Abstract
BACKGROUND AND PURPOSE: Our aim was to evaluate the hypothesis that water diffusion alterations are present in normal-appearing white matter of patients with relapsing-remitting multiple sclerosis (RRMS) and to assess their change with time.
MATERIALS AND METHODS: Fifty-four subjects with clinically diagnosed RRMS, with disease duration of less than 12 months and an expanded disability status scale (EDSS) score of <3.5, underwent a diffusion 3T MR imaging study. The apparent diffusion coefficient (ADC) maps generated were compared with those of 18 control subjects. Eighteen of the 54 patients underwent MR imaging assessment at 3 and 6 months after baseline evaluation. Remitting patients were clinically and MR imaging stable for the 2 months before the study. All patients were drug-free for the 3 months before the study, and in the relapsing patients, the MR imaging was always performed before beginning treatment.
RESULTS: Mean ADC values showed significant differences when relapsing, remitting, and control patients were compared. The relapsing or remitting phase showed significant difference when compared both with controls (P < .01) and between them (P < .05). Comparing mean ADC values of patients with clinical disability (EDSS <2 versus EDSS ≥2) also provided significant differences with the control group (P < .01). The data of patients showing a relapsing episode during the longitudinal part of the study showed a significant difference compared with data from their remitting phase (P < .01).
CONCLUSION: Brain microstructural changes can be detected and correlate with clinical impairment during the stages of MS. These changes modify with time in the relapsing group.
The early clinical course of multiple sclerosis (MS) is usually marked by a relapsing-remitting (RR) phase characterized by episodes of acute worsening of symptoms followed by recovery, with a stable course between relapses. The diagnosis at presentation may be difficult, so the initial clinical suspicion must be confirmed by appropriate imaging and laboratory testing such as MR imaging, CSF examination, and evoked potentials studies.
Valuable data from technical advancement have increased the sensitivity of conventional MR imaging, allowing a better visualization of both acute and chronic brain lesions, monitoring of the disease progression, and detection of the appearance of new lesions and the real extension of damage in white/gray matter.1–4 In addition, imaging techniques such as diffusion-weighted imaging (DWI) have been widely applied in the study of normal-appearing white matter (NAWM).5–10 These studies helped to support the recent evidence that MS is a diffuse disease of the central nervous system (CNS).11–13
Because of a lack of a clear understanding of the causes of MS, treatments cannot stop the disease progression. Current cornerstones of therapy are corticosteroids and immunomodulatory drugs. The first reduce the inflammatory component of the acute phases of MS, whereas the second, such as interferon-β (IFN-β) or glatiramer acetate (GA), slow the disease progression. High-dose glucocorticosteroids show a nongenomic effect that probably damages the cells,14,15 whereas IFN-β has an immunomodulatory effect and seems to act on the brain endothelial cells by blocking the migration of inflammatory cells into the CNS.16 GA, on the other hand, can induce specific suppressor cells that migrate to the brain and lead to in situ bystander suppression.17 All these effects could probably influence the random motion of water molecules in the NAWM. Therefore, during the progression of MS, either medications or severe pathologic disruptions would influence the diffusivity. This possibility highlights the value of DWI evaluation of NAWM in drug-free patients. This approach would allow the detection of the subtle changes that probably characterize the initial clinical phase of MS.
The aim of our study was therefore to test the possible NAWM diffusivity changes in drug-free patients with MS. Furthermore, to uncover a correlation between clinical data and NAWM pattern, we evaluated the longitudinal MS-related NAWM changes during 6 months of disease course.
Materials and Methods
Subjects
The NAWM of 54 drug-free patients with RRMS (35 women and 19 men; mean age, 32.4 years; range, 18–46 years) and with a mean disease duration of less than 12 months (mean, 237 days) admitted to the neurologic department of our university hospital were studied by DWI.
The diagnosis of MS was established by clinical, laboratory, and MR imaging criteria and matched the criteria of Poser et al18 and McDonald et al.19 In all instances, patients underwent detection of oligoclonal banding in the CSF. MR imaging confirmed the diagnosis. Inclusion criteria were the following: 1) clinically definite diagnosis of RRMS, 2) Expanded Disability Status Scale (EDSS)20 scores between 0 (absence of neurologic signs) and 3.5 (neurologic signs with moderate disability), 3) history of at least 2 clearly documented relapses, and 4) mean disease duration of less than 12 months.
Relapsing patients had a new symptom or a recrudescence of an old one; this was combined with either appearance of a new enhancing lesion or with an old one showing contrast enhancement. Patients showing stable clinical and MR imaging conditions in the 2 months before the study were defined as “remitting.” Patients undergoing the study were not on any disease-modifying drugs and were free from steroid therapy for the previous 3 months. In each patient with an acute relapse, the MR imaging was performed within 4 days of the onset of symptoms and always before beginning a corticosteroids treatment.
Eighteen of 54 subjects entered a longitudinal study and were examined at 3 (T3 point) and 6 (T6 point) months after baseline (T0 point) evaluation. These patients refused immunosuppressant or immunomodulatory treatments because of unbearable side effects, desire of pregnancy, or other personal reasons. The remaining patients started standard MS-specific treatments (80% IFN-β 1a or 1b subcutaneously; 20% GA subcutaneously) after the first MR imaging. Eighteen healthy volunteers (11 women and 7 men; mean age, 31 ± 3.6 years) served as controls.
Informed consent was obtained from all patients, and the study was approved by the local ethics committee.
MR Imaging
Imaging was performed in all subjects by using a 3T MR imaging clinical unit (Achieva, Philips Medical Systems, Eindhoven, the Netherlands). All subjects were examined by using the same protocol, which included axial and sagittal spin-echo (SE) T1-weighted sequences (2000 ms/10–15 ms/2[TR/TE/NEX]; section thickness, 4 mm; gap, 1 mm), axial SE proton-density and T2-weighted sequences (2000 ms/80 ms/1[TR/TE/NEX]), and axial fluid-attenuated inversion-recovery T2-weighted sequences (11 000 ms/125 ms/2[TR/TE/NEX]). Single-shot echo-planar DWI sequences (TR, 3906 ms; TE, 55 ms; thickness, 4 mm; matrix, 256 × 256; FOV, 230 mm; gap, 1 mm; b factor, 0–1000) were performed with enhanced gradient mode and SE acquisition and sensitivity encoding (SENSE; factor, 2). Diffusion gradients were applied to each of the 3 orthogonal directions (x, y, z), and the apparent diffusion coefficient (ADC) maps were generated (trace ADC = [ADCx + ADCy + ADCz ]/3). Postcontrast (gadolinium-diethylene-triaminepentaacetic acid, 0.2 mmol/Kg) axial, sagittal, and coronal T1-weighted images (2000/10–15/2[TR/TE/NEX]) were acquired with a delay time of 4 minutes.
MR Imaging Analysis
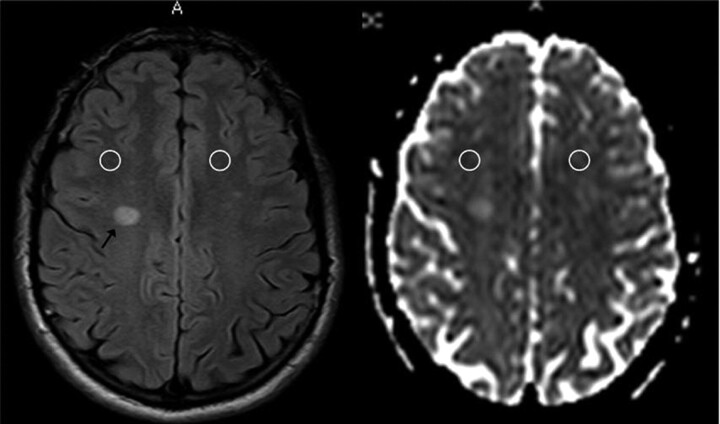
An experienced neuroradiologist, blinded to the clinical details of patients with MS, performed the MR image analysis on a dedicated workstation. Small elliptic regions of interest (ROIs) of approximately 50 mm2 (corresponding to approximately 60 pixels) were placed bilaterally over the NAWM, distant from MS lesions and at different levels, including the centrum semiovale, the frontal white matter (WM), the occipital WM, and the brachium pontis. The ROIs were first drawn on the T2-weighted images and then superimposed at the same anatomic level on the ADC maps by using the software provided by the scanner producer (Easy Vision, Philips Medical Systems, Best, the Netherlands) (Fig 1). To avoid partial volume artifacts and considering the distortion of the echo-planar imaging (EPI) sequence, we placed ROIs in large areas of NAWM, remote from MS lesions as well as distant from the ventricles, and examined the 2 sections above and below each ROI to avoid closeness with MS lesions and contacts with areas other than NAWM.
Fig 1.
Example of ROIs of approximately 50 mm2 placed over the NAWM of the centrum semiovale and then superimposed on the ADC maps.
Considering the lack of spatial correspondence between diffusion and T2-weighted images to fit into corresponding NAWM, we determined the positioning of the ROIs from careful visual comparison between the T2-weighted image and the ADC trace map, thus manually adjusting them. A similar method was applied at T3 and T6 for the analysis of the 18 patients who entered the longitudinal study.
ROIs of approximately 50 mm2 were drawn in images of the age-matched control subjects at the same anatomic levels as those of the MS group.
Statistical Analysis
Data were analyzed by using the 1-way analysis of variance (ANOVA; Statsoft 1999, Statistica, Tulsa, Okla) between and within factors groups with 3 levels. We analyzed the following groups: remitting versus relapsing versus control subjects, patients with a low EDSS (<2; n = 32) versus patients with a high EDSS (≥2; n = 22) versus controls, and patients at different time points of study. Differences with a P value of less than 0.05 were considered significant.
Results
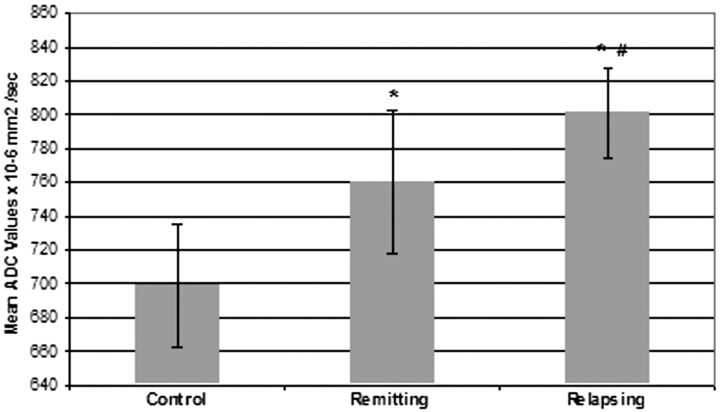
Statistically significant differences in mean ADC values obtained from all the studied regions were noted when comparing relapsing, remitting, and control patients (mean ADC: 801 ± 27 × 10−6 mm2/s; 760 ± 43 × 10−6 mm2/s; 699 ± 36 × 10−6 mm2/s in relapsing, remitting, and controls, respectively). Patients in both relapsing or remitting phases showed significant differences when compared with controls and between them as shown in Fig 2 (relapsing versus controls, P < .01; relapsing versus remitting, P < .05).
Fig 2.
Comparison of relapsing versus remitting versus control patients (mean ADC: 801 × 10−6 mm2/s ± 27; 760 × 10−6 mm2/s ± 43; 699 × 10−6 mm2/s ± 36 in relapsing, remitting, and controls respectively). Asterisk indicates P < .01 versus controls; number symbol, P < .05 versus remitting.
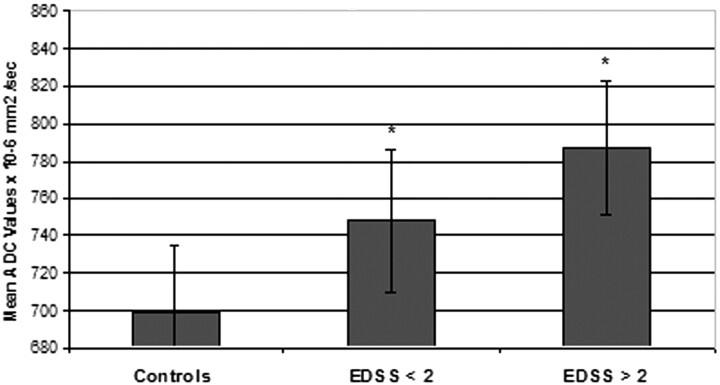
Significant differences were also noted when comparing mean ADC values of patients with clinical disability (EDSS <2 versus EDSS ≥2; ADC: 748 ± 38 versus 787 ± 36 × 10−6 mm2/s; controls, ± 36). In fact, as in Fig 3, both EDSS groups showed notable differences with controls but not between them (EDSS versus controls, P < .01).
Fig 3.
Mean ADC values comparison of patient groups with different EDSS scores and controls (ADC: EDSS <2 versus EDSS ≥2, 748 ± 38 versus 787 ± 36 × 10−6 mm2/s; controls, 699 × 10−6 mm2/s ± 36). Asterisk indicates P < .01 versus controls.
Patients enrolled in the longitudinal study (n = 18), when compared at the different time points, showed a slight increase with time in mean ADC values. Such increase, however, did not appear to be of statistical significance (mean ADC: T0, 759 ± 39; T3, 767 ± 43; T6, 772 ± 34 × 10−6 mm2/s).
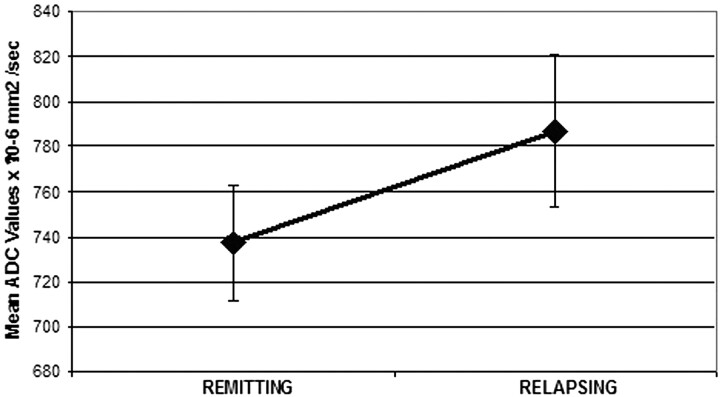
The patients who had 1 relapsing episode (n = 7) during the longitudinal part of the study did not show any statistically significant difference when their mean ADC values were compared at the 3 time points (T0: 770 ± 43; T3: 763 ± 61; T6: 781 ± 31 × 10−6 mm2/s). However, in this subgroup of patients, when the ADC values of those having 1 relapsing episode were compared with the ADC values of the same subjects during their remitting phase, a statistically significant difference was observed (mean ADC: relapsing, 787 ± 34; remitting, 737 ± 26 × 10−6 mm2/s; P < .01) (Fig 4).
Fig 4.
Comparison between patients participating in the longitudinal study during their relapsing and their remitting phase (mean ADC: relapsing, 787 ± 34; remitting, 737 ± 26 × 10−6 mm2/s; P < .01).
Discussion
This study shows that drug-free patients with RRMS have an abnormal water diffusivity in NAWM compared by ANOVA with control subjects and that the diffusivity changes correlate significantly with clinical disability expressed as an EDSS score. The study also shows a change in water diffusivity between remitting and relapsing patients and between patients with low disability or high disability.
Our data are consistent with previous reports describing similar findings. All previous similar studies, however, have used a more heterogeneous family of patients—that is, patients with either low EDSS but longer disease duration or with shorter disease duration but higher EDSS.21–23 Other authors have, instead, followed in time patients with a clinical isolated syndrome.9
The main interest of our study, however, is that it shows an effect of the disease beyond the lesion areas since the very beginning of its course. An important observation that emerges from our data is the fact that even at an early stage and in the absence of other apparent signs of disease activity, the bases for its further evolution are already somehow set into the NAWM. The reason for the difference in severity and speed in the further evolution of the disease in different patients, however, remains unclear. The water diffusivity is determined by the total amount of water in the extracellular/intracellular space and by the cell attenuation. A drug-free population of patients was required to avoid the effect that treatment could have on the extracellular space and, therefore, on water diffusivity.24,22
DWI, in fact, is a far more sensitive sequence than T1- and T2-weighted images in detecting water changes at a molecular level. Therefore, it is not surprising that Brainin et al,24 in the evaluation of T1 and T2 values of the NAWM after steroid therapy, found a significant T2 decrease only in 1 case.
What is causing the diffusivity changes detected in patients with early MS remains unknown. Recent pathologic investigations have identified processes such as glial pathology, axonal loss, or perivascular cuffing as the main contributors to NAWM alterations12; however, the timing and degree of expression of such processes and their relationship to the clinical phenotypes are still poorly understood.
Our data, although not supported by histologic correlates, could suggest that the previously mentioned pathologic changes occur even during the early phases of the disease; and because the changes were observed in all positioned ROIs and correlated with clinical disability, they strengthen the concept that MS is a diffuse CNS disease.11
Moreover, it is tempting to speculate that the diffusivity changes observed during the remitting phase could be due to axonal and glial pathology, whereas the higher ADC values detected during the relapsing phases would be explained by the contribution of the inflammatory reaction on an already partially damaged tissue.
This could be supported by recent findings by Barnett and Prineas,25 who have proposed oligodendrocytes apoptosis as the initial event in new-lesion formation, later followed by an inflammatory response, with leukocyte recruitment necessary to remove the huge quantity of apoptotic membrane.
In the longitudinal study, we observed that the ADC changes of the early phase of the disease tend to increase with time, however, without statistically significant differences, possibly because of the short time period between the longitudinal points analysis. The averaging effect of comparing the mean ADC values of patients who had 1 relapsing episode during the longitudinal part of the study probably explains why the values did not show any statistically significant difference.
However, a significant difference in ADC values was noted in those patients participating in the longitudinal study and having a relapsing episode. This confirms that the acute inflammatory episode has a definite and progressive influence on the WM so that DWI could be considered a valuable tool to properly stage the extension and effective progression of damage in the WM outside the visible lesions.
The decision to use the more sensitive isotropic diffusion method over other MR imaging techniques was based on previous data.26 In addition, ROI analysis in this study was preferred over the global histogram analysis because our purpose was to assess by DWI the NAWM remote from MS lesions. With the histogram analysis, periplaque WM, which is known to express different diffusivity values from those of distant regions,27 might influence the reproducibility of the measurements for our specific target.
One potential limitation in our study was the well-known distortion of ROIs as superimposed on the DWI as a result of the intrinsic characteristic of EPI sequences. However, because our purpose did not require a millimetric correspondence between the T2-weighted images and DWIs because ROIs were placed over large areas of NAWM, remote from lesions, ventricles, and other small structures (eg, ROIs were not positioned over the internal capsule, corpus callosum, and so forth), possible millimetric noncorrespondence would not influence the overall results. In any case, the distortion was minimized by using the SENSE mode, which allowed us to reduce the length of EPI train, thereby reducing susceptibility-related distortion and blurring effect. In addition, manual correction with careful comparison of T2-weighted images was performed in cases of lack of spatial correspondence.
A major limitation of the present study was the small number of patients who underwent the longitudinal analysis. Almost all studied patients, in fact, were treated with MS-specific immunomodulatory agents in the next months after the diagnosis. Immunomodulatory treatments in MS are efficacious only at the beginning of the disease; thus, it is essential not to postpone treatment from the first manifestation of the disease.
In conclusion, this study suggests that subtle structural brain changes are already present during the early stages of MS, when clinical impairment and EDSS are low, and that such changes correlate with clinical impairment since the very beginning of the disease. The use of a drug-free population permitted us to determine that the modifications detected are to be attributed to the disease itself and not to external causes.
References
- 1.Filippi M. Magnetization transfer imaging to monitor the evolution of individual multiple sclerosis lesions. Neurology 1999;53(Suppl 3):S18–22 [PubMed] [Google Scholar]
- 2.Filippi M, Campi A, Dousset V, et al. A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology 1995;45:478–82 [DOI] [PubMed] [Google Scholar]
- 3.Filippi M, Arnold DL, Comi G. Magnetic Resonance Spectroscopy in Multiple Sclerosis. Milan, Italy: Springer-Verlag;2001
- 4.Ge Y, Grossman RI, Udupa JK, et al. Magnetization transfer ratio histogram analysis of gray matter in relapsing-remitting multiple sclerosis. AJNR Am J Neuroradiol 2001;22:470–75 [PMC free article] [PubMed] [Google Scholar]
- 5.Cercignani M, Inglese M, Pagani E, et al. Mean diffusivity and fractional anisotropy histograms of patients with multiple sclerosis. AJNR Am J Neuroradiol 2001;22:952–58 [PMC free article] [PubMed] [Google Scholar]
- 6.Ciccarelli O, Werring DJ, Wheeler-Kingshott CA, et al. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology 2001;56:926–33 [DOI] [PubMed] [Google Scholar]
- 7.Filippi M, Inglese M. Overview of diffusion-weighted magnetic resonance studies in multiple sclerosis. J Neurol Sci 2001;186:S37–S43 [DOI] [PubMed] [Google Scholar]
- 8.Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology 2005;65:1526–32 [DOI] [PubMed] [Google Scholar]
- 9.Caramia F, Pantano P, Di Legge S, et al. A longitudinal study of MR diffusion changes in normal appearing white matter of patients with early multiple sclerosis. Magn Reson Imaging 2002;20:383–88 [DOI] [PubMed] [Google Scholar]
- 10.Cassol E, Ranjeva JP, Ibarrola D, et al. Diffusion tensor imaging in multiple sclerosis: a tool for monitoring changes in normal-appearing white matter. Mult Scler 2004;10:188–96 [DOI] [PubMed] [Google Scholar]
- 11.Filippi M, Rocca MA. MRI evidence for multiple sclerosis as a diffuse disease of the central nervous system. J Neurol 2005;252 (suppl):v16–v24 [DOI] [PubMed] [Google Scholar]
- 12.Miller DH, Thompson AJ, Filippi M. Magnetic resonance studies of abnormalities in the normal appearing white matter and grey matter in multiple sclerosis. J Neurol 2003;250:1407–19 [DOI] [PubMed] [Google Scholar]
- 13.Hafler DA, Slavik JM, Anderson DE, et al. Multiple sclerosis. Immunol Rev 2005;204:208–31 [DOI] [PubMed] [Google Scholar]
- 14.Diem R, Hobom M, Maier K, et al. Methylprednisolone increases neuronal apoptosis during autoimmune CNS inflammation by inhibition of an endogenous neuroprotective pathway. J Neurosci 2003;23:6993–7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao AB, Richert N, Howard T, et al. Methylprednisolone effect on brain volume and enhancing lesions in MS before and during IFNbeta-1b. Neurology 2002;59:688–94 [DOI] [PubMed] [Google Scholar]
- 16.Kraus J, Ling AK, Hamm S, et al. Interferon-beta stabilizes barrier characteristics of brain endothelial cells in vitro. Ann Neurol 2004;56:192–205 [DOI] [PubMed] [Google Scholar]
- 17.Arnon R, Aharoni R. Mechanism of action of glatiramer acetate in multiple sclerosis and its potential for the development of new applications. Proc Natl Acad Sci U S A 2004;101(suppl 2):14593–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–31 [DOI] [PubMed] [Google Scholar]
- 19.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–27 [DOI] [PubMed] [Google Scholar]
- 20.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–52 [DOI] [PubMed] [Google Scholar]
- 21.Griffin CM, Chard DT, Parker GJ, et al. The relationship between lesion and normal appearing brain tissue abnormalities in early relapsing remitting multiple sclerosis. J Neurol 2002;249:193–99 [DOI] [PubMed] [Google Scholar]
- 22.Cercignani M, Iannucci G, Rocca MA, et al. Pathologic damage in MS assessed by diffusion-weighted and magnetization transfer MRI. Neurology 2000;54:1139–44 [DOI] [PubMed] [Google Scholar]
- 23.Gallo A, Rovaris M, Riva R, et al. Diffusion-tensor magnetic resonance imaging detects normal-appearing white matter damage unrelated to short-term disease activity in patients at the earliest clinical stage of multiple sclerosis. Arch Neurol 2005;62:803–08 [DOI] [PubMed] [Google Scholar]
- 24.Brainin M, Neuhold A, Reisner T, et al. Changes within the “normal” cerebral white matter of multiple sclerosis patients during acute attacks and during high-dose cortisone therapy assessed by means of quantitative MRI. J Neurol Neurosurg Psychiatry 1989;52:1355–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 2004;55:458–68 [DOI] [PubMed] [Google Scholar]
- 26.Castriota-Scanderbeg A, Fasano F, Hagberg G, et al. Coefficient D(av) is more sensitive than fractional anisotropy in monitoring progression of irreversible tissue damage in focal nonactive multiple sclerosis lesions. AJNR Am J Neuroradiol 2003;24:663–70 [PMC free article] [PubMed] [Google Scholar]
- 27.Guo AC, MacFall JR, Provenzale JM. Multiple sclerosis: diffusion tensor MR imaging for evaluation of normal-appearing white matter. Radiology 2002;222:729–36 [DOI] [PubMed] [Google Scholar]