed and purely lytic lesions.
Cement Leaks
We reported 52 leaks (50.5%); this rate is higher than in the series of pure lytic metastases of Weill et al,4 which was 38%, but it is lower than that of Barragan et al,5 which was 423 leaks for 304 vertebrae (139%).
In the present study, the 4 cases of epidural tumoral extension were not compressive, but all of them were associated with a leak: this underlines the potential severity of an epidural tumoral extension, even if it is not a contraindication if not compressive (ie, without any associated neurologic symptom).
According to Weill,4 the presence of a posterior wall disruption induces a potential risk of leak in the spinal canal, but this risk seems to be controllable among the vertebrae without any epidural extension because we did not observe any intracanalar leak in these cases. The leak occurrence was not related to the type of vertebral structure (mixed or pure blastic). Each type of vertebra presents different risks. The injection pressure is low during the filling of mixed vertebrae and the leaks occur at the end of the injection, so that the operator has time to stop it. The injection pressure is maximal for pure blastic vertebrae, and the leaks occur suddenly in the beginning of the injection and are often unpredictable.
Clinical Complications
The occurrence of clinical complications was not correlated with the type of vertebral structure, thanks to the carefulness of the operator, who adapted the technique of injection to the nature of the vertebra; he decreased the injection pressure in case of too high resistance of a pure blastic vertebra and of posterior wall disruption, which may explain the filling failure.
The 2 cases with general complications represented 3.4% of the 59 procedures, which is an intermediate result between the rate of 5% found by Weill et al4 and the rate of 1.7% found by Barragan et al.5 In the present series, the mortality rate was nil. Here, both pulmonary embolisms were related to a vascular cement leak into the azygous vein. The corresponding vertebrae presented no peculiarities and usual precautions had been taken, yet the procedures were more risky because of the number of levels treated at the same time (4 vertebrae in both cases); usually it is recommended not to exceed 3 adjacent vertebrae per procedure, though this has never been proved. Furthermore, the occurrence of pulmonary embolisms, which initially were thought to be a rare occurrence, are more and more frequent.7-11
The local complications rate was 8.5%, which is lower than the rate of 12.5% found by Weill et al4 but higher than the rate of 5.1% found by Barragan et al.5 The local complications were the consequence of a cement-related compression in all cases. We insist on the risk represented by a vertebra with tumoral epidural extension even if it is not associated with any neurologic symptoms; among the 4 cases, we observed the occurrence of 1 durable radiculalgia and 1 cauda equina syndrome. This is in contradiction with the study of Shimony et al,12 which found no significant differences in pain or mobility outcomes and in clinical complications for patients who present a “mild or moderate” epidural tumoral involvement.
The case of hemothorax is, to our knowledge, the first to be reported after vertebroplasty.
Filling Rate
Filling Failures.
Among the 5 filling failures, 4 cases occurred in case of posterior wall disruption, probably because the operator wanted to avoid intracanalar leakage of cement. In fact, we think that posterior wall disruption is not a contraindication to perform vertebroplasty.
Filling failures were more frequent among pure blastic vertebrae because of their stiffness; in these cases, the operator preferred a filling failure to an eventual leak occurrence (especially when the resistance to the pressure was too high).
Filling Quality and Analgesic Efficacy.
We found a statistical relationship between the filling quality and the analgesic efficacy of vertebroplasty, which is controversial. Cotten et al6 evaluated the analgesic effect after 30 procedures for lytic metastases and 10 procedures for myeloma and found that pain relief was not proportional to the percentage of lesion filling. Our findings contradict this, and our data are supportive of the 3 biomechanisms that allow the analgesic effect of vertebroplasty (the stabilization of microfractures, the consolidation of which allows the reduction of mechanic forces, and the destruction of the peritumoral nervous ramifications) depend on the filling quality.
Filling Volume and Analgesic Efficacy.
In this study, no relationship was found between the filling volume and the analgesic efficacy; thus, 4 patients had an excellent analgesic result for a vertebra filled with 2 mL, and 3 patients for a vertebra filled with only 1 mL. This must not hide the intrinsic analgesic efficacy of the vertebroplasty, where the most dramatic proof is its almost immediate occurrence after the procedure, which allowed for all patients to remove or at least to decrease the major analgesic medications. A possible explanation is that the analgesic efficacy depends on the filling quality, which is obtained by various filling volume according to the vertebral volume and to the type of vertebral structure (pure blastic or mixed).
This absence of relationships between filling volume and analgesic effect is a new datum that has to be considered. It explains why no minimal effective volume has been found—in this series and in the literature. A minimal effective volume of 2 mL was experimentally found by Belkoff et al13 on osteoporotic vertebrae, but only for restoring a “good strength.” Jensen et al14 showed, on osteoporotic vertebrae, that the effective volumes could be very different (from 2.2 to 11 mL) with an acceptable analgesic efficacy for 90% of the patients. According to Baroud,15 the predominant paradigm of “maximum filling” should be shifted for the paradigm of “sufficient filling,” all the more because it is known that the filling of a vertebra increases the pressure on the adjacent levels with a risk of secondary fracture if this pressure is too high.15-17
Our study has several limitations. First, owing to its retrospective aspect, selection bias was unavoidable. Second, neither the radiodensity of the metastases nor their volume was measured. A strict classification of pure blastic lesions could have given some guidelines for their eventual treatment by vertebroplasty. On the other hand, it is very difficult to measure with precision such types of vertebrae, which are often heterogeneous and unfairly limited.
Conclusions
Vertebroplasty for osteoblastic and mixed metastases allows a satisfactory analgesic efficacy, which was statistically independent of the type of vertebral structure (mixed or pure blastic); this supports the treatment of pure blastic vertebrae as well as the other vertebral structures. In these types of vertebrae, the cement fills first the lytic zones in mixed vertebrae, and micro fractures that are present in the periphery and sometimes inside pure blastic lesions. Thus, in both cases, the obtained stiffness seemed to be sufficient for inducing a significant analgesic effect.
In the present series, the clinical complication rate was acceptable. We insist on the high risk of intracanalar leak concerning the vertebrae, which presents an epidural tumoral extension.
References
- 1.Kallmes DF, Jensen ME. Percutaneous vertebroplasty. Radiology 2003;229:27–36 [DOI] [PubMed] [Google Scholar]
- 2.Gangi A, Kastler BA, Dietemann JL. Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy. AJNR Am J Neuroradiol 1994;15:83–6 [PMC free article] [PubMed] [Google Scholar]
- 3.Cotten A, Boutry N, Cortet B, et al. Percutaneous vertebroplasty: state of the art. Radiographics 1998;18:311–21 [DOI] [PubMed] [Google Scholar]
- 4.Weill A, Chiras J, Simon JM, et al. Spinal metastases: indication for and results of percutaneous injection of acrylic chirurgical cement. Radiology 1996;199:241–47 [DOI] [PubMed] [Google Scholar]
- 5.Barragan HM, Vallee JN, Lo D, et al. Percutaneous vertebroplasty for spinal metastases: complications. Radiology 2006;238:354–62 [DOI] [PubMed] [Google Scholar]
- 6.Cotten A, Dewatre F, Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of filling and the leakage of methyl methylacrylate at clinical follow-up. Radiology 1996;200:525–30 [DOI] [PubMed] [Google Scholar]
- 7.Seo JS, Kim YJ, Choi BW, et al. MDCT of pulmonary embolism after percutaneous vertebroplasty. AJR Am J Roentgenol 2005;184:1364–65 [DOI] [PubMed] [Google Scholar]
- 8.Choe du H, Marom EM, Ahrar K, et al. Pulmonary embolism of polymethyl methacrylate during percutaneous vertebroplasty and kyphoplasty. AJR Am J Roentgenol 2004;183:1097–102 [DOI] [PubMed] [Google Scholar]
- 9.Chen HL, Wong CS, Ho ST, et al. A lethal pulmonary embolism during percutaneous vertebroplasty. Anesth Analg 2002;95:1060–62 [DOI] [PubMed] [Google Scholar]
- 10.Jang JS, Lee SH, Jung SK. Pulmonary embolism of polymethylmethacrylate after percutaneous vertebroplasty: a report of three cases. Spine 2002;27:E416–18 [DOI] [PubMed] [Google Scholar]
- 11.Padovani B, Kasriel O, Brunner Ph. Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJNR Am J Neuroradiol 1999;20:375–77 [PMC free article] [PubMed] [Google Scholar]
- 12.Shimony JS, Gilula LA, Zeller AJ, et al. Percutaneous vertebroplasty for malignant compression fractures with epidural involvement. Radiology 2004;232:846–53 [DOI] [PubMed] [Google Scholar]
- 13.Belkoff SM, Mathis JM, Jasper LE, et al. The biomechanics of vertebroplasty: the effects of cement volume on mechanical behavior. Spine 2001;26:1537–41 [DOI] [PubMed] [Google Scholar]
- 14.Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol 1997;18:1897–904 [PMC free article] [PubMed] [Google Scholar]
- 15.Baroud G, Heini P, Nemes J, et al. Biomechanical explanation of adjacent fractures following vertebroplasty [letter]. Radiology 2003;229:606–07 [DOI] [PubMed] [Google Scholar]
- 16.Berlemann U, Ferguson SJ, Nolte LP, et al. Adjacent vertebral failure after vertebroplasty. A biomechanical investigation. J Bone Joint Surg Br 2002;84:748–52 [DOI] [PubMed] [Google Scholar]
- 17.Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol 2006;27:217–23 [PMC free article] [PubMed] [Google Scholar]