Abstract
Purpose
To assess the comparative diagnostic accuracy of cardiac CT and transesophageal echocardiography (TEE) in the detection of valvular and perivalvular complications in infective endocarditis.
Materials and Methods
PubMed and Embase databases were systematically searched until July 2019 for original articles published in English. Studies were included if (a) they used CT and/or TEE as an index test, (b) data were provided as infective endocarditis valvular complications (classified as abscess or pseudoaneurysm, vegetation, leaflet perforation, and paravalvular leakage), and (c) they used surgical findings as the reference standard.
Results
Eight studies fulfilled the inclusion criteria. The sensitivity was higher for CT than TEE for abscess or pseudoaneurysm detection, 78% (95% confidence interval [CI]: 70%, 85%) (112 of 142) versus 69% (95% CI: 62%, 76%) (94 of 135) (P = .052) and increased to 87% (95% CI: 78%, 93%) (70 of 79) when restricted to multiphase CT studies, the difference being significant (P = .04). The sensitivity was significantly higher for TEE than CT for vegetation detection, 94% (95% CI: 92%, 96%) (363 of 383) versus 64% (95% CI: 57%, 70%) (151 of 237) (P < .001) and leaflet perforation detection, 81% (95% CI: 71%, 88%) (74 of 91) versus 41% (95% CI: 25%, 59%) (14 of 35) (P = .02).The sensitivity for paravalvular leakage was 69% (95% CI: 58%, 79%) (56 of 80) versus 44% (95% CI: 30%, 59%) (21 of 48) for TEE and CT, respectively (P = .27).
Conclusion
CT performs better than TEE in the detection of abscess or pseudoaneurysm whereas TEE gives superior results for vegetation detection, leaflet perforation, and paravalvular leakage.
Supplemental material is available for this article.
© RSNA, 2020
Summary
Given their complementary value for detecting infective endocarditis valvular abnormalities, the combined use of CT and transesophageal echocardiography could become the preferred imaging strategy performed in patients suspected of having infective endocarditis valvular complications.
Key Points
■ CT demonstrates a sensitivity of 78% compared with 69% for transesophageal echocardiography (TEE) (P = .052) for the detection of periannular extension of infective endocarditis, which increased to 87% in multiphase-gated CT studies (P = .04).
■ TEE is more sensitive than CT for vegetation detection (94% vs 64%) (P < .001), leaflet perforation (81% vs 41%) (P = .02), and paravalvular leakage (69% vs 44%) (P = .27).
■ As multiphase-gated CT acquisition improves the detection of infective endocarditis valvular complications, retrospective electrocardiogram-gated protocol should be the preferred technique.
Introduction
Infective endocarditis is a severe life-threatening disease characterized by a high risk of complications and a mortality rate around 25% after 1-year clinical follow-up. Surgical treatment is required during the acute phase in about 25%–50% of the cases because of heart failure, uncontrolled infection, or to prevent embolization (1). Valvular and perivalvular complications such as abscess or pseudoaneurysm, vegetation, leaflet perforation, and paravalvular leakage require particularly frequent and urgent surgical management.
Infective endocarditis diagnosis is based on modified Duke criteria including clinical, biologic, and echocardiographic findings (2). Because transthoracic echocardiography has demonstrated limited sensitivity in the diagnosis of infective endocarditis, transesophageal echocardiography (TEE) is now usually performed and considered the reference imaging technique (3). However, TEE is limited by practitioner skill, patient morphology, and artifacts related to valve calcifications or mechanic prosthetic valves (especially for the diagnosis of anterior aortic abscess). Moreover, TEE may be contraindicated (esophageal disease) or poorly tolerated by the patient. Finally, findings from echocardiography are negative or inconclusive in up to 30% of patients with subsequently proven infective endocarditis.
There have been encouraging results in terms of cardiac CT in detecting infective endocarditis valvular and perivalvular complications, and some recent reviews including a few studies that have suggested that cardiac CT should be included in the routine imaging strategy (4–7). However, in international guidelines for the management of infective endocarditis, the usefulness of cardiac CT is restricted to difficult cases with inadequate transthoracic echocardiography or TEE. Moreover, there is no specific recommendation for the systematic use of cardiac CT once the diagnosis has been made (8,9).
The purpose of our systematic review and meta-analysis was to determine the comparative diagnostic accuracy of cardiac CT and TEE in the detection of valvular and perivalvular complications (abscess or pseudoaneurysm, vegetation, valve perforation, perivalvular leak) in patients with infective endocarditis.
Materials and Methods
Methods
The meta-analysis was performed according to standard guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-analysis (10).
Search Strategy
A database search was performed by two investigators independently (M.O., Michèle Hamon) in PubMed and EMBASE databases until July 2019 for studies published in English. Our research included the terms “infective endocarditis,” “computed tomography,” “echocardiography,” “echocardiography, transesophageal,” and their related terms.
We also explored references from retrieved articles and reviews. The retrieved studies were carefully examined by the same two investigators (M.O., Michèle Hamon) to exclude potentially duplicated or overlapping data. Meeting abstracts were excluded because they did not provide adequately detailed data and their results might not be final. Disagreements were resolved by consensus between the same two authors.
Study Selection
Studies were included if (a) they used CT and/or TEE as index test, (b) data were provided as infective endocarditis valvular complications (classified as abscess or pseudoaneurysm, vegetation, leaflet perforation, and paravalvular leakage), and (c) they used surgical findings as the reference standard. Patients with native and prosthetic valves were included. Only studies providing data on true-positive, false-positive, true-negative, and false-negative results or studies providing enough detailed data to allow their calculation were taken into account. The studies based solely on clinical follow-up were excluded. Case reports, letters, and reviews were excluded.
Data Extraction and Quality Assessment
The same two investigators (M.O., Michèle Hamon) independently reviewed the titles and abstracts of the research results to determine the studies presenting the inclusion criteria and then performed the data extraction. The discrepancies were resolved by consensus. The following information was extracted from each study: author; journal and year of publication; design of the study; inclusion period; study population characteristics; number of patients assessed; mean age; percentage male; number of valves, position and type, and distinction between native and prosthetic valves; time interval between imaging examinations (CT and TEE) and between imaging and surgery; CT technical characteristics including the type of machine used, parameters for acquisition, technical protocol, slice thickness, radiation dose (in millisieverts), medication used (β-blocker); and coronary artery disease assessed with CT.
The quality of each study was judged using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) (11) method assessing the risk of bias and the clinical applicability through four key areas: patient selection, test index, standard reference, and flow and time.
Data Synthesis and Statistical Analysis
Imaging complications of infective endocarditis were classified as abscess or pseudoaneurysm, vegetation, leaflet perforation, and paravalvular leakage. Given their similar clinical and surgical implications, abscess and pseudoaneurysm were analyzed together. By means of true-positive, true-negative, false-positive, and false-negative rates, we computed sensitivity and specificity for the diagnosis of each infective endocarditis valvular complication. Subanalysis was performed according to CT protocol used. All data were extracted from published articles without access to individual patient-level data excluding specific adjustments on some patient characteristics such as age, risk factors, or medical history.
We computed all statistics for the first individual and then combined studies using a random-effects model, weighting each point estimated from calculations to the inverse of the sum of its variance and the between-study variance. The between-study statistical heterogeneity was evaluated using the Cochran Q χ2 and the inconsistency index (I2) test. When P was < .10 for the Q χ2, the heterogeneity was considered as being significant. The statistical variation between studies could be explained using the inconsistency index (I2) which ranged between 0% (no heterogeneity) and 100% (maximal heterogeneity). A funnel plot was drawn to assess publication bias (Fig E1 [supplement]). Statistical computations were performed with Meta-Disc 1.4 (Clinical Biostatistics Unit, Hospital Ramon y Cajal, Madrid, Spain) (12), and SAS software 7.1 (SAS Institute, Cary, NC). Comparison of estimated sensitivity and specificity was performed using a mixed effect model, with SAS 7.1.
Results
The review process is described in Figure 1. We ultimately included eight studies (13–20) in this systematic review, with 355 and 510 patients evaluated with CT and TEE, respectively.
Figure 1:
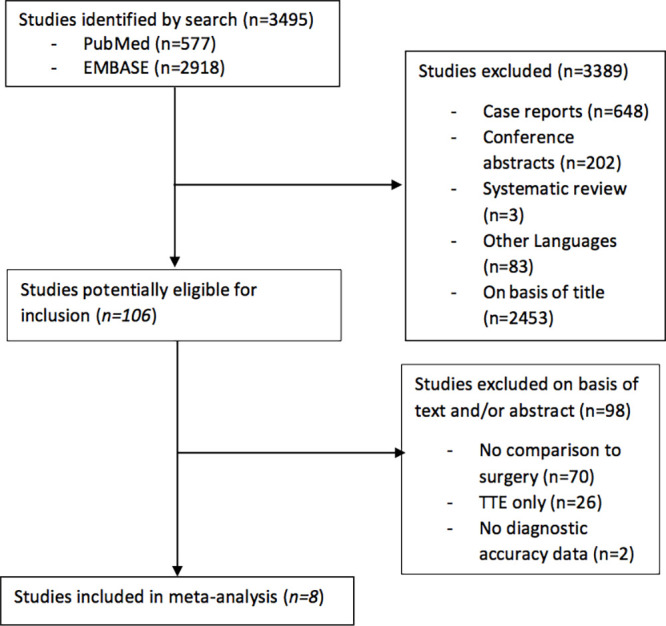
Flowchart describes the publication search and selection of eligible studies.
Studies’ Characteristics
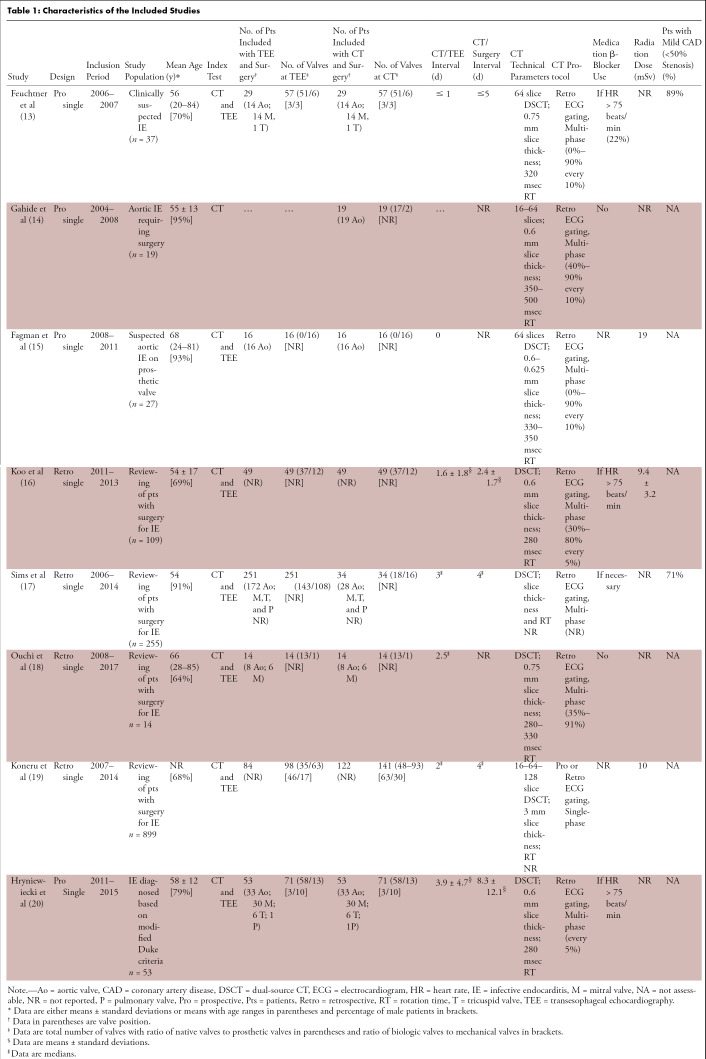
Characteristics of the studies are reported in Table 1. All studies were published between 2009 and 2019, with an inclusion period ranging from 2004 to 2017. Seven studies analyzed patients with native and prosthetic valves (13,14,16–20), whereas patients with prosthetic valves only were analyzed in one study (15). Four studies had a waiting period of fewer than 5 days between CT and surgery (13,16,17,19); one study had an average waiting period of 8 days (20).
Table 1:
Characteristics of the Included Studies
Only three studies reported a radiation dose at CT, ranging from 9 to 19 mSv (15,16,19). Two studies reported results of coronary CT angiography (13,17).
Quality Assessment
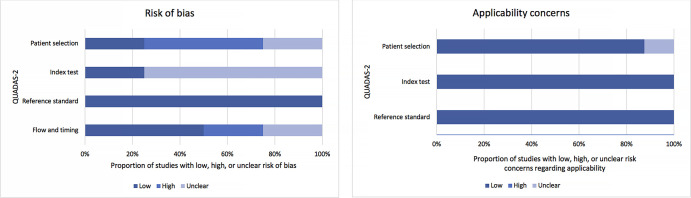
The overall quality assessment of the studies according to the QUADAS-2 tool is reported in Figure 2. Three studies did not mention the time interval between the index test and the reference standard (14,15,18). Data were prospectively collected in 50% (four of eight) of studies (13–15,20).
Figure 2:
Quality assessment of studies included with QUADAS-2 revised criteria. Stacked bars represent the proportion of studies with low, uncertain, or high risk of bias with regard to patient selection, reference standard used, and imaging modality (index test). QUADAS = Quality Assessment of Diagnostic Accuracy Studies.
Pooled Diagnostic Accuracy for Infective Endocarditis–related Valvular Complication Diagnosis
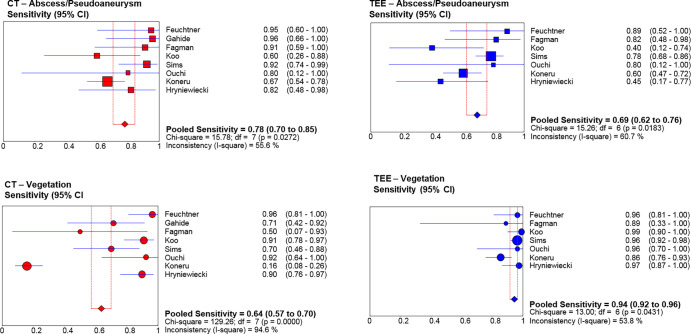
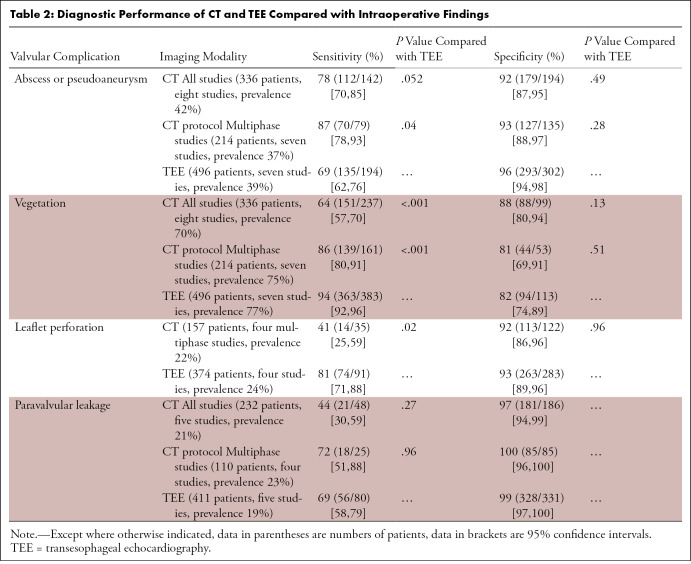
Abscess or pseudoaneurysm.—In included studies, the prevalence of abscess or pseudoaneurysm was 42% and 39% for CT and TEE, respectively. The pooled sensitivity tended to be higher for CT (78%; 95% confidence interval [CI]: 70%, 85%) (112 of 142) than for TEE (69%; 95% CI: 62%, 76%) (135 of 194) (P = .052) (Fig 3). The pooled specificity was 92% (95% CI: 87%, 95%) (179 of 194) and 96% (95% CI: 94%, 98%) (293 of 302) for CT and TEE, respectively (P = .49). Heterogeneity was present for sensitivity for both CT and TEE (P < .10).
Figure 3:
Forest plots for abscess or pseudoaneurysm pooled sensitivity and vegetation pooled sensitivity. CT (red dots), transesophageal echocardiography (TEE) (blue dots). Square dots represent abscess or pseudoaneurysm, round dots represent vegetation. CI = confidence interval, df = degrees of freedom.
Vegetation.—In the included studies, the vegetation prevalence was 70% and 77% for CT and TEE, respectively. CT demonstrated lower pooled sensitivity (64%; 95% CI: 57%, 70%) (151 of 237) than TEE (94%; 95% CI: 92%, 96%) (363 of 383) (P < .001) (Fig 3). Pooled specificity was 88% (95% CI: 80%, 94%) (88 of 99) and 82% (95% CI: 74%, 89%) (94 of 113) for CT and TEE, respectively (P = .13). Heterogeneity was present for sensitivity for both CT and TEE and for specificity for TEE (P < .10).
Leaflet perforation.—The perforation prevalence was 22% and 24%, respectively, for CT and TEE in included studies.
Pooled sensitivity and specificity of CT was 41% (95% CI: 25%, 59%) (14 of 35) and 92% (95% CI: 86%, 96%) (113 of 122), respectively. With a sensitivity of 81% (95% CI: 71%, 88%) (74 of 91), TEE was more sensitive than CT (P = .02). Heterogeneity was present for specificity for TEE (P < .10).
Paravalvular leakage.—The paravalvular leakage prevalence was 21% for CT and 19% for TEE in included studies. Pooled sensitivity and specificity of CT was 44% (95% CI: 30%, 59%) (21 of 48) and 97% (95% CI: 94%, 99%) (181 of 186), respectively. Sensitivity of TEE was 69% (95% CI: 58%, 79%) (56 of 80). Heterogeneity was present for sensitivity for both CT and TEE (P < .10).
Subgroup Analysis
We performed a subanalysis according to the CT protocol used. When only studies using multiphase protocol were analyzed (seven of eight studies), CT sensitivity increased up to 87% (95% CI: 78%, 93%) (70 of 79), 86% (95% CI: 80%, 91%) (139 of 160), and 72% (95% CI: 51%, 88%) (18 of 25) for abscess or pseudoaneurysm, vegetation, and paravalvular leakage detection, respectively (13–18,20). All results are reported in Table 2.
Table 2:
Diagnostic Performance of CT and TEE Compared with Intraoperative Findings
Publication Bias
The asymmetric funnel plot suggested some potential publication bias (Fig E1 [supplement]).
Discussion
Our meta-analysis indicates that CT is superior to TEE in the detection of abscess or pseudoaneurysm, whereas TEE achieves higher performance in the detection of vegetation, leaflet perforation, and paravalvular leakage.
CT demonstrates a higher sensitivity than TEE for detecting abscess and pseudoaneurysm (78% vs 69%, increasing up to 87% when only multiphase CT studies are pooled); hence, a CT with a negative finding performs better than TEE to rule out abscess and pseudoaneurysm. Paravalvular abscess and pseudoaneurysm are frequent (up to 40% of infective endocarditis patients) and associated with an increased risk of death and require an early surgical management (1,21). These complications occur predominantly in the aortic valve area and are usually localized in the mitral-aortic intervalvular region. In this area, the potential complications are easily depicted (anatomic location and extent) using CT compared with TEE (15). As one-third of the abscess and pseudoaneurysm can be missed using TEE, our results suggest that CT should be systematically performed in addition to TEE for detecting abscess and pseudoaneurysm.
For the other infective endocarditis valvular complications, TEE is more sensitive than CT. The specificity is similar between the two imaging modalities (when multiphase studies are included). However, it has been suggested that CT has lower limitations compared with TEE in case of potential artifacts related to prosthetic valves or calcifications (13,15,16).
It should be noted that the sensitivity of CT in the detection of vegetation is highly dependent on the CT acquisition mode, ranging from 50% to 96% when using a retrospective multiphasic analysis (13–18,20) to only 16% with a single-phase analysis (19). This might be explained by the ability to evaluate the mobility of the vegetation and to identify the best phase in a multiphasic protocol for visualizing the pathologic feature.
Unfortunately, in the present meta-analysis, owing to unavailable data, we were not able to perform a subanalysis of the variable size of vegetations. In their study, Gahide et al (14) demonstrate 100% sensitivity and specificity of CT for the detection of vegetation greater than 10 mm, which is usually the cutoff for rapid surgical management, given the high embolic risk (22).
Owing to its dynamic and flow analysis ability, TEE demonstrated better sensitivity than CT for detecting perforation and paravalvular leakage. Small leaflet defects and leakage missed at CT can be diagnosed by using color Doppler imaging during TEE.
Although recent guidelines have underlined the potential role of CT in specific situations, they do not lead to specific and formal recommendations (8,9). Our meta-analysis demonstrates a complementary role of CT and TEE for the detection of infective endocarditis valvular and perivalvular complications in both native and prosthetic valves. In one included study, Hryniewiecki et al (20) performed a combined analysis and obtained a sensitivity of 100% for the combined use of CT and TEE for detecting abscess or pseudoaneurysm and vegetation. Additionally, in a previous meta-analysis limited to prosthetic valve infective endocarditis, adding CT to TEE improved sensitivity for abscess or pseudoaneurysm detection from 86% (TEE) to 100% (TEE plus CT), and vegetation detection from 82% (TEE) to 88% (TEE plus CT) (23). Those results are in accordance with our current review, suggesting that the combined use of CT and TEE could become the preferred imaging strategy in patients with suspected valvular complications of infective endocarditis. Further studies are required to confirm this result.
CT is a safe, noninvasive technique requiring minimal patient cooperation. In addition to the detection of valvular infective endocarditis complications, CT may provide much additional extravalvular information over TEE. For the surgical approach, and in addition to the location and extent of perivalvular infections, CT can precisely assess the course of bypass grafts in patients with prior surgical revascularization, and the thoracic aorta analysis is essential in the perspective of the extracorporeal circulation. Furthermore, CT can also evaluate the coronary anatomy during the same examination and avoid preoperative invasive coronary angiography. In the current international guidelines on the management of valvular heart disease, coronary angiography is recommended prior to surgery in patients with one or more cardiovascular risk factors; with history of cardiovascular disease, suspected of having myocardial ischemia, or left ventricular systolic dysfunction; in men older than 40 years; and in postmenopausal women (24,25). However, in this clinical setting the invasive coronary angiography can lead to serious complications such as cerebral or peripheral embolization, and therefore it should be limited to patients with high pretest likelihood. In patients with low to intermediate probability of coronary artery disease, CT should be preferred over invasive imaging. In our review, only two studies reported results of coronary CT angiography in patients suspected of having infective endocarditis and found a low prevalence of coronary artery disease (13,17).
CT has limitations related to radiation exposure (ranging from 9 to 19 mSv in the three studies reporting this data in our review) and contraindications such as iodinated contrast material allergy and renal insufficiency. However, those limitations have to be balanced with the high mortality and morbidity rate of infective endocarditis. Finally, tachycardia and arrhythmia are limitations for cardiac-gated CT that can be reduced by the use of β-blockers and high temporal resolution CT.
TEE is a more invasive examination, which may be poorly tolerated by the patient but provides no radiation. TEE requires sedation and a trained practitioner and may be limited by the morphology and echogenicity of the patient and the presence of prosthetic material or heavy valve calcification. However, compared with CT, TEE is not limited by renal failure, iodinated contrast material allergy, or abnormal cardiac rhythm.
Our meta-analysis includes more contemporary CT technology and is in agreement with a recent systematic review by Gomes et al (6) who proposed to integrate CT in the standard diagnostic workup of patients in addition to the echocardiographic evaluation, and to perform CT as a substitute for TEE in patients in whom TEE is not feasible.
Our study had limitations. Only studies with patients requiring surgical confirmation of findings consistent with infective endocarditis were included in our meta-analysis. As mentioned in Table 1, some studies have limited sample size, and four had a retrospective design. The time interval between the imaging test and surgery was either not reported (14,15,18) or was sometimes several days (20), limiting reliability of surgical confirmation. The asymmetrical funnel plot (Fig E1 [supplement]) suggested some potential publication bias that could be explained by the fact that smaller studies reporting low diagnostic performance may be less likely to be submitted or accepted for publication. Hence, the overall diagnostic odds ratio could have been slightly overestimated. Only studies published in the English language were included. However, the restrictions in language are unlikely to have excluded any relevant studies, as these are almost invarariably published in English-language Medline-referenced journals.
Finally, we were not able to perform meta-regression analysis based on valve position (mitral vs aortic), on valve type (native vs prosthetic, prosthetic mechanic vs biologic), and on vegetation size because of the small number of data available or because this information could not be extracted from the studies. Future studies should report results on per-valve analysis according to position and type (native vs prosthetic).
In conclusion, our results indicate that CT performs better than TEE in the detection of abscess or pseudoaneurysm, whereas TEE is superior for the detection of vegetation, leaflet perforation, and paravalvular leakage.
When compared with a single phase, multiphase-gated CT acquisition allows dynamic and multiphase analysis and improves the detection of infective endocarditis valvular complications.
Given their complementary value for detecting infective endocarditis valvular abnormalities, the combined use of CT and TEE could become the preferred imaging strategy in patients suspected of having infective endocarditis valvular complications.
However, future large multicenter prospective comparative studies between CT and TEE are required to substantiate and validate the clinical utility of complementary CT and TEE in various clinical scenarios in patients with suspected infective endocarditis before definite recommendations for an optimal imaging strategy for diagnosis and management can be made.
SUPPLEMENTAL FIGURES
Disclosures of Conflicts of Interest: M.O. disclosed no relevant relationships. L.G. disclosed no relevant relationships. Martial Hamon disclosed no relevant relationships. Michèle Hamon disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- TEE
- transesophageal echocardiography
References
- 1.Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation 2010;121(9):1141–1152. [DOI] [PubMed] [Google Scholar]
- 2.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30(4):633–638. [DOI] [PubMed] [Google Scholar]
- 3.Afonso L, Kottam A, Reddy V, Penumetcha A. Echocardiography in Infective Endocarditis: State of the Art. Curr Cardiol Rep 2017;19(12):127. [DOI] [PubMed] [Google Scholar]
- 4.Wong D, Rubinshtein R, Keynan Y. Alternative Cardiac Imaging Modalities to Echocardiography for the Diagnosis of Infective Endocarditis. Am J Cardiol 2016;118(9):1410–1418. [DOI] [PubMed] [Google Scholar]
- 5.Kim IC, Chang S, Hong GR, et al. Comparison of Cardiac Computed Tomography With Transesophageal Echocardiography for Identifying Vegetation and Intracardiac Complications in Patients With Infective Endocarditis in the Era of 3-Dimensional Images. Circ Cardiovasc Imaging 2018;11(3):e006986. [DOI] [PubMed] [Google Scholar]
- 6.Gomes A, Glaudemans AWJM, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017;17(1):e1–e14. [DOI] [PubMed] [Google Scholar]
- 7.Cahill TJ, Baddour LM, Habib G, et al. Challenges in Infective Endocarditis. J Am Coll Cardiol 2017;69(3):325–344. [DOI] [PubMed] [Google Scholar]
- 8.Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36(44):3075–3128. [DOI] [PubMed] [Google Scholar]
- 9.Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015;132(15):1435–1486. [DOI] [PubMed] [Google Scholar]
- 10.McInnes MDF, Moher D, Thombs BD, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018;319(4):388–396. [DOI] [PubMed] [Google Scholar]
- 11.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155(8):529–536. [DOI] [PubMed] [Google Scholar]
- 12.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009;53(5):436–444. [DOI] [PubMed] [Google Scholar]
- 14.Gahide G, Bommart S, Demaria R, et al. Preoperative evaluation in aortic endocarditis: findings on cardiac CT. AJR Am J Roentgenol 2010;194(3):574–578. [DOI] [PubMed] [Google Scholar]
- 15.Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012;22(11):2407–2414. [DOI] [PubMed] [Google Scholar]
- 16.Koo HJ, Yang DH, Kang JW, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018;19(2):199–207. [DOI] [PubMed] [Google Scholar]
- 17.Sims JR, Anavekar NS, Chandrasekaran K, et al. Utility of cardiac computed tomography scanning in the diagnosis and pre-operative evaluation of patients with infective endocarditis. Int J Cardiovasc Imaging 2018;34(7):1155–1163. [DOI] [PubMed] [Google Scholar]
- 18.Ouchi K, Sakuma T, Ojiri H. Cardiac computed tomography as a viable alternative to echocardiography to detect vegetations and perivalvular complications in patients with infective endocarditis. Jpn J Radiol 2018;36(7):421–428. [DOI] [PubMed] [Google Scholar]
- 19.Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018;8(4):439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hryniewiecki T, Zatorska K, Abramczuk E, et al. The usefulness of cardiac CT in the diagnosis of perivalvular complications in patients with infective endocarditis. Eur Radiol 2019;29(8):4368–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagman E, Flinck A, Snygg-Martin U, Olaison L, Bech-Hanssen O, Svensson G. Surgical decision-making in aortic prosthetic valve endocarditis: the influence of electrocardiogram-gated computed tomography. Eur J Cardiothorac Surg 2016;50(6):1165–1171. [DOI] [PubMed] [Google Scholar]
- 22.Jacob S, Tong AT. Role of echocardiography in the diagnosis and management of infective endocarditis. Curr Opin Cardiol 2002;17(5):478–485. [DOI] [PubMed] [Google Scholar]
- 23.Habets J, Tanis W, Reitsma JB, et al. Are novel non-invasive imaging techniques needed in patients with suspected prosthetic heart valve endocarditis? A systematic review and meta-analysis. Eur Radiol 2015;25(7):2125–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falk V, Baumgartner H, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2017;52(4):616–664. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(22):2438–2488 [Published correction appears in J Am Coll Cardiol 2014;63(22):2489.]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.