Abstract
BACKGROUND AND PURPOSE: The organic solvent dimethyl-sulfoxide (DMSO), as a commonly used vehicle for nonadhesive liquid embolics, is not devoid of local angiotoxic effects. We compared microvascular toxicities of superselective infusions of DMSO with potentially more compatible solvents in swine rete mirabile.
METHODS: Fourteen swine underwent angiography for superselective catheterization of 28 arteries of the rete while electrocardiography and intra-arterial pressure were continuously monitored. The investigated solvents were DMSO, dimethyl isosorbide (DMI), ethyl lactate, glycofurol 75, N-methyl pyrrolidone (NMP), and solketal. Control infusion of saline ruled out catheter induced vasospasm in all cases. Each artery of the rete was infused only once with 0.8 mL of one of the solvents over 60 seconds. Acute angiographic and hemodynamic consequences were evaluated. Blood samples were assessed for signs of intravascular hemolysis. Brains and retia were harvested for gross and histopathologic investigation.
RESULTS: On the basis of the angiographic data, DMSO induced the most pronounced vasospasm with the longest recovery period of all solvents investigated. Ethyl lactate, glycofurol 75, and solketal elicited less severe vasospasms and accordingly resolved much more quickly. DMI and NMP induced only minimal vasospasms with comparably short duration. No solvent caused significant hemodynamic alterations or hemolysis. Gross inspection of brains showed no abnormalities, whereas histopathologic examination revealed mostly nonspecific findings. One rete exposed to solketal displayed possible causal histotoxic changes.
CONCLUSION: DMI and NMP produced far less vasospasm than DMSO. No changes in hemodynamic or hemolytic parameters and no histopathologic findings were observed with infusion of these solvents.
Precipitating liquid embolics used for embolization of cerebral aneurysms or arteriovenous malformations (AVMs) are based on solutions of preformed polymers dissolved in organic, water-miscible solvents.1–6 After intravascular injection, the liquid embolic precipitates and forms a nonadhesive solid cast as a result of a rapid exchange of the solvent with surrounding physiologic fluids. A growing clinical experience in the treatment of cerebral aneurysms and AVMs is gained with Onyx (Micro Therapeutics, Irvine, Calif), which is currently the only commercially available nonadhesive liquid embolic agent and uses dimethyl-sulfoxide (DMSO) as the carrier solvent.7–11 Nevertheless, several systemic side effects have been attributed to the use of DMSO for other therapeutic applications.12–21 As for rapid intra-arterial injections, fatal solvent-related adverse effects, such as severe vasospasm, angionecrosis, endothelial denuding, and internal elastic lamina disruption, have been reported; subarachnoid hemorrhage, stroke, and death are frequent clinical sequelae.1,5,22–26 Although the use of low injection rates may avoid structural vascular damages, even slow DMSO injections are still not devoid of mild to moderate vasospasm,27 which may compromise proper deployment of liquid embolic to the target during AVM embolization. Furthermore, the requirement for a low infusion rate may limit its use for high volume vascular structures, for which dose-dependent toxicity levels have not yet been evaluated. Moreover, DMSO dissolves many routinely used microcatheters, so that its application is restricted to the use with especially designed DMSO-resistant catheters.
Therefore, alternative water-miscible solvents for liquid embolics to circumvent these limitations were proposed.28,29 Solvents such as dimethyl isosorbide (DMI), ethyl lactate, glycofurol 75, N-methyl pyrrolidone (NMP) or solketal have been used as pharmaceutical excipients in humans for intravenous (ethyl lactate, solketal), intramuscular (glycofurol 75, NMP), or topical (DMI, NMP) administrations to facilitate the dissolution of poorly water-soluble drugs.28,30,31 The goal of this study was to compare local angiographic (degree of vasospasm), systemic hemodynamic (blood pressure, heart rate, and electrocardiography [ECG]), clinical laboratory (hemolytic parameters), and histopathologic implications of superselective injections of these solvents and DMSO into the swine carotid rete mirabile, which has previously been used to evaluate angiographic effects of DMSO.5,22,27
Materials and Methods
Materials
Solvents tested were DMI (Arlasolve; Uniqema, New Castle, Del), DMSO (KIC Chemicals, Armonk, NY), ethyl lactate (Fluka, Buchs, Switzerland), glycofurol 75 (tetrahydrofurfuryl alcohol polyethylene glycol ether; Hoffman-la-Roche, Basel, Switzerland), NMP (Pharmasolve; ISP, Wayne, NJ), and solketal (d,l-α,β-isopropylidene-glycerol; Fluka). With the exception of solketal, all solvents were of pharmaceutical grade. Before use, solvents were heat-sterilized at 121°C for 20 minutes.
Methods
All animal experiments and handling were carried out in accordance with the national laws for animal protection and were approved by the Review Board for Care of Animals. Fifteen female swine were used for this study, 14 of which received angiography; 1 animal served as a control. The animals were 4–5 months old, weighed 40–55 kg, and were maintained on a standard laboratory diet. After an overnight fast, each swine was premedicated with intramuscular ketamine (20 mg/kg). Endotracheal intubation was performed, and general anesthesia was maintained with ketamine 20 mg/kg/h and fentanyl 0.025 mg/kg/h. Pancuronium 0.1 mg/kg/h was given for muscle relaxation.
Digital subtraction angiography (DSA) was performed with a angiography unit (Integris V; Philips Medical Systems, Best, the Netherlands). Continuous hemodynamic monitoring was measured through a 6F guiding catheter using a standard, precalibrated intra-arterial fluid pressure transducer (Abbott Critical Care Systems, Sligo, Ireland). Blood pressure as well as heart rate and ECG were continuously monitored and intermittently charted (Sirecust 732; Siemens, Erlangen, Germany). 5F and 6F sheaths were placed in the left and right femoral arteries and attached to pressurized saline infusions. Afterward, 5000 IU heparin was given. A 6F guide catheter (Envoy; Cordis, Warren, NJ) was placed in the aortic arch and connected to the fluid pressure transducer; continuous blood pressure monitoring was started. A 4F guide catheter (Vertebral; Terumo Europe, Leuven, Belgium) was used for catheterization of the common carotid artery (CCA). Owing to organic solvent incompatibility with certain commonly used polymers for constructing microcatheters, all superselective catheterizations were performed with a Rebar 14 (Micro Therapeutics), which has previously been determined to be resistant to the organic solvents tested in this study. Superselective catheterization of the ascending pharyngeal artery (ie, artery of the rete) was performed by coaxial placement of the microcatheter/microguidewire combination through the guiding catheter. The tip of the microcatheter was placed distal to the pharyngeal branch of the ascending pharyngeal artery to ensure that infusions were delivered to the rete only (Fig 1). Preinfusion DSA was used to ensure proper positioning of the microcatheter and to study the anatomic configuration and blood flow pattern of the rete mirabile. For all solvents, a total volume of 0.8 mL was infused over 60 seconds. These parameters have been shown to evoke a profound but reversible vasospasm of the ascending pharyngeal artery and rete after superselective infusions of DMSO.27 Serial superselective microcatheterization of each artery of the rete (ascending pharyngeal) was performed in 14 swine using standard techniques. One swine that had had no prior angiographic procedures served as the virgin rete control. Superselective infusion of 0.8 mL of normal saline over 60 seconds was performed before each solvent infusion. Postinfusion DSA was obtained through CCA injection at 3 minutes to rule out changes of the retial circulation. In no case was vasospasm detected after saline infusion, so that superselective microcatheterization of the ascending pharyngeal artery was performed once more, followed by infusion of 0.8 mL of one of the organic solvents over 60 seconds. No solvent was used twice in the same animal. Because the solvents investigated are known to display hemolytic activity and induce morphologic changes of red blood cells,32 blood was drawn from one of the sheaths before and after solvent infusion to evaluate hemolytic parameters (hemoglobin [Hb], free hemoglobin [fHb], and lactate dehydrogenase [LDH]). The hemodynamic (blood pressure, heart rate, and ECG), angiographic, and histopathologic consequences of superselective injections of the solvents were evaluated using the following standardized protocol. Measurements of blood pressure, heart rate, and ECG were obtained immediately before infusion (ie, baseline) and were subsequently repeated immediately, 1 minute, 3 minutes, and 5 minutes after infusion. Postinfusion DSA was obtained, in which changes in the anatomic appearance or hemodynamics of the retial circulation as a result of vasospasm were assessed. If vasospasm was detected, serial follow-up DSA every 5 minutes was subsequently performed until vasospasm resolved or a total of 30 minutes had elapsed. These hemodynamic and angiographic protocols were repeated in the contralateral rete.
Fig 1.
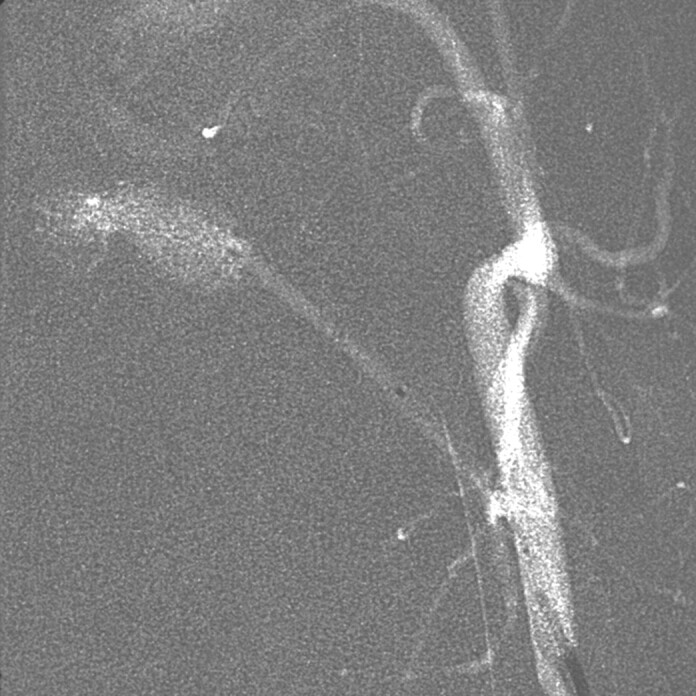
Superselective catheterization of the artery of the rete.
The microcatheter is positioned in the ascending pharyngeal artery (arrow) distally to the muscular branch for infusion of 0.8 mL of solvent over 60 seconds.
To be able to better compare these with previously observed results for the severity of vasospasm, a 5-point grading system was used, as described previously.27 This system was derived from previous subjective angiographic grading systems used for quantifying the severity of blood-flow alterations associated with vasospasm and thromboembolic occlusion of coronary and cerebral arteries,18–21 including the system developed for the Thrombolysis in Myocardial Infarction (TIMI) clinical trials. The following grades were defined specific to involvement of the ascending pharyngeal and retial arteries: grade 0, no vasospasm; grade 1, minimal narrowing (≤25%) of the ascending pharyngeal artery with normal runoff and no involvement of the retial arteries; grade 2, mild narrowing (26%–50%) of the ascending pharyngeal artery with normal runoff and no involvement of the retial arteries; grade 3, moderate narrowing (51%–75%) of the ascending pharyngeal artery with minor or no significant decrease in runoff (TIMI grade 2 or 3) and minimal or no involvement of the retial arteries; grade 4, severe narrowing of the ascending pharyngeal artery (>75%) with diminished runoff (TIMI grade 1) and vasospasm involving the retial arteries; and grade 5, profound vasospasm of the ascending pharyngeal and retial arteries resulting in loss of anterograde flow (TIMI grade 0). After DSA, the swine were sacrificed while under general anesthesia using standard approved procedures, followed by immediate necropsy. Each rete was carefully harvested from the cavernous sinus as described previously.22 The retia were grossly inspected for any evidence of histotoxicity, such as thrombosis or hemorrhage. The specimens were placed in 4% formalin for fixation. Standard techniques were used for preparing sections of the retia for light microscopy and sections were stained with hematoxylin-eosin, Van Gieson’s elastic (EvG), and phosphotungstic acid. The prepared slides were evaluated by an experienced neuropathologist who was blinded to the solvents used. The retial samples were carefully scrutinized for a variety of possible histopathologic changes, including angionecrosis, intimal injuries, fibrin, acute inflammatory infiltrate, disruption of internal elastic lamina, and hemorrhage in arterial walls or perivascular spaces.
Results
Hemodynamics and Laboratory Parameters
No significant changes in blood pressure, heart rate, ECG, or hemolytic parameters were observed that could be attributable to superselective infusion of any of the solvents investigated. There was actually considerable variation in baseline measurements of both blood pressure and heart rate, measuring between 90/60 and 160/110 mm Hg, and between 110 and 160 beats per minute, respectively. Such variations can be attributed to natural variations in individual animals and to the frequent variability and instability in the depth of the anesthesia achieved in swine. Despite these large interindividual differences, the measurements of blood pressure and heart rate in the individual swine before and after the infusion of each solvent were in the range of approximately 5%. Taking variations of baseline hemodynamics and intrinsic errors in their measurements with the devices used in this study into account, it has been proposed that a minimum of 10% change was necessary to reflect a potentially real difference in heart rate or blood pressure that may be attributed to effects of the solvents.27 Using this criterion, we found no significant changes in blood pressure or heart rate for any solvent investigated.
The baseline ECGs showed normal sinus rhythm or occasionally a mild sinus tachycardia. No significant arrhythmias or ectopia were observed at baseline, though several subjects had nonspecific ST and T wave changes, which are commonly seen in swine placed under general anesthesia. After infusion of saline or any of the solvents, there was no evidence of increased ectopia, tachyarrhythmia, bradyarrhythmia, or myocardiac ischemia (eg, Q waves, ST wave elevations, inverted T waves).
No significant increases in Hb, fHb, or LDH, parameters that would indicate intravascular hemolysis, were found in any of the animals infused with the solvents. Mean values for Hb were 9.0 ± 0.9 and 8.9 ± 1.1 g/dL before and after embolization, respectively; mean values for fHb were 17.2 ± 8.7 and 15.9 ± 10.2 mg/dL before and after embolization, respectively; and mean values for LDH were 521.4 ± 104.7 and 501.4 ± 80.7 U/L before and after embolization, respectively (n = 14). These values were of no statistical significance (Wilcoxon test: P = .646 for Hb, P = .929 for fHb, and P = .347 for LDH).
Angiography
No significant technical difficulties were encountered with the superselective catheterizations and infusion of solvents of the ascending pharyngeal artery. Superselective infusion of saline did not result in angiographically documented vasospasm at postinfusion DSA in any of the specimens. In contrast, all of the solvents investigated displayed a variable degree of vasospasm, that was typically transient within the observation interval. In only 1 swine in which DMSO was infused was there residual vasospasm (grade 1) at the end of the 30-minute period of follow-up prescribed by the protocol.
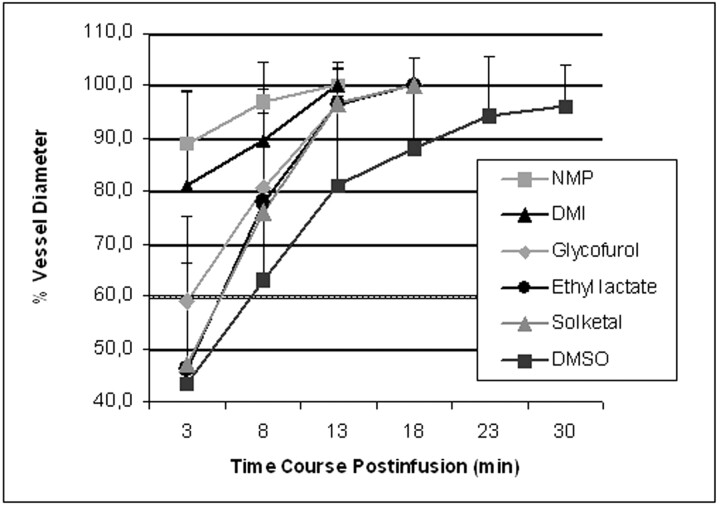
To facilitate the discrimination of different properties of the solvents investigated, the individual vasospasm score, average vasospasm score, and average duration of vasospasm were determined. These results are summarized in Table 1. DMSO induced the most severe average vasospasm after 3 minutes with the longest average duration among all solvents investigated. The average vasospasm score after 3 minutes for DMSO was 2.9, whereas the average DMSO-induced vasospasm resolved not before 17.3 minutes (Fig 2). These data were in good agreement with values reported earlier.27 The vasospasm associated with the infusion of solketal, ethyl lactate, and glycofurol 75 was moderate and was less severe compared with the vasospasm induced by DMSO. The average vasospasm scores after 3 minutes were 2.6 for ethyl lactate and solketal and 2.0 for glycofurol 75. In contrast to DMSO, the vasospasm induced by those solvents resolved much more quickly, with an average duration of vasospasm of 8.0 minutes for solketal and glycofurol 75 and 9.3 minutes for ethyl lactate. The solvents that evoked the weakest and most transient vasospasms were DMI and NMP. The average vasospasm scores 3 minutes after solvent infusion were 1.0 for DMI and 0.8 for NMP. Average duration of vasospasm after infusion of DMI or NMP was 4.8 or 3.7 minutes, respectively (Fig 3). In addition, of all solvents investigated, only infusions of DMI and NMP evoked no vasospasm at all in 2 cases each. Temporal declines of solvent-induced vasospasms are illustrated in Fig 4.
Table 1:
Experimental data
| Swine No. | Side | Solvent Infused (0.8 mL/60 s) | % of Vessel Narrowing/Vasospasm Score After: |
|||||
|---|---|---|---|---|---|---|---|---|
| 3 min | 8 min | 13 min | 18 min | 23 min | 28 min | |||
| 1 | Right | DMSO | 50/2.5 | 51/2.5 | 41/2 | 36/2 | 23/2 | 15/1 |
| Left | Glycofurol 75 | 65/3 | 45/2 | 0/0 | 0/0 | |||
| 2 | Right | DMI | 0/0 | 0/0 | 0/0 | |||
| Left | NMP | 28/2 | 18/1 | 0/0 | ||||
| 3 | Right | Solketal | 50/2.5 | 20/1 | 0/0 | 0/0 | ||
| Left | Ethyl lactate | 50/2.5 | 19/1 | 0/0 | 0/0 | |||
| 4 | Right | DMSO | 82/4 | 35/2 | 0/0 | 0/0 | ||
| Left | DMI | 32/2 | 21/1 | 0/0 | ||||
| 5 | Right | NMP | 0/0 | 0/0 | 0/0 | |||
| Left | Solketal | 41/2 | 0/0 | 0/0 | ||||
| 6 | Right | Glycofurol 75 | 45/2 | 23/1 | 18/1 | 0/0 | ||
| Left | Ethyl lactate | 82/4 | 23/1 | 0/0 | 0/0 | |||
| 7 | Right | DMSO | 36/2 | 21/1 | 17/1 | 0/0 | 0/0 | |
| Left | NMP | 14/1 | 0/0 | 0/0 | ||||
| 8 | Right | Glycofurol 75 | 21/1 | 0/0 | 0/0 | |||
| Left | DMI | 23/1 | 15/1 | 0/0 | ||||
| 9 | Right | Ethyl lactate | 40/2 | 20/1 | 0/0 | 0/0 | ||
| Left | NMP | 0/0 | 0/0 | 0/0 | ||||
| 10 | Right | DMSO | 68/3 | 40/2 | 18/1 | 11/1 | 0/0 | 0/0 |
| Left | Solketal | 53/3 | 50/2.5 | 13/1 | 0/0 | |||
| 11 | Right | DMI | 39/0 | 0/0 | 0/0 | |||
| Left | Ethyl lactate | 42/2 | 26/1.5 | 14/1 | 0/0 | |||
| 12 | Right | Glycofurol 75 | 36/2 | 16/1 | 0/0 | 0/0 | ||
| Left | Solketal | 59/3 | 25/1.5 | 0/0 | 0/0 | |||
| 13 | Right | NMP | 17/1 | 0/0 | 0/0 | |||
| Left | DMI | 0/0 | 0/0 | 0/0 | ||||
| 14 | Right | NMP | 10/1 | 0/0 | 0/0 | |||
| Left | Glycofurol 75 | 38/2 | 18/1 | 0/0 | 0/0 | |||
Note:—DMSO indicates dimethyl sulfoxide; DMI, dimethyl isosorbide; NMP, N-methyl-2-pyrollidone.
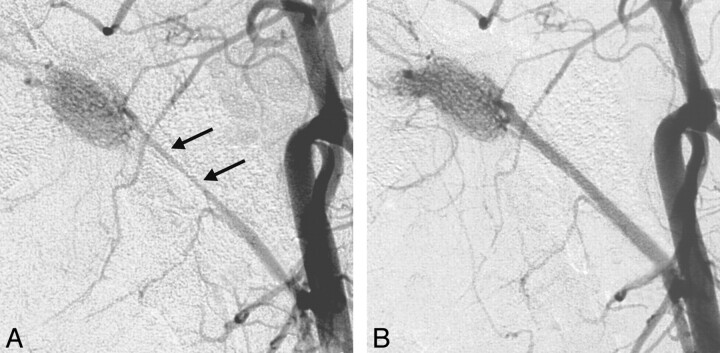
Fig 2.
Vasospasm of the artery of the rete after infusion of DMSO.
A, Postinfusion DSA 3 minutes after infusion of DMSO reveals severe grade 3 vasospasm of the ascending pharyngeal artery (arrows).
B, Repeat DSA shows complete resolution of vasospasm at 23 minutes after DMSO infusion.
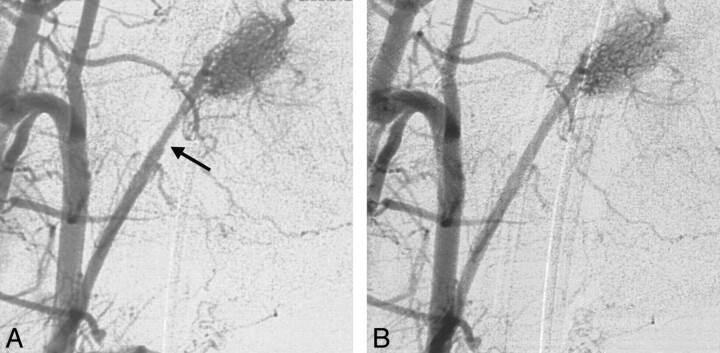
Fig 3.
Vasospasm of the artery of the rete after infusion of DMI.
A, Postinfusion DSA 3 minutes after the injection of DMI demonstrates mild grade 1 vasospasm of the ascending pharyngeal artery (arrow).
B, Repeat DSA 8 minutes after DMI infusion confirms complete resolution of vasospasm.
Fig 4.
Temporal courses of solvent induced changes in diameters of swine artery of the rete (n = 28) expressed as a percentage of baseline values before solvent injections. Among all solvents investigated, DMSO provoked the most severe initial vasospasm that resolved the most slowly, whereas DMI and NMP induced only weak vasospasms that resolved quickly.
Gross and Histopathologic Evaluation
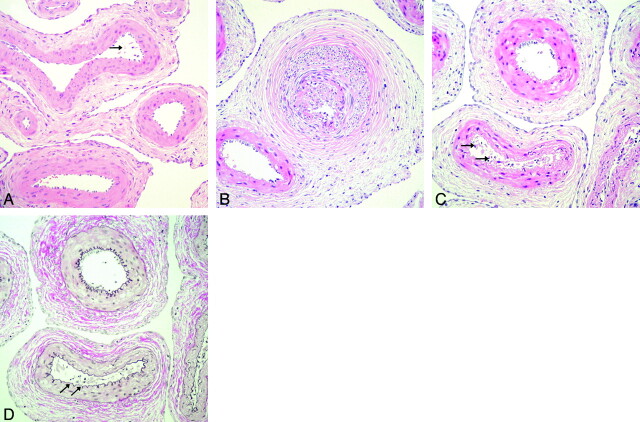
All resected retia appeared grossly normal with no signs of focal thrombosis or angionecrosis. The harvested brains were sectioned and investigated macroscopically for softening, encephalomalacia, or subarachnoid hemorrhage. In none of the specimens investigated was an abnormal appearance of the brain found. Microscopic evaluation of retia revealed normal retial microarteries in most cases, and histopathologic changes were mostly unspecific. The most frequent nonspecific finding was partial endothelial separation in scattered retial arteries (Fig 5A). In addition, 1 side of a rete exposed to glycofurol 75 displayed a small number of retial arteries with arterial wall hyperplasia of all vessel layers (Fig 5B). This finding cannot be attributed to acute angiotoxic effects of solvent infusion. Several microarteries showed more pronounced endothelial denuding than the endothelial separation described previously but only on a single retial side that had been exposed to solketal (Fig 5C). Thus, this histopathologic finding could possibly be related to infusion of this solvent. EvG staining excluded disruption of the internal elastic lamina or an arterial wall injury (Fig 5D). Histopathologic signs of angionecrosis, fibrin deposition, hemorrhage, disruption of internal elastic lamina, or acute cellular inflammation were not observed in any of the specimens investigated.
Fig 5.
Histopathologic changes of retia after solvent infusion.
A, Separation of few endothelial cells in 1 retial artery (arrow) (hematoxylin-eosin; original magnification, 200×). Rete was exposed to 0.8 mL of DMSO per 60 seconds.
B, Right-sided rete of swine 8 exposed to 0.8 mL of glycofurol 75 per 60 seconds shows arterial wall hyperplasia, including all vessel layers (hematoxylin-eosin; original magnification, 200×).
C, Left-sided rete of swine 12 exposed to 0.8 mL of solketal shows endothelial denuding of several microarteries (arrows) (hematoxylin-eosin; original magnification, 200×).
D, Note intact internal elastic lamina within these vessels (arrows) (Van Gieson’s elastic; original magnification, 200×).
Discussion
In this comparative study, we evaluated the potential angiographic, hemodynamic, clinical laboratory, and histopathologic implications of infusions of DMSO and the water-miscible organic solvents DMI, ethyl lactate, glycofurol 75, NMP, and solketal into the swine carotid rete. This experimental AVM model has been used previously for the assessment of the angiotoxic actions of DMSO,5,22,27 though it must be pointed out that vasospasm as a kind of physiologic effect is not necessarily a marker of angiotoxicity. We chose a solvent volume of 0.8 mL to be infused within 60 seconds (dose rate of 13.3 μL/s), because prior investigations have shown that identical injections of DMSO induced, on the average, a moderate degree of vasospasm (range from mild to severe) that was always fully reversible within the observation interval of 30 minutes.27
On the basis of the angiographic findings, the investigated substances can be subdivided into 3 groups: (1) DMSO induced the most severe vasospasm with the longest duration of all solvents investigated; (2) ethyl lactate, glycofurol 75, and solketal all induced a slightly lower initial degree of vasospasm that, in contrast to DMSO, resolved much more quickly; and (3) DMI and NMP elicited only minimal initial vasospasms that resolved very quickly.
Moreover, DMI and NMP also display favorable general toxicity properties for their use in liquid embolics. DMI has a low systemic toxicity, with an LD50 of more than 5.4–6.9 g/kg of body weight.33 It is known as a skin penetration enhancer34,35 and is used for pharmaceutical and cosmetic applications.36 Furthermore, it has been proposed for the treatment of aphthous stomatitis.37 Although few toxicologic data are available about this solvent, DMI is not reported to be carcinogenic by any US agencies38,39 or mutagenic using Ames assays, or clastogenic to human lymphocytes.33 NMP also has a low systemic toxicity in experimental animals, with an LD50 of 2.6–7.0 g/kg body weight,40 thus progressively replacing more toxic and highly volatile solvents in occupational and environmental settings. In addition, NMP has some pharmaceutical uses because of its ability as a potent skin penetration enhancer.41–43 In human volunteer studies, no adverse effects were observed either after an 8-hour inhalation exposure at 10–50 mg/m3 44 or after ingestion of 100 mg of NMP.45 Furthermore, NMP showed no carcinogenic activity in a 2-year study in rats46 or mutagenic activity in the Ames assay in vivo47 in either mice or hamsters.48 Although some trials have shown that NMP might induce aneuploidy in yeast,49,50 NMP is not considered as mutagen or carcinogen. Developmental toxicity studies revealed that NMP can be teratogenic,51 but only at dose levels causing maternal toxicity.52,53 More recently, a drug-delivery system that can be used for both parenteral and site-specific drug delivery based on a polymeric matrix formulation dissolved in NMP was approved by the FDA (Atrigel Implant Drug Delivery Technology, Atrix Laboratories, Fort Collins, Colo).
The acute toxicity levels of the alternative solvents as measured by the intravenous LD50 are reported to lie in the range of 2.7–6.8 g/kg body weight; DMSO has an IV LD50 of 5.4 g/kg in rats.28 Because these doses are typically 2–3 orders of magnitude higher than the injected doses for cerebrovascular embolizations, solvent-related systemic effects such as hemodynamic alterations would not be expected. Nevertheless, because none of the solvents except DMSO has previously been tested intra-arterially, blood pressure, heart rate, and ECG were monitored in each specimen, but no significant changes of these parameters or evidence of increased ectopia or myocardiac ischemia were noted. Similar observations have been made for slow infusions of DMSO into the rete.27
Anemia, hemoglobinuria, and bilirubinuria mimicking a hemolytic transfusion reaction as well as sulfhemoglobinemia have been reported in humans after dermal and intravenous applications of DMSO.13,20 This remarkably high hemolytic activity of DMSO has been investigated on red blood cells in vitro together with the other organic solvents tested in this study. DMSO and ethyl lactate displayed the highest hemolytic activity, glycofurol 75, NMP, and solketal were ranked moderate, and DMI was the solvent with the lowest potential for hemolysis.32 This low hemolytic activity of DMI has been reported previously.54 Despite infusing 2 different solvents (1 for each rete) in each specimen, no significant changes of hemolytic parameters were registered that were attributable to the infusions of any of the solvents investigated. Nonetheless, even if this experimental setup revealed no solvent-related hemolysis, solvents with low or moderate hemolytic activity should be preferred for the use as integral components of liquid embolics.
Microscopic histopathologic analysis confirmed normal microarterial wall assembly or only unspecific changes on both sides of most exposed retia. These changes were similar to those reported previously.22,27 Only on one side of a rete exposed to solketal, several microarteries showed endothelial denuding. Infusion of solketal into this artery of the rete induced an initial grade 3 vasospasm that resolved completely after 18 minutes without angiographic signs of alterations to the exposed retial system. Although this histopathologic finding can be considered as possible histotoxic effect of solvent infusion, no similar histologic findings were observed after infusion of Solketal or any other solvent.
Conclusion
From the data provided by superselective organic solvent injections into swine carotid rete mirabile, DMI and NMP induced the lowest vasospasm of all substances investigated. In addition, no hemodynamic changes, no signs of intravascular hemolysis, and no acute histopathologic findings were found to be associated with infusions of these solvents.
Table 2:
Statistical analysis
| Solvents | % of Vessel Narrowing at 3 min | Average % of Vessel Narrowing at 3 min | Vasospasm Score at 3 min | Average Vasospasm Score at 3 min | Average Duration of Vasospasm (min) |
|---|---|---|---|---|---|
| DMSO | 50, 82, 36, 68 | 59.0 | 2.5, 4, 2, 3 | 2.9 | 19.1 |
| Ethyl lactate | 50, 82, 40, 42 | 53.5 | 2.5, 4, 2, 2 | 2.6 | 11.8 |
| Solketal | 50, 41, 53, 59 | 50.8 | 2.5, 2, 3, 3 | 2.6 | 10.5 |
| Glycofurol | 65, 45, 21, 36, 38 | 41.0 | 3, 2, 1, 2, 2 | 2.0 | 10.5 |
| DMI | 0, 32, 23, 39, 0 | 18.8* | 0, 2, 1, 2, 0 | 1.0 | 5.9* |
| NMP | 28, 0, 14, 0, 17, 10 | 11.5* | 2, 0, 1, 0, 1, 1 | 0.8 | 5.0* |
Note:—DMSO indicates dimethyl sulfoxide; DMI, dimethyl isosorbide; NMP, N-methyl-2-pyrollidone.
P < .05, Mann-Whitney test, comparison with corresponding values obtained for DMSO.
References
- 1.Yang X, Wu Z, Li Y, et al. Re-evaluation of cellulose acetate polymer: angiographic findings and histological studies. Surg Neurol 2001;55:116–22 [DOI] [PubMed] [Google Scholar]
- 2.Mandai S, Kinugasa K, Ohmoto T. Direct thrombosis of aneurysms with cellulose acetate polymer. Part I: Results of thrombosis in experimental aneurysms. J Neurosurg 1992;77:497–500 [DOI] [PubMed] [Google Scholar]
- 3.Taki W, Yonekawa Y, Iwata H, et al. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol 1990;11:163–68 [PMC free article] [PubMed] [Google Scholar]
- 4.Hamada J, Kai Y, Morioka M, et al. A mixture of ethylene vinyl alcohol copolymer and ethanol yielding a nonadhesive liquid embolic agent to treat cerebral arteriovenous malformations: initial clinical experience. J Neurosurg 2002;97:881–88 [DOI] [PubMed] [Google Scholar]
- 5.Murayama Y, Vinuela F, Ulhoa A, et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery 1998;43:1164–75 [DOI] [PubMed] [Google Scholar]
- 6.Murayama Y, Vinuela F, Tateshima S, et al. Endovascular treatment of experimental aneurysms by use of a combination of liquid embolic agents and protective devices. AJNR Am J Neuroradiol 2000;21:1726–35 [PMC free article] [PubMed] [Google Scholar]
- 7.Jahan R, Murayama Y, Gobin YP, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery 2001;48:984–95 [DOI] [PubMed] [Google Scholar]
- 8.Mawad ME, Cekirge S, Ciceri E, et al. Endovascular treatment of giant and large intracranial aneurysms by using a combination of stent placement and liquid polymer injection. J Neurosurg 2002;96:474–82 [DOI] [PubMed] [Google Scholar]
- 9.Molyneux AJ, Cekirge S, Saatci I, et al. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol 2004;25:39–51 [PMC free article] [PubMed] [Google Scholar]
- 10.Nishi S, Taki W, Nakahara I, et al. Embolization of cerebral aneurysms with a liquid embolus, EVAL mixture: report of three cases. Acta Neurochir (Wien) 1996;138:294–300 [DOI] [PubMed] [Google Scholar]
- 11.Saatci I, Cekirge HS, Ciceri EF, et al. CT and MR imaging findings and their implications in the follow-up of patients with intracranial aneurysms treated with endosaccular occlusion with onyx. AJNR Am J Neuroradiol 2003;24:567–78 [PMC free article] [PubMed] [Google Scholar]
- 12.Davis JM, Rowley SD, Braine HG, et al. Clinical toxicity of cryopreserved bone marrow graft infusion. Blood 1990;75:781–86 [PubMed] [Google Scholar]
- 13.Samoszuk M, Reid ME, Toy PT. Intravenous dimethylsulfoxide therapy causes severe hemolysis mimicking a hemolytic transfusion reaction. Transfusion 1983;23:405. [DOI] [PubMed] [Google Scholar]
- 14.Stroncek DF, Fautsch SK, Lasky LC, et al. Adverse reactions in patients transfused with cryopreserved marrow. Transfusion 1991;31:521–26 [DOI] [PubMed] [Google Scholar]
- 15.Smith DM, Weisenburger DD, Bierman P, et al. Acute renal failure associated with autologous bone marrow transplantation. Bone Marrow Transplant 1987;2:195–201 [PubMed] [Google Scholar]
- 16.Hameroff SR, Otto CW, Kanel J, et al. Acute cardiovascular effects of dimethyl sulfoxide. Ann N Y Acad Sci 1983;411:94–99 [DOI] [PubMed] [Google Scholar]
- 17.Rapoport AP, Rowe JM, Packman CH, et al. Cardiac arrest after autologous marrow infusion. Bone Marrow Transplant 1991;7:401–03 [PubMed] [Google Scholar]
- 18.Styler MJ, Topolsky DL, Crilley PA, et al. Transient high grade heart block following autologous bone marrow infusion. Bone Marrow Transplant 1992;10:435–38 [PubMed] [Google Scholar]
- 19.Baum CM, Weissman IL, Tsukamoto AS, et al. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci U S A 1992;89:2804–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess JL, Hamner AP, Robertson WO. Sulfhemoglobinemia after dermal application of DMSO. Vet Hum Toxicol 1998;40:87–89 [PubMed] [Google Scholar]
- 21.Topacoglu H, Karcioglu O, Ozsarac M, et al. Massive intracranial hemorrhage associated with the ingestion of dimethyl sulfoxide. Vet Hum Toxicol 2004;46:138–40 [PubMed] [Google Scholar]
- 22.Chaloupka JC, Vinuela F, Vinters HV, et al. Technical feasibility and histopathologic studies of ethylene vinyl copolymer (EVAL) using a swine endovascular embolization model. AJNR Am J Neuroradiol 1994;15:1107–15 [PMC free article] [PubMed] [Google Scholar]
- 23.Laurent A, Wassef M, Zhang HQ, et al. Solutions gélifiantes pour embolisation: etude animale de la toxicité cardiovasculaire d’un constituant, le diméthylsulfoxyde. J Neuroradiol 1997;24:314 [Google Scholar]
- 24.Sampei K, Hashimoto N, Kazekawa K, et al. Histological changes in brain tissue and vasculature after intracarotid infusion of organic solvents in rats. Neuroradiology 1996;38:291–94 [DOI] [PubMed] [Google Scholar]
- 25.Hamada J, Kai Y, Morioka M, et al. A nonadhesive liquid embolic agent composed of ethylene vinyl alcohol copolymer and ethanol mixture for the treatment of cerebral arteriovenous malformations: experimental study. J Neurosurg 2002;97:889–95 [DOI] [PubMed] [Google Scholar]
- 26.Laurent A, Dufaux J, Honiger J, et al. Injectable gel-giving solutions for embolization: hydrodynamic and animal studies. Proceedings of the Société Française de Neuroradiologie (SFNR) 1997;25:46 [Google Scholar]
- 27.Chaloupka JC, Huddle DC, Alderman J, et al. A reexamination of the angiotoxicity of superselective injection of DMSO in the swine rete embolization model. AJNR Am J Neuroradiol 1999;20:401–10 [PMC free article] [PubMed] [Google Scholar]
- 28.Mottu F, Laurent A, Rufenacht DA, et al. Organic solvents for pharmaceutical parenterals and embolic liquids: a review of toxicity data. PDA J Pharm Sci Technol 2000;54:456–69 [PubMed] [Google Scholar]
- 29.Mottu F, Gailloud P, Massuelle D, et al. In vitro assessment of new embolic liquids prepared from preformed polymers and water-miscible solvents for aneurysm treatment. Biomaterials 2000;21:803–11 [DOI] [PubMed] [Google Scholar]
- 30.Powell MF, Nguyen T, Baloian L. Compendium of excipients for parenteral formulations. PDA J Pharm Sci Technol 1998;52:238–311 [PubMed] [Google Scholar]
- 31.Heintz C, Boymond C. Aspects pharmaco-toxicologiques et utilisation thérapeutique de quelques solvants injectables non aqueux miscibles à l’eau. STP Pharma 1989;5:548–60 [Google Scholar]
- 32.Mottu F, Stelling MJ, Rufenacht DA, et al. Comparative hemolytic activity of undiluted organic water-miscible solvents for intravenous and intra-arterial injection. PDA J Pharm Sci Technol 2001;55:16–23 [PubMed] [Google Scholar]
- 33.National Industrial Chemicals Notification and Assessment Scheme. Canberra, Australia, 2004. Full report on Arlasolve DMI. Available at: http://www.nicnas.gov.au/publications/CAR/new/Std/stdFULLR/std1000FR/std1052FR.pdf. Accessed2004. Oct 17
- 34.Simonsen L, Petersen MB, Groth L. In vivo skin penetration of salicylic compounds in hairless rats. Eur J Pharm Sci 2002;17:95–104 [DOI] [PubMed] [Google Scholar]
- 35.Squillante E, Needham T, Maniar A, et al. Codiffusion of propylene glycol and dimethyl isosorbide in hairless mouse skin. Eur J Pharm Biopharm 1998;46:265–71 [DOI] [PubMed] [Google Scholar]
- 36.Arlasolve (Dimethyl isosorbide). ICI Technical Note,1995
- 37.Hodosh M, Hodosh SH, Hodosh AJ. Treatment of aphthous stomatitis with saturated potassium nitrate/dimethyl isosorbide. Quintessence Int 2004;35:137–41 [PubMed] [Google Scholar]
- 38.Hazardous Substances Data Bank. Bethesda, MD: National Library of Medicine (US), 2004. Dimethyl isosorbide. Available at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB. Accessed2004. Oct 17
- 39.Report on carcinogens, 10th ed. Triangle Park, NC: US Department of Health and Human Services, Public Health Service, National Toxicology Program;2002
- 40.Hazardous Substances Data Bank. Bethesda, MD: National Library of Medicine (US), 2004. 1-Methyl-2-pyrollidone. Available at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB. Accessed2004. Oct 17
- 41.Priborsky J, Muhlbachova E. Evaluation of in-vitro percutaneous absorption across human skin and in animal models. J Pharm Pharmacol 1990;42:468–72 [DOI] [PubMed] [Google Scholar]
- 42.Koizumi A, Fujii M, Kondoh M, et al. Effect of N-methyl-2-pyrrolidone on skin permeation of estradiol. Eur J Pharm Biopharm 2004;57:473–78 [DOI] [PubMed] [Google Scholar]
- 43.Payan JP, Boudry I, Beydon D, et al. Toxicokinetics and metabolism of N-[14C]N-methyl-2-pyrrolidone in male Sprague-Dawley rats: in vivo and in vitro percutaneous absorption. Drug Metab Dispos 2003;31:659–69 [DOI] [PubMed] [Google Scholar]
- 44.Akesson B, Paulsson K. Experimental exposure of male volunteers to N-methyl-2-pyrrolidone (NMP): acute effects and pharmacokinetics of NMP in plasma and urine. Occup Environ Med 1997;54:236–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akesson B, Jonsson BA. Major metabolic pathway for N-methyl-2-pyrrolidone in humans. Drug Metab Dispos 1997;25:267–69 [PubMed] [Google Scholar]
- 46.Pharmasolve (N-methyl-2-pyrrolidone). ISP Technical Note,1990
- 47.Wells DA, Thomas HF, Digenis GA. Mutagenicity and cytotoxicity of N-methyl-2-pyrrolidinone and 4-(methylamino)butanoic acid in the Salmonella/microsome assay. J Appl Toxicol 1988;8:135–39 [DOI] [PubMed] [Google Scholar]
- 48.Engelhardt G, Fleig H. 1-Methyl-2-pyrrolidinone (NMP) does not induce structural and numerical chromosomal aberrations in vivo. Mutat Res 1993;298:149–55 [DOI] [PubMed] [Google Scholar]
- 49.Zimmermann FK, Holzwarth UL, Scheel I, et al. Aprotic polar solvents that affect porcine brain tubulin aggregation in vitro induce aneuploidy in yeast cells growing at low temperatures. Mutat Res 1988;201:431–42 [DOI] [PubMed] [Google Scholar]
- 50.Mayer VW, Goin CJ, Taylor-Mayer RE. Aneuploidy induction in saccharomyces cerevisiae by two solvent compounds, 1-methyl-2-pyrrolidinone and 2-pyrrolidinone. Environ Mol Mutagen 1988;11:31–40 [DOI] [PubMed] [Google Scholar]
- 51.Lee KP, Chromey NC, Culik R, et al. Toxicity of N-methyl-2-pyrrolidone (NMP): teratogenic, subchronic, and two-year inhalation studies. Fundam Appl Toxicol 1987;9:222–35 [DOI] [PubMed] [Google Scholar]
- 52.Saillenfait AM, Gallissot F, Langonne I, et al. Developmental toxicity of N-methyl-2-pyrrolidone administered orally to rats. Food Chem Toxicol 2002;40:1705–12 [DOI] [PubMed] [Google Scholar]
- 53.Saillenfait AM, Gallissot F, Morel G. Developmental toxicity of N-methyl-2-pyrrolidone in rats following inhalation exposure. Food Chem Toxicol 2003;41:583–88 [DOI] [PubMed] [Google Scholar]
- 54.Reed KW, Yalkowsky SH. Lysis of human red blood cells in the presence of various cosolvents. J Parenteral Sci Technol 1985;39:64–68 [PubMed] [Google Scholar]