Abstract
BACKGROUND AND PURPOSE: Brain creatine (Cr) deficiencies (BCr-d) are rare disorders of creatine biosynthesis and transport. We performed consecutive measures of total Cr (tCr) and of its phosphorylated fraction, phosphocreatine (PCr), in the brains of children affected by Cr synthesis defects during a long period of therapy. The aim was to identify the optimal treatment strategy for these disorders.
MATERIALS AND METHODS: Two patients with guanidinoacetate methyltransferase defect (GAMT-d) were treated with different amounts of Cr and with diet restrictions aimed at reducing endogenous guanidinoacetate (GAA) synthesis. Three patients with arginine:glycine amidinotransferase defect (AGAT-d) were treated with different Cr intakes. The patients’ treatments were monitored by means of 1H- and 31P-MR spectroscopy.
RESULTS: Cr and PCr replenishment was lower in GAMT-d than in AGAT-d even when GAMT-d therapy was carried out with a very high Cr intake. Cr and especially PCr replenishment became more efficient only when GAA blood values were reduced. Adenosine triphosphate (ATP) was increased in the baseline phosphorous spectrum of GAMT-d, and it returned to a normal value with treatment. Brain pH and brain Pi showed no significant change in the AGAT-d syndrome and at any Cr intake. However, 1 of the 2 GAMT-d patients manifested a lower brain pH level while consuming the GAA-lowering diet.
CONCLUSIONS: AGAT-d treatment needs lower Cr intake than GAMT-d. Cr supplementation in GAMT-d treatment should include diet restrictions aimed at reducing GAA concentration in body fluids. 1H- and especially 31P-MR spectroscopy are the ideal tools for monitoring the therapy response to these disorders.
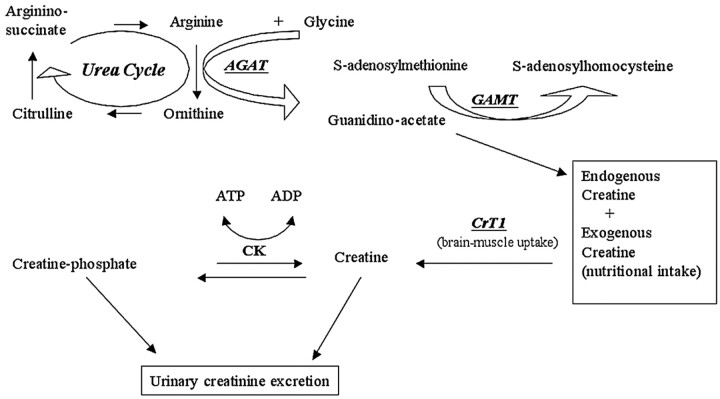
Brain creatine (Cr) deficiencies (BCr-d) are inborn disorders of Cr biosynthesis and Cr transport discovered over the last 10 years and first revealed by proton spectroscopy (1H-MR spectroscopy [1H-MRS]).1 Following the metabolic pathway of creatine (Fig 1), 2 different groups of disorders may result in the brain creatine depletion: disorders deriving from de novo Cr biosynthesis defect and disorders of Cr transport at the level of the blood-brain barrier.
Fig 1.
Metabolic pathway of creatine/phosphocreatine.
The first group includes the enzymatic defects of arginine:glycine amidinotransferase (AGAT-d)2,3 and of guanidinoacetate methyltransferase (GAMT-d),1,4–10 which are the first 2 steps in Cr synthesis. These patients have a systemic depletion of Cr, but in GAMT-d, there is a concomitant endogenous overproduction of guanidinoacetate (GAA), a guanidine compound known for its neurotoxic action11 and its epileptogenic potential.12,13 On the other hand, patients with Cr transport disorder (CT1-d) have normal Cr levels in the body fluids accompanied by an increased creatine-creatinine urinary excretion.14–17 The common clinical aspect of BCr-d is developmental delay, mental retardation, and a prominent disturbance of cognitive and expressive speech. Some patients with GAMT-d may have intractable epilepsy and extrapyramidal movement disorders, probably related to GAA accumulation in tissues and body fluids.18–20 MR imaging of the brain in GAMT-d reveals either pallidum hyperintensity on T2-weighted images,1,6,8 myelination delay,9 or no MR imaging changes9,10; posterior periventricular white matter T2-weighted hyperintensity or brain atrophy are described in some CT1-d cases16 whereas MR imaging is normal in children with AGAT-d.21 Inheritance of AGAT-d and of GAMT-d is autosomal recessive,2,22 whereas CT1-d is X-linked.23
Consecutive 1H-MRS studies have demonstrated that Cr depletion, under Cr oral administration, is reversible in GAMT-d and AGAT-d but irreversible in CT1-d. In the index case of a male patient with GAMT-d, therapy was monitored over 26 months; the authors demonstrated a 75%–90% replenishment of Cr in the brain with a biphasic trend.4 Then, 2 sisters with AGAT-d were monitored for 16 months, and a progressive increase in brain Cr, up to 90%–100% of the normal brain Cr concentration, was measured.21
The brain and muscle metabolism is characterized by a high and fluctuating energy demand, where phosphocreatine (PCr) plays the important role of a temporal and spatial buffer for ATP cellular homeostasis. Therefore, the metabolic and clinical improvement recorded in these patients with Cr assumption is most likely related to the degree of PCr recovery.24
Brain PCr can be measured in vivo only by 31P-MRS; so far, no studies have been carried out to determine how substitution therapy of BCr-d increases the Cr pool stored in the form of PCr through the creatine kinase (CK) system reaction.
In this study, we measured, by 1H- and 31P-spectroscopy, the time-course of brain total Cr (tCr) and PCr restoration in children with GAMT-d and in children with AGAT-d. MRS measurements were taken during a long period of therapy performed with different Cr intakes and, in the GAMT-d patients, with a diet aimed at lowering GAA endogenous synthesis.
Because treatment of these disorders relies upon lifelong Cr ingestion, this study aimed at identifying the minimum amount of Cr necessary for these children. Another purpose of this work was to assess whether brain tCr might affect the rate of the brain's CK reaction and thus the total amount of brain PCr.
Materials and Methods
Patients and Control Subjects.
The study group included 2 children with GAMT-d (patients 1 and 2) and 3 children with AGAT-d (patients 3, 4, and 5). Four of them have been described previously. In particular, patient 1 was diagnosed at the age of 4 by brain 1H-MRS.8 Diagnosis of patient 2, a 12-year-old girl, was based on biochemical abnormalities (increased GAA in blood and urine).25 The children with AGAT-d were 3 members of the same family, 2 sisters (patients 3 and 4) and 1 male cousin (patient 5), diagnosed by brain 1H-MRS.2,3,21 Table 1 summarizes the clinical presentation and genetic and MR imaging features of the patients.
Table 1:
Presentation of the patients
| Patients (Age of diagnosis) | Clinical Presentation | MRI | Mutation |
|---|---|---|---|
| 1. GAMT-d (4 years) | Psychomotor delay with absence of speech; mild hypotonia; dyskinetic movements; drug resistant seizures | Bilateral pallidal alterations | c.491insG/IVS5–3C>G |
| 2. GAMT-d (12 years) | Mental retardation; partial seizures; autism-like behavior | Normal | L197P/L197P |
| 3. AGAT-d (6 years) | Mental retardation; behavioral disorders; absence of speech; reduced somatic growth and microcephalia | Normal | W149X/W149X |
| 4. AGAT-d (4 years) | Mental retardation; behavioral disorders; absence of speech; reduced somatic growth and microcephalia | Normal | W149X/W149X |
| 5. AGAT-d (2 years) | Psychomotor delay; absence of language; behavioral disorders. | Normal | W149X/W149X |
Note:—GAMT-d indicates guanidinoacetate methyltransferase defect; AGAT-d, arginine:glycine amidinotransferase defect.
The control subjects were healthy volunteers, children of the local staff (n = 10; 3 boys and 7 girls; age range, 7–14 years).
MR Spectroscopy.
MRS studies were performed with a 1.5T clinical scanner (Signa Horizon LX 1.5; GE Healthcare, Milwaukee, Wis). 1H-MRS was acquired by using a standard quadrature head coil and a single-voxel point-resolved spectroscopy sequence (TE, 35 ms; TR, 2 seconds; 128 averages on a volume of interest [VOI] of 3.4 mL placed in the parietal cortical gray matter).
31P-MRS was performed in the same session and with the same MR system operating at 25.86 MHz. Data were collected with a surface flexible spectroscopy coil placed around the head at its maximum diameter, applying a hard pulse. The spectral width was 2500 Hz, and 1024 complex data points were sampled with a TR of 4 seconds and a flip angle of 60° for a total of 128 averages.
Signal Intensity Analysis.
Total brain Cr was measured by single-voxel 1H-MRS in terms of absolute concentration, expressed in millimoles per liter VOI using the LCModel.26 A further calibration by a phantom containing 50 mmol/L of NAA was performed before each session to check calibration factors and system stability. Phosphocreatine and the other high-energy phosphates such as inorganic phosphate (Pi) and nucleoside triphosphates (γ-ATP, α-ATP, and β-ATP) were measured in terms of a ratio with respect to the phosphodiester signal intensity (PDE). The PDE signal intensity was chosen as an internal reference because this metabolite is not included in any known metabolic circuit in cellular ATP production. The same metabolite was also chosen for the spectral frequency offset registration because its resonance frequency is not affected by the chemical-shift phenomena related to possible pH variations. The spectra were processed with zero-order phase correction, zero filling to 4048 points, and spectral apodization with a Gaussian line-broadening at 8 Hz.27 The intraindividual reliability upon re-examination of each patient (in term of variance σ2intra)28 was in the range of 2%–4% for 1H-MRS (measured on NAA) and in the range of 3%–5% for 31P-MRS (measured on phosphomonoester [PME]/PDE ratio).
Treatment Protocols and MRS Laboratory Monitoring.
This study protocol was approved by the Ethical Committee of the Stella Maris Scientific Institute and informed consent was obtained from the children’ parents.
In patient 1 with GAMT-d, 1H-/31P-MRS measurements were recorded after a 1-year treatment with Cr supplementation at 800 mg/kg of body weight (bw)/day; after 2 years, while consuming Cr at 400 mg/kg/bw/day and ornithine (Orn; 100 mg/kg/bw/day), and then after another year of a diet that also included arginine (Arg) restriction (45 mg/kg/bw/day).
Patient 2 with GAMT-d was examined at the baseline and after 8 months of treatment including Cr (400 mg/kg/bw/day), and Orn (100 mg/kg/bw/day) intake, as well as protein and Arg restriction (1 g/kg/ bw/day and 15 mg/kg/bw/day, respectively).
The 3 patients with AGAT-d were supplied with 400 mg/kg/bw/day of Cr during the first 2 years of evaluation, and the time course of brain Cr replenishment of this period was monitored only by 1H- MRS.21 To evaluate brain PCr and brain tCr in a baseline condition and under therapy, Cr supplementation in these children was then ceased for 3 months and re-introduced at 400 mg/kg/bw/day for a 6-month period followed by a 200 mg/kg for another 6 months. 1H- and 31P-MRS were performed at the end of the washout therapy (and are therefore considered the baseline evaluation) after 3 and 6 months of 400 mg/kg/bw/day Cr reintake and after 3 and 6 months of 200 mg/kg/ bw/day Cr intake.
Creatine monohydrate was used in these studies and the total daily amount of Cr was administered in 5 single dosages regularly distributed throughout the day. Plasmatic Cr levels were assessed at the time of each MRS session to confirm the Cr fluctuations expected in response to the Cr regimen applied. Blood GAA concentration was measured using the reversed phase-high-performance liquid chromatography analysis,29 and it was computed as the median value of consecutive measurements performed over each period of therapy changes.
Results
MRS and laboratory measurements of each patient and of normal subjects are reported in Table 2. The patients assumed the prescribed amount of Cr regularly because the plasmatic modifications observed during the study were proportionate to the protocols adopted. No negative effects were observed during the period of Cr discontinuation in AGAT-d patients.
Table 2:
Laboratory and MRS measurements of the patients and of the control subjects
| Patients | Cr per Os (mg/kg/bw/day) | Plasmatic Cr (μmol/L) | Plasmatic GAA (μmol/L) | Brain Cr (mmol/L) | PCr/PDE | Pi/PDE | ATP/PDE | pH |
|---|---|---|---|---|---|---|---|---|
| GAMT-d | ||||||||
| Patient 1 | 800 | 487 | 11.4 | 3.86 | 1.03 | 0.76 | 0.58 | 7.22 |
| 400* | 598 | 11.1 | 3.10 | 0.91 | 0.75 | 0.59 | 6.85 | |
| 400** | 512 | 7.9 | 3.31 | 1.19 | 0.70 | 0.65 | 7.43 | |
| Patient 2 | 0 | 24 | 40.3 | 0.93 | 0.37† | 0.72 | 0.80 | 7.08 |
| 400** | 431 | 7.4 | 3.76 | 1.02 | 0.78 | 0.57 | 6.75 | |
| AGAT-d | ||||||||
| Patient 3 | 0 | 6 | 1.10 | 0.73 | 0.84 | 0.63 | 7.31 | |
| 400 | 776 | 3.87 | 1.24 | 0.85 | 0.61 | 7.21 | ||
| 200 | 203 | 3.65 | 1.19 | 0.82 | 0.70 | 7.20 | ||
| Patient 4 | 0 | 6 | 1.05 | 0.76 | 0.87 | 0.64 | 7.31 | |
| 400 | 588 | 4.00 | 1.49 | 0.90 | 0.65 | 7.17 | ||
| 200 | 178 | 3.85 | 1.15 | 0.81 | 0.77 | 7.36 | ||
| Patient 5 | 0 | 8 | 1.33 | 0.76 | 0.87 | 0.64 | 7.22 | |
| 400 | 283 | 3.99 | 1.32 | 0.87 | 0.64 | 7.04 | ||
| 200 | 93 | 3.52 | 0.83 | 0.76 | 0.62 | 7.54 | ||
| 300 | 246 | 3.61 | 0.93 | 0.77 | 0.54 | 7.22 | ||
| Reference values | n.v. ± SD | 64 ± 14 | 1.48 ± 0.51 | 4.37 ± 0.44 | 1.40 ± 0.14 | 0.87 ± 0.08 | 0.64 ± 0.06 | 7.15 ± 0.12 |
Note:—Os indicates oral intake; Cr, creatine; bw, body weight; GAA, guanidinoacetate; PCr, phosphocreatine; PDE, phosphodiester; Pi, inorganic phosphate; GAMT-d indicates guanidinoacetate methyltransferase defect; AGAT-d, arginine:glycine amidinotransferase defect; n.v., normal value.
Coupled with ornithine supplementation.
Coupled with ornithine supplementation and arginine restriction.
Resonance frequency of this signal was at −0.5 ppm and was assigned to GAA-P.
Patients with GAMT-d.
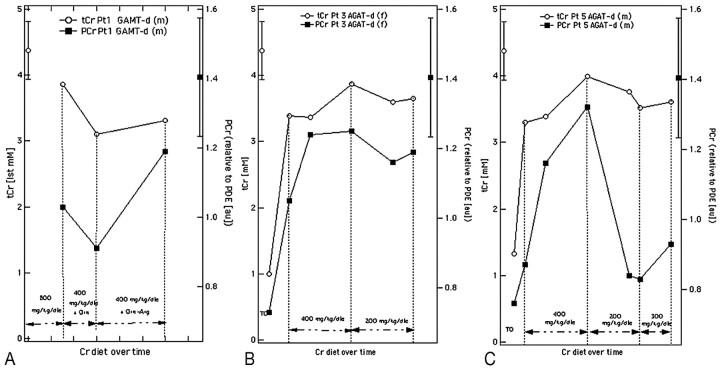
Brain Cr before therapy in patient 1 was undetectable and blood GAA level was markedly elevated (18.6 μmol/L; normal value [n.v.], 0.86 ± 0.27). After a 1-year supplementation with 800 mg tCr increased to 3.86 mmol/L (88% of the normal level), PCr/PDE ratio was 1.03 (74% of the normal level) and blood GAA concentration was 11.4 μmol/L. As Cr administration was reduced to 400 mg/kg/bw/day and Orn was added, tCr and PCr decreased to 3.10 mmol/L and 0.91, respectively (71% and 65% of the normal level), and blood GAA was 11.1 μmol/L. When a further arginine and protein restriction was included, tCr and PCr increased to 3.31 mmol/L and 1.19 (76% and 85%), whereas GAA blood values decreased to 7.9 μmol/L (Fig 3A).
Fig 3.
Total Cr (right axis, empty dot) and PCr (left axis, solid square) modifications recorded in patients 1 (A), 3 (B), and 5 (C) with different Cr daily amounts. Normative data in the bars are presented as mean ± SD.
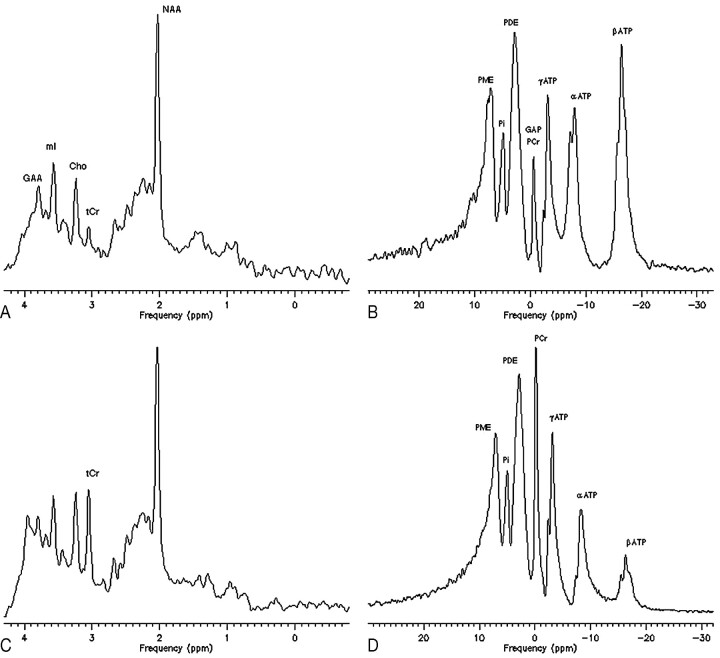
The brain Cr of patient 2 rose after 8 months of therapy from 0.93 to 3.76 mmol/L. The basal 1H-MRS study showed a signal intensity at 3.8 ppm (Fig 2A) and the basal 31P-MRS study detected a peak shift of δ = −0.5 ppm, which, on the basis of previous reports1,5,30–32 were respectively interpreted as brain accumulation of GAA (at 3.8 ppm) and of its phosphate form, GAA-P (at −0.5 ppm) (Fig 2B). In the posttreatment control, the PCr/PDE ratio was restored to 1.02 (73% of the normal level). The basal GAA blood concentration was 40.3 μmol/L, and it declined to 7.4 μmol/L after treatment. In this patient, ATP signals were markedly increased at the baseline 31P-MRS acquisition (ATP/PDE, 0.80; n.v., 0.64 ± 0.06) (Fig 2B) and normalized in the control (Fig 2D). In the GAMT-d boy, 31P-MRS was not performed at the baseline, and the ATP signal intensity was measured with the spectra obtained under therapy and was normal.
Fig 2.
Single-voxel proton spectroscopy (1H-MRS) and whole-brain phosphorous spectroscopy (31P-MRS) of patient 2, before (A and B) and after (C and D) 6 months of therapy with Cr at 400 mg/kg/bw/day and guanidinoacetate (GAA)-lowering diet restrictions.
The baseline studies demonstrate, besides a strong reduction of tCr and PCr peaks, an abnormal peak of guanidinoacetate (GAA) at 3.8 ppm on proton spectrum (A), of GAA-phosphate (GAA-P) at −0.5 ppm on phosphorous spectrum (B), and a high level of brain ATP. In contrast, in the on-therapy spectra, the GAA and GAA-P peak are not resolved, ATP turns to normal but there is an incomplete recovery of the tCr and PCr signal intensity.
Patients with AGAT-d.
1H- and 31P-MRS studies performed after the 3-month period of Cr washout revealed similar tCr and PCr measurements in the patients with AGAT-d (Table 2). In fact, tCr and PCr significantly increased after Cr supplementation, with no difference between administration at 400 and 200 mg/kg/bw/day (Fig. 3B). However, an unexpected drop in the PCr/PDE ratio from 1.32 to 0.83 was detected in patient 5 when the Cr was cut down to 200 mg/kg/ bw/day (Fig. 3C).
The Pi, ATP, and pH of the brain were stable and within normal values in AGAT patients during all the above mentioned treatments.
Discussion
The normal human requirement of Cr is approximately 2 to 3 g/day and this comes from food and endogenous synthesis (Fig 1).24 Blood Cr is taken up by brain via the Cr transport, a saturable sodium and chloride active process33 and converted into PCr in a reversible reaction catalyzed by 1 of the 5 CK isoenzymes that are heterogeneously distributed throughout the brain.34,35
Brain Cr deficiencies are rare neurometabolic syndromes. So far, 20 patients from different families with GAMT-d have been reported, whereas only 1 family with 3 AGAT-d affected children has been identified.
The clinical picture in GAMT-d is more severe than in AGAT-d, because it includes not only mental retardation and speech impairment (as in AGAT-d) but also epilepsy and extrapyramidal disorders.
Both defects lead to the depletion of Cr and PCr, but in GAMT-d, GAA accumulates in excess levels in body fluids and brain. There is a large amount of evidence suggesting that GAA is chiefly responsible for epilepsy and extrapyramidal disorders.11–13,20 In addition, GAA is a Cr analog and therefore acts as a Cr agonist both at the level of the brain Cr transport system and that of the CK phosphorylation system.24,33,36
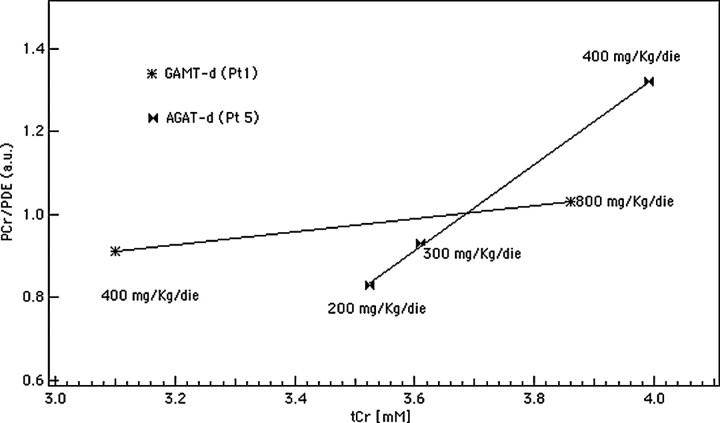
The values of the cerebral tCr and PCr we have recorded by spectroscopy in our patients are in line with the above-mentioned biochemical considerations. The level of Cr and PCr rose to 90% of normal when the 3 AGAT-d children were supplemented with a Cr intake of 400 mg/kg/bw/day. On the other hand, the male patient with GAMT-d treated with the same amount of Cr showed lower levels of brain tCr and PCr, corresponding to 71% and 65% of the mean normal values, respectively. Figure 4 compares tCr and PCr values of GAMT-d (patient 1 initially treated with mere Cr and then with Cr plus Orn) and of AGAT-d (patient 5). It reveals the discrepancy in PCr restoration from one syndrome to another and therefore illustrates the difference in CK system efficacy expressed, as a raw measure, by the angular coefficient (b) of the fitting line. At the beginning of the study, our MR system was not equipped with the coil and software required for 31P-MRS acquisition; therefore, in this comparison, the baseline PCr measure is missing in patient 1. Despite this bias, the resulting line slopes clearly suggest that the tCr and PCr are lower in GAMT-d than in AGAT-d, and that the rate of Cr phosphorylation is related to the cytosolic concentration of Cr in both defects. An amelioration of PCr in patient 1 was achieved only when there was an Arg restriction in the diet, which in turn determined a decrease in the blood GAA concentration. A satisfying brain PCr increase and blood GAA decrease were immediately obtained in the other GAMT-d patient, who followed a diet with Cr intake and protein restriction aimed at reducing the synthesis of GAA from the beginning.
Fig 4.
AGAT-d versus GAMT-d CK reaction rate evaluation. The angular coefficient (b value) of the fitting lines is an index of the CK system efficacy (b [pt 1] = 0.16 and b [pt 5] = 1.04). The CK system is down-regulated in GAMT-d (pt 1) with respect to AGAT-d (pt 5) because PCr remains low even when tCr availability is almost 90% of normal. These measures were recorded when patient 1 was treated only with Cr.
Phosphorous spectra measurements of the latter depicted an increase of the 3 nucleoside triphosphate signals, especially of the β-ATP peak (Fig 2B), the triplet at 16.26 ppm whose signal intensity contribution is related only to ATP nucleosides,27 which normalized after therapy and with PCr restoration (Fig 2D). This same signal intensity abnormality is also revealed in the spectral profiles published by Schulze et al5 and by Renema et al,31 who performed brain 31P-MRS in a patient with GAMT-d and in a gene knockout GAMT-d mouse model, respectively, though no comment was provided by any of the authors.
Because ATP signal intensity normalizes with PCr recovery, we can speculate that the lack of Cr would shift the kinetics of the CK reaction (ATP + Cr ↔ ADP + PCr) toward the left, producing an accumulation of free ATP molecules. 31P-MRS also showed a signal intensity shift of −0.5 ppm with respect to the 0-ppm assignment of PCr (ie, the frequency commonly related to the phosphorylated fraction of GAA [GAA-P]), whereas 1H-MRS revealed a small GAA signal intensity at 3.8 ppm that is difficult to resolve because it occurs in a spectral area with several overlapping resonances.30–32 Brain GAA and GAA-P value discrepancies have already been found in 2 GAMT-d patients by Schulze et al5,10 and in the mouse model for GAMT-d.31 These data could support the hypothesis of a prevalence of GAA-P fraction over the total GAA, confirming the competitive and injurious effect of GAA on the CK function.
The 31P-MRS signal acquisition and analysis technique used in this study may harbor some potential sources of errors related to scalp/muscle voxel contamination and to the absence of external phosphorus reference for absolute PCr quantification. Estimations of the volume of extracranial tissue relative to that of the brain within a volume such as the one we used have given evidence of brain spectra with extremely low contamination from other tissues.37,38 In addition, because this potential error of the signal amplitude is maintained unchanged in the consecutive studies concerning each and every patient, the results should not be affected in any way. The drawback of the absence of the external phosphorus reference was addressed using the PDE signal as internal reference. This metabolite is not included in any known metabolic circuit of ATP production and its frequency is not affected by chemical-shift phenomena. The PDE ratios of all the other metabolites (ie, Pi and PME) turned out to be stable in all the consecutive measurements and patients. The latter has also been useful as control measure for problems due to noise related to technical factors in surface coil loading or in signal gain attenuation.
According to our MRS results, Cr intake was fixed as 200 mg/kg/day for the 2 sisters with AGAT-d and 400 mg/kg/day for both patients with GAMT-d. Because of the PCr drop detected with Cr supplementation at 200 mg, the male patient with AGAT-d was supplemented with 300 mg/kg/day and after 6 months of this regimen, the tCr and PCr attained 84% and 64% of the normal level, respectively.
Oral supplementation of Cr had beneficial effects on the clinical evolution of our patients. Indeed, the extrapyramidal signs and seizures disappeared in the patients with GAMT-d; however, they remained severely retarded. In the patients with AGAT-d, clinical improvement was more remarkable because patients learned to speak, and their autism-like behavior faded, though they still show a mild mental retardation. The favorable outcome of AGAT defect has only been demonstrated in this small cohort of patients coming from the same family and therefore sharing identical AGAT mutation. It is possible that other AGAT-d patients do not respond similarly when supplemented with Cr. These children increased in body weight over the 6-year period of this study. Indeed the body mass index (BMI) of the 2 sisters went from 13.7 to 29.55 and from 12.17 to 25.42, and the boy increased from 15.22 to 24.10 (BMI n.v. 18–25). This weight gain, however, could depend on genetic and/or environmental factors.
Our AGAT-d patients are thus far the only ones reported, and it is known that their treatment requires a life-long ingestion of Cr. Because some adverse effects of long-term Cr administration have been reported,39–41 the primary objective of this study was to identify the minimum amount of Cr necessary for this treatment to be effective. Therefore, should any concern arise on our decision of suspending the treatment for a 3-month period, the reduction of the initial Cr dosage, supported by the results of this study, could act as a justification. Moreover, this protocol was carried out under clinical monitoring, and no complications were reported.
This is the first comparative study of the GAMT and AGAT defect performed with 1H- and 31P-MRS of the brain, before and during treatment. In this study, we have provided the “in vivo” demonstration of the Cr-competitive action of GAA in the brain by measuring PCr fluctuations correlated to the different therapeutic strategies.
Conclusion
AGAT-d shows milder clinical symptoms than GAMT-d, and its long-term therapy may be carried out with lower Cr administration than that suggested for GAMT-d. The combined effect of Cr deficiency and GAA accumulation might be responsible for the more severe phenotype of GAMT-d. Therefore, in addition to the Cr intake, a dietary treatment to achieve reduction of GAA endogenous synthesis is recommended. This is supported by our demonstration that after the reduction of blood GAA, there is a better brain PCr restoration. PCr is essential for ATP cellular homeostasis and, because GAA might interfere on the CK reaction, slowing down brain PCr synthesis, we suggest 31P-MRS as the ideal tool for treatment monitoring, especially in GAMT-d.
Footnotes
This work was supported by Grants RF2/02 and RC4/04 from the Italian Ministry of Health and by Telethon-application GGP04092.
Paper previously presented in part at International Society of Magnetic Resonance in Medicine Meeting; May 15–21, 2004; Kyoto, Japan.
References
- 1.Stöckler S, Holzbach U, Hanefeld F, et al. Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr Res 1994;36:409–13 [DOI] [PubMed] [Google Scholar]
- 2.Item CB, Stöckler-Ipsiroglu S, Stromberger C, et al. Arginine:glycine amidinotransferase deficiency: the third inborn error of creatine metabolism in man. Am J Hum Genet 2001;69:1127–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battini R, Leuzzi V, Carducci CA, et al. Creatine depletion in a new case with AGAT deficiency: clinical and genetic study in a large pedigree. Mol Gen Metab 2002;77:326–31 [DOI] [PubMed] [Google Scholar]
- 4.Stöckler S, Hanefeld F, Frahm J. Creatine replacement therapy in guanidinoacetate methyltransferase deficiency, a novel inborn error of metabolism. Lancet 1996;348:789–90 [DOI] [PubMed] [Google Scholar]
- 5.Schulze A, Hess T, Wevers R, et al. Creatine deficiency syndrome caused by guanidinoacetate methyltransferase deficiency: diagnostic tools of a new inborn error of metabolism. J Pediatr 1997;131:626–31 [DOI] [PubMed] [Google Scholar]
- 6.Ganesan V, Johnson A, Connelly A, et al. Guanidinoacetate methyltransferase deficiency: new clinical features. Pediatr Neurol 1997;17:155–57 [DOI] [PubMed] [Google Scholar]
- 7.Von K Figura, F Hanefeld, D Isbrandt, et al. Guanidinoacetate methyltransferase deficiency. In: Scriver CR, Beaudet AL, Sly WS, et al, eds. The Metabolic and Molecular Basis of Inherited disease, 6th ed. New York: McGraw Hill;2000. :1897–908
- 8.Leuzzi V, Bianchi MC, Tosetti M, et al. Brain creatine depletion: a new case report of guanidinoacetate methyltransferase (improving with creatine supplementation). Neurology 2000;55:1407–09 [DOI] [PubMed] [Google Scholar]
- 9.van der Knaap MS, Verhoeven NM, Maaswinkel-Mooij P, et al. Mental retardation and behavioral problems as presenting signs of a creatine synthesis defect. Ann Neurol 2000;47:540–43 [DOI] [PubMed] [Google Scholar]
- 10.Schulze A, Bachert P, Schlemmer H, et al. Lack of creatine in muscle and brain in an adult with GAMT deficiency. Ann Neurol 2003;53:248–51 [DOI] [PubMed] [Google Scholar]
- 11.Mori A. Biochemistry and neurotoxicology of guanidino compounds. History and recent advances. Pavlov J Biol Sci 1987;22:85–94 [DOI] [PubMed] [Google Scholar]
- 12.D’Hooge R, Pei YQ, Marescau B, et al. Convulsive action and toxicity of uremic guanidine compounds: behavioral assessment and relation to brain concentration in adult mice. J Neurol Sci 1992;112:96–115 [DOI] [PubMed] [Google Scholar]
- 13.Zugno AI, Franzon R, Chiarani F, et al. Evaluation of the mechanism underlying the inhibitory effect of guanidinoacetate on brain Na+,K+-ATPase activity. Int J Dev Neurosci 2004;22:191–96 [DOI] [PubMed] [Google Scholar]
- 14.Cecil KM, Salomons GS, Ball WS Jr., et al. Irreversible brain creatine deficiency with elevated serum and urine creatine: A creatine transporter defect? Ann Neurol 2001;49:401–04 [DOI] [PubMed] [Google Scholar]
- 15.Salomons GS, van Dooren SJ, Verhoeven NM, et al. X-linked creatine-transporter gene (SLC6A8) defect: a new creatine-deficiency syndrome. Am J Hum Genet 2001;68:1497–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.deGrauw TJ, Salomons GS, Cecil KM, et al. Congenital creatine transporter deficiency. Neuropediatrics 2002;33:232–38 [DOI] [PubMed] [Google Scholar]
- 17.Verhoeven NM, Salomons GS, Jakobs C. Laboratory diagnosis of defects of creatine biosynthesis and transport. Clin Chim Acta 2005;361:1–9 [DOI] [PubMed] [Google Scholar]
- 18.Leuzzi V. Inborn errors of creatine metabolism and epilepsy: clinical features, diagnosis and treatment. J Child Neurol 2002;17 (Suppl 3):S89–97 [PubMed] [Google Scholar]
- 19.Schulze A. Creatine deficiency syndromes. Mol Cell Biochem 2003;244:143–50 [PubMed] [Google Scholar]
- 20.Schulze A, Ebinger F, Rating D, et al. Improving treatment of guanidinoacetate methyltransferase deficiency: reduction of guanidinoacetic acid in body fluids by arginine restriction and ornithine supplementation. Mol Genet Metab 2001;74:413–19 [DOI] [PubMed] [Google Scholar]
- 21.Bianchi MC, Tosetti M, Fornai F, et al. Reversible brain creatine deficiency in two sisters with normal blood creatine level. Ann Neurol 2000;47:511–13 [PubMed] [Google Scholar]
- 22.Chae YJ, Chung CE, Kim BJ, et al. The gene encoding guanidinoacetate methyltransferase (GAMT) maps to human chromosome 19 at band p13.3 and to mouse chromosome 10. Genomics 1998;49:162–64 [DOI] [PubMed] [Google Scholar]
- 23.Gregor P, Nash SR, Caron MG, et al. Assignment of the creatine transporter gene (SLC6A8) to human chromosome Xq28 telomeric to G6PD. Genomics 1995;25:332–33 [DOI] [PubMed] [Google Scholar]
- 24.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev 2000;80:1107–213 [DOI] [PubMed] [Google Scholar]
- 25.Leuzzi V, Carducci C, Bianchi MC, et al. A new case of guanidinoacetate methyltransferase (GAMT) deficiency. Clinical, molecular and brain 1H-31P-magnetic resonance spectroscopy (MRS) features. IX International Congress on Inborn Errors of Metabolism; 2–6 September2003; Brisbane, Australia.
- 26.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–79 [DOI] [PubMed] [Google Scholar]
- 27.de Graaf RA. In Vivo NMR Spectroscopy: Principles and Technique. Chichester, UK: Wiley & Sons Ltd;1998. :61–67
- 28.Schirmer T, Auer DP. On the reliability of quantitative clinical magnetic resonance spectroscopy of the human brain. NMR Biomed 2000;13:28–36 [DOI] [PubMed] [Google Scholar]
- 29.Carducci Ca, Leuzzi V, Carducci Cl, et al. Two new severe mutations causing guanidinoacetate methyltransferase deficiency. Mol Gen Metab 2000;71:633–38 [DOI] [PubMed] [Google Scholar]
- 30.Frahm J, Requardt M, Helms G, et al. Creatine deficiency in the brain: a new treatable inborn error of metabolism identified by proton and phosphorus MR spectroscopy in vivo. In: Proceedings of the Second Annual Meeting of the Society of Magnetic Resonance, San Francisco, Calif, August 16–19,1994
- 31.Renema WK, Schmidt A, van Asten JJ, et al. MR spectroscopy of muscle and brain in guanidinoacetate methyltransferase (GAMT)-deficient mice: validation of an animal model to study creatine deficiency. Magn Reson Med 2003;50:936–43 [DOI] [PubMed] [Google Scholar]
- 32.Schmidt A, Marescau B, Boehm EA, et al. Severey altered guanidino compound levels, disturbed body weight homeostasis and impaired fertility in a mouse model of guanidinoacetate N-methyltransferase (GAMT) deficiency. Hum Mol Genet 2004;13:905–21 [DOI] [PubMed] [Google Scholar]
- 33.Braissant O, Henry H, Loup M, et al. Endogenous synthesis and transport of creatine in the rat brain: an in situ hybridization study. Mol Brain Res 2001;86:193–201 [DOI] [PubMed] [Google Scholar]
- 34.Wallimann T, Wyss M, Brdiczka D, et al. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the “phosphocreatine circuit” for cellular energy homeostasis. Biochem J 1992;281:21–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallimann T, Hemmer W. Creatine kinase in non-muscle tissues and cells. Mol and Cell Biochem 1994;133–134:193–220 [DOI] [PubMed] [Google Scholar]
- 36.Ohtsuki S, Tachikawa M, Takanaga H, et al. The blood-brain barrier creatine transporter is a major pathway for supplying creatine to the brain. J Cereb Blood Flow Metab 2002;22:1327–35 [DOI] [PubMed] [Google Scholar]
- 37.Pettegrew J, Minshew NJ, Diehl J, et al. Anatomical considerations for interpreting topical 31P-NMR. Lancet 1983;2(8355):913. [DOI] [PubMed] [Google Scholar]
- 38.Tofts PS, Cady EB, Delpy DT, et al. Surface coil NMR spectroscopy of brain. Lancet 1984;i:459. [DOI] [PubMed] [Google Scholar]
- 39.Pritchard NR, Kalra PA. Renal dysfunction accompanying oral creatine supplements. Lancet 1998;351:1252–53 [DOI] [PubMed] [Google Scholar]
- 40.Koshy KM, Griswold E, Schneeberger EE. Interstitial nephritis in a patient taking creatine. N Engl J Med 1999;340:814–15 [DOI] [PubMed] [Google Scholar]
- 41.Persky AM, Brazeau GA. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev 2001;53:161–76 [PubMed] [Google Scholar]