Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to assess the incidence of de novo aneurysm formation, the incidence of subarachnoid hemorrhage (SAH), and the growth of existing untreated aneurysms in 52 patients after therapeutic carotid artery balloon occlusion for carotid aneurysms.
PATIENTS AND METHODS: Between January 1996 and August 2004, 52 patients were treated with carotid artery balloon occlusion for carotid aneurysms. In June 2005, all patients, their next of kin, or family physicians were contacted and questioned concerning episodes of headache or hospital admissions that could be attributed to SAH. In addition, MR imaging and MR angiography (MRA) at 3T were performed in 26 of 44 surviving patients after a mean follow-up period of 50.2 months (median, 43.5 months; range, 14–107 months). MR imaging and MRA studies were compared with the digital subtraction angiograms at the time of carotid artery occlusion.
RESULTS: During clinical follow-up of 52 patients at a mean of 50.3 months (median, 42.5 months; range, 0–107 months), no episodes of SAH were reported (0%; 97.5% confidence interval [CI], 0–8.2%). In the 26 patients with follow-up MR imaging, no de novo aneurysms were detected (0%; 97.5 CI, 0–13.2%). Five existing untreated small aneurysms in 5 patients had not enlarged after a mean follow-up of 40 months.
CONCLUSION: In this study, therapeutic carotid artery occlusion was not associated with development of new aneurysms or enlargement of existing untreated aneurysms with time.
Therapeutic carotid artery balloon occlusion is a simple, safe, and effective method in the treatment of carotid aneurysms that are not suitable for surgical clipping or selective occlusion with coils.1–4 After carotid occlusion, hemodynamic alterations will occur in the circle of Willis with increased flow over the contralateral carotid artery and anterior and/or posterior communicating arteries. There is some concern, expressed in anecdotal reports, that these hemodynamic changes predispose for the formation of de novo aneurysms or enlargement of existing untreated aneurysms, exposing the patient to a risk of subarachnoid hemorrhage (SAH).5–9
In this study, MR imaging and MR angiography (MRA) at 3T10 were used to assess the incidence of de novo aneurysm formation and the long-term occurrence of enlargement of existing untreated aneurysms after therapeutic carotid artery balloon occlusion. In addition, we assessed the incidence of SAH during an average follow-up period of 50.3 months.
Materials and Methods
Patients
Between January 1996 and August 2004, 52 patients were treated with carotid artery balloon occlusion for carotid aneurysms. In June 2005, a follow-up survey was performed. During the follow-up, 8 patients had died: 1 patient died shortly after carotid occlusion of initial SAH, 1 patient died of trauma, 2 elderly patients died in the hospital of pneumonia and multiple organ failure, 1 patient died in the hospital of chronic obstructive pulmonary disease, 1 patient died of lung cancer, 1 patient died in the hospital of cardiac infarction, and 1 patient died at home of “old age.” None of these patients died of SAH. Of the remaining 44 surviving patients, 37 were contacted and asked about episodes of headache or hospital admissions that could be attributed to SAH. Seven patients could not be traced, but previous clinical and MR imaging follow-up was available in medical records.
Follow-up MRA was offered to these 37 patients and 28 (76%) agreed to participate in this study. Between January and June 2005, MR imaging and MRA were performed in 26 of 28 patients. In 2 patients, MR imaging could not be performed because of claustrophobia in 1 and severe cervical kyphosis in the other patient. There were 21 women and 5 men with a mean age of 60.6 years (range, 28–81 years). The mean follow-up period of 26 patients after carotid occlusion was 50.2 months (median, 43.5 months; range, 14–107 months).
Clinical follow-up of all 52 patients, including the deceased patients and patients who could not be traced but who had previous follow-up, was at a mean of 50.3 months (median, 42.5 months; range, 0–107 months).
This study was approved by the ethics committees of both participating hospitals.
MR Imaging Protocol
MR imaging and MRA were performed on a 3T system (Intera R10; Philips Medical Systems, Best, the Netherlands) by using a phased-array head coil (MR Imaging Devices, Gainesville, Fla) with sensitivity encoding.
The MR imaging protocol included axial and coronal T2-weighted fast spin-echo, coronal T1-weighted spin-echo, and high-resolution multiple overlapping thin-slab acquisition (MOTSA) 3D time of flight (TOF) MRA sequences. Imaging parameters for the T1-weighted spin-echo sequence were the following: TR/TE, 570/12; 256 × 256 matrix (reconstructed to 512 × 512); 180-mm FOV; 90% rectangular FOV; and 3-mm-thick sections with 0.3-mm gap. Parameters for the T2-weighted fast spin-echo sequence were the following: TR/TE, 3394/80; 400 × 400 matrix (reconstructed to 512 × 512); 230-mm FOV; 70% rectangular FOV; 3-mm-thick sections with 0.5-mm gap. For the MOTSA 3D-TOF MR image, the parameters were as follows: 3D fast-field echo T1-weighted sequence; TR/TE, 21/4; flip angle, 20°; 512 × 512 matrix (reconstructed to 1024 × 1024); 200-mm FOV; 85% rectangular FOV; 1.0-mm-thick sections, interpolated to 0.5-mm; and 160 sections acquired in 8 slabs. The measured voxel size of the MOTSA 3D-TOF MR image was 0.39 × 0.61 × 1 mm, and the reconstructed voxel size was 0.2 × 0.2 × 0.5 mm.
MR imaging and MRA studies were interpreted by 2 experienced neuroradiologists in consensus and were compared with the digital subtraction angiograms at the time of carotid artery occlusion to assess the incidence of de novo aneurysm formation and growth of additional aneurysms. All patients and their family physicians were informed on the imaging findings.
Statistical Analysis
We recorded the overall number of de novo aneurysms and the overall number of episodes of SAH and calculated cumulative incidence rates with corresponding 95% confidence intervals (CI).
Results
In the 26 patients, no de novo aneurysms (cumulative incidence rate, 0%; 97.5% CI, 0–13.2%) were identified on MRA after a mean follow-up of 50.2 months (median, 43.5 months; range, 14–107 months; 109 patient years). Five of 26 patients had 1 untreated additional aneurysm on the following locations: anterior communicating artery, 2 mm (Fig 1); left ophthalmic artery, 3 mm (Fig 2); left middle cerebral artery, 5 mm; right M2-M3 junction, 3 mm; and left superior cerebellar artery, 2 mm. None of these 5 small additional aneurysms had enlarged after a mean follow-up of 40 months (range, 21–60 months; 16.6 patient years).
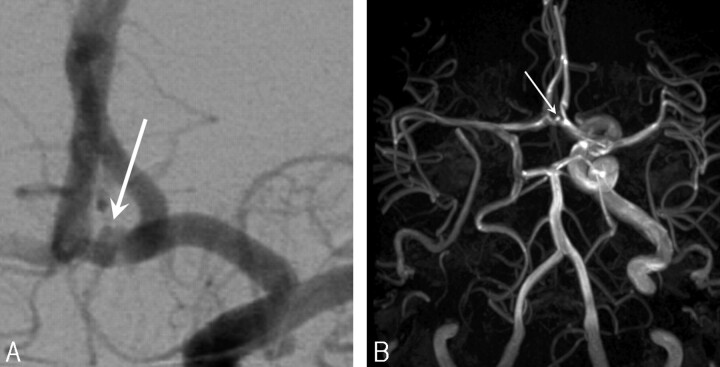
Fig 1.
A, Additional 1.7-mm anterior communicating artery aneurysm (arrow) in a 71-year-old woman with a giant right cavernous sinus aneurysm treated with carotid artery occlusion.
B, MRA 28 months after carotid artery occlusion demonstrates unchanged size (arrow).
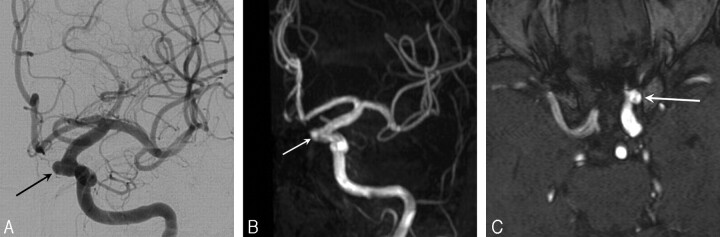
Fig 2.
A, Left internal carotid angiogram at the time of right internal carotid artery occlusion for a giant ophthalmic aneurysm in a 43-year-old woman shows additional small mirror ophthalmic aneurysm with a wide neck (arrow).
B, MRA maximum-intensity-projection image after 27 months demonstrates unchanged size (arrow).
C, MRA source image after 27 months demonstrates unchanged size (arrow).
During clinical follow-up of all 52 patients at a mean of 50.3 months (median, 42.5 months; range, 0–107 months; 218 patient years), no episodes of headache or hospital admissions that could be attributed to SAH were reported (cumulative incidence rate, 0%; 97.5% CI, 0–8.2%).
Discussion
There is some concern that increased hemodynamic stress in the circle of Willis after therapeutic balloon occlusion predisposes to the formation of de novo aneurysms or enlargement of existing aneurysms. Because no systematic follow-up data are available, frequency is not well established and publications are anecdotal.5–9 In a review6 of 19 articles published between 1962 and 2000 describing 30 cases with angiographically proved de novo aneurysm formation after carotid occlusion, incidence in 7 large series (including more than 30 carotid occlusions) varied from 0.7% to 3.4% (average, 2%), whereas 2 other smaller series (with fewer than 30 procedures) reported an incidence of 10% and 11%, respectively. On the other hand, other reviews of carotid ligation (some including more than 100 patients), failed to disclose new aneurysms, so cumulative incidence may be lower than 2%,6 with an unknown annual incidence rate. The interval between carotid occlusion and the onset of symptoms due to de novo aneurysms specified in 28 cases varied from 3 to 25 years with an average of 9.6 years with 20 of 28 (71%) cases between 3 and 10 years. The location of 27 de novo aneurysms was the anterior communicating artery in 11 (41%), the carotid artery in 14 (52%), and the vertebral artery in 2 (7%).
The incidence of de novo aneurysm formation and recurrent SAH in patients with aneurysms treated by clipping was recently established in large studies.11–13 In these studies, CT angiography was used to detect long-term de novo aneurysms and long-term enlargement of existing aneurysms after surgical clipping of a ruptured aneurysm. In 610 patients, 19 definitive de novo aneurysms were found in 14 patients (after a mean interval of 9.1 years) and 42 probable de novo aneurysms were found in 34 patients. This corresponds to an overall incidence of de novo aneurysm formation between 2.3% and 10% and an annual incidence between 0.37 and 1.20%. Of 19 definitive de novo aneurysms, 12 (63%) were located on the middle cerebral artery, 2 (11%) on the anterior communicating artery, and 5 (26%) on the carotid artery. Of these 19 aneurysms, 18 (95%) were smaller than 5 mm. In the same study, 4 of 18 (22%) existing aneurysms had enlarged in the first 5 years of follow-up, and 13 of 53 (25%), in the first 10 years. In a study of 752 patients with previously clipped ruptured aneurysms, the cumulative incidence of recurrent SAH was 3.2% in the first 10 years after initial SAH13 and was 22 times higher than that expected in populations with comparable age and sex.
The relatively high cumulative incidence of 2.3%–10% of de novo aneurysm formation in patients with clipped aneurysms and the cumulative incidence of recurrent SAH of 3.2% in the first 10 years seem higher than the estimated incidence of less than 2% of symptomatic or asymptomatic de novo aneurysm formation after carotid artery occlusion, though patients with carotid artery occlusion have not been studied systematically. Therapeutic carotid occlusion probably does not increase the incidence of new aneurysm formation with time compared with patients with clipped aneurysms but may influence the location of these new aneurysms. After carotid occlusion, new aneurysms occur almost exclusively on the contralateral internal carotid artery and anterior communicating artery; these vessels are the site of main hemodynamic changes with increased blood flow to supply the contralateral carotid circulation. In patients with clipped aneurysms, new aneurysms most frequently develop on the middle cerebral artery, a location seldom encountered after carotid occlusion.
Our prospective study, though small and with limited follow-up, confirms the assumption that carotid artery occlusion by itself does not seem to induce new aneurysm formation. However, longer follow-up will be needed for definitive conclusions because new aneurysms may develop after as long as 25 years. Our MRA protocol on a 3T system10 is a suitable screening tool for intracranial aneurysms. High resolution images of cerebral vasculature without administration of contrast material are obtained, and aneurysms of 2 mm or even smaller (Fig 1) can be depicted with confidence.
Balloon or coil occlusion of the carotid artery is generally used in symptomatic large and giant carotid aneurysms and is a simple, safe, and effective treatment. Tolerance to carotid occlusion can be tested reliably by clinical and angiographic test occlusion protocols, even in patients under general anesthesia.1,14 Fear of inducing new aneurysm formation by hemodynamic stress is probably unfounded and should not be an argument to proceed to alternative therapies that preserve patency of the carotid artery but are technically more challenging. These alternative therapies, such as direct surgical clipping, bypass surgery, or endovascular treatment with coils or liquid embolics with assistance of a supporting balloon or endovascular stent, are associated with higher complication rates.15–18 In our practice, these therapies are restricted to patients who cannot tolerate carotid artery occlusion.
Conclusion
In this study, therapeutic carotid artery occlusion was not associated with development of new aneurysms or enlargement of existing untreated aneurysms with time. Longer follow-up will be needed to draw more definitive conclusions.
Footnotes
This work was supported by Maatschap Radiologie, St. Elisabeth Ziekenhuis, Tilburg, the Netherlands and by Onderzoeksschool Neurowetenschappen, Amsterdam, the Netherlands.
References
- 1.van Rooij WJ, Sluzewski M, Slob MJ, et al. Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol 2005;26:175–78 [PMC free article] [PubMed] [Google Scholar]
- 2.van Rooij WJ, Sluzewski M, Metz NH, et al. Carotid balloon occlusion for large and giant aneurysms: evaluation of a new test occlusion protocol. Neurosurgery 2000;47:116–21 [DOI] [PubMed] [Google Scholar]
- 3.van der Schaaf IC, Brilstra EH, Buskens E, et al. Endovascular treatment of aneurysms in the cavernous sinus: a systematic review on balloon occlusion of the parent vessel and embolization with coils. Stroke 2002;33:313–18 [DOI] [PubMed] [Google Scholar]
- 4.Larson JJ, Tew JM Jr, Tomsick TA, et al. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery 1990;36:23–30 [PubMed] [Google Scholar]
- 5.Timperman PE, Tomsick TA, Tew JM Jr, et al. Aneurysm formation after carotid occlusion. ANJR Am J Neuroradiol 1995;16:329–31 [PMC free article] [PubMed] [Google Scholar]
- 6.Briganti F, Cirillo S, Caranci F, et al. Development of “de novo” aneurysms following endovascular procedures. Neuroradiology 2002;44:604–09 [DOI] [PubMed] [Google Scholar]
- 7.Clark WC, Ray MW. Contralateral intracranial aneurysm formation as a late complication of carotid ligation. Surg Neurol 1982;18:485–62 [DOI] [PubMed] [Google Scholar]
- 8.Dyste GW, Beck DW. De novo aneurysm formation following carotid ligation: case report and review of the literature. Neurosurgery 1989;24:88–92 [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara S, Fujii K, Fukui M. De novo aneurysm formation and aneurysm growth following therapeutic carotid occlusion for internal carotid artery (ICA) aneurysms. Acta Neurochirur (Wien) 1993;120:20–25 [DOI] [PubMed] [Google Scholar]
- 10.Majoie CB, Sprengers ME, van Rooij WJ, et al. MR angiography at 3T versus digital subtraction angiography in the follow-up of intracranial aneurysms treated with detachable coils. AJNR Am J Neuroradiol 2005;26:1349–56 [PMC free article] [PubMed] [Google Scholar]
- 11.van der Schaaf IC, Velthuis BK, Wermer MJ, et al, ASTRA Study Group. New detected aneurysms on follow-up screening in patients with previously clipped intracranial aneurysms: comparison with DSA or CTA at the time of SAH. Stroke 2005;36:1753–58 [DOI] [PubMed] [Google Scholar]
- 12.Wermer MJ, van der Schaaf IC, Velthuis BK, et al, ASTRA Study Group. Follow-up screening after subarachnoid haemorrhage: frequency and determinants of new aneurysms and enlargement of existing aneurysms. Brain 2005;128:2421–29 [DOI] [PubMed] [Google Scholar]
- 13.Wermer MJ, Greebe P, Algra A, et al. Incidence of recurrent subarachnoid hemorrhage after clipping for ruptured intracranial aneurysms. Stroke 2005;36:2394–99 [DOI] [PubMed] [Google Scholar]
- 14.Abud DG, Spelle L, Piotin M, et al. Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol 2005;26:2602–09 [PMC free article] [PubMed] [Google Scholar]
- 15.Brilstra EH, Rinkel GJ, Klijn CJ, et al. Excimer laser-assisted bypass in aneurysm treatment: short-term outcomes. J Neurosurg 2002;97:1029–35 [DOI] [PubMed] [Google Scholar]
- 16.Kessler IM, Mounayer C, Piotin M, et al. The use of balloon-expandable stents in the management of intracranial arterial diseases: a 5-year single-center experience. AJNR Am J Neuroradiol 2005;26:2342–48 [PMC free article] [PubMed] [Google Scholar]
- 17.Lubicz B, Piotin M, Mounayer C, et al. Selective endovascular treatment of intracranial aneurysms with a liquid embolic: a single-center experience in 39 patients with 41 aneurysms. AJNR Am J Neuroradiol 2005;26:885–93 [PMC free article] [PubMed] [Google Scholar]
- 18.Sluzewski M, van Rooij WJ, Beute GN, et al. Balloon-assisted coil embolization of intracranial aneurysms: incidence, complications, and angiography results. J Neurosurg 2006;105:369–99 [DOI] [PubMed] [Google Scholar]