Abstract
BACKGROUND AND PURPOSE: Cortical hyperdensity was observed in the immediate postembolization CT scans of some patients with intracranial aneurysms following uneventful endovascular treatments. The clinical significance and possible underlying mechanism were evaluated.
MATERIAL AND METHODS: Ninety-three consecutive patients with a total of 100 intracranial aneurysms, treated by endosaccular packing, were studied. Seventy-four aneurysms were treated with balloon assistance, and the remaining aneurysms were treated without balloon assistance. All patients underwent cranial CT just before and immediately after the endovascular treatment. If the post-treatment CT showed any new finding, an immediate MR imaging and a repeat CT 4–6 hours after the initial posttreatment CT were performed. Several parameters were investigated related to the presence of cortical hyperdensity.
RESULTS: Cranial CT showed focal cortical hyperdensity following the treatment of 40/74 aneurysms (54%) with balloon remodeling and 9/26 aneurysms (34.6%) without balloon assistance. None of these patients were symptomatic, and cortical hyperdensity resolved in the repeat CT scans. A statistically significant relationship was observed between the presence of this finding and the total amount of contrast material, microcatheter time, number of balloon inflations, and total balloon inflation time.
CONCLUSION: Immediate postembolization CT may show focal cortical hyperdensity following uneventful endovascular aneurysm treatment, most likely caused by blood-brain barrier disruption resulting in accumulation of contrast medium. The hyperdensity was more frequent when balloon assistance was used but was also seen in the patients with no balloon use. It is important to differentiate this clinically insignificant finding from possible hemorrhage, which would affect patients’ immediate postprocedural medical management.
Endovascular treatment of intracranial aneurysms has improved with recent advances in endovascular techniques. In addition to parent artery occlusion and endosaccular coiling, the use of supporting devices or techniques such as balloon remodeling or stents; as well as an endosaccular liquid polymer, Onyx (Micro Therapeutics, Irvine, Calif); and packing have proved to be safe and important in the treatment of intracranial aneurysms.1–17 However, aneurysmal rupture, parent artery occlusion, coil migration, and so forth are still being reported to be procedure-related complications of endovascular treatment.18,19
Thromboembolic events are one of the most frequently seen and serious complications of endovascular treatments. They can occur during or after the treatment: during cerebral angiography, while introducing catheters and/or placing endosaccular coils. Symptomatic or asymptomatic thromboembolic events have been reported to occur in a range of 1%–78% of cases.3,18,20–22 To prevent potential thromboembolic events, we systemically anticoagulate patients during and after these procedures.
This study reports a finding of focal cortical hyperattenuation observed in the immediate postembolization CT scans of patients with intracranial aneurysms, following uneventful endovascular treatments. It is crucial to differentiate this clinically insignificant finding from possible hemorrhage, which would affect a patient’s immediate postprocedural management, such as anticoagulation and/or antiaggregant treatment.
Methods
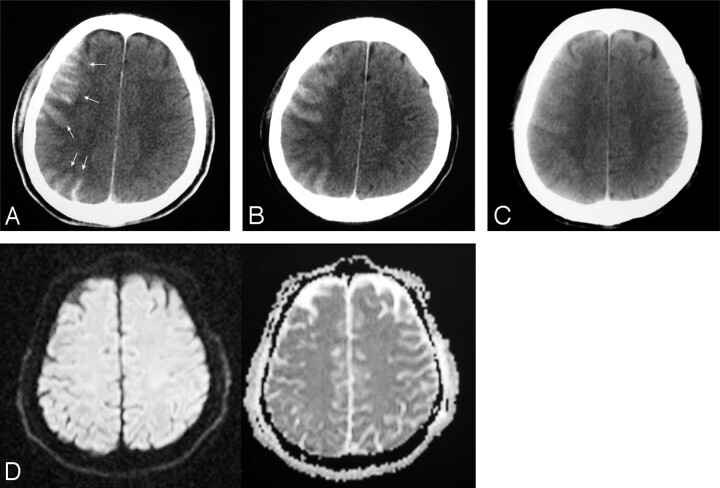
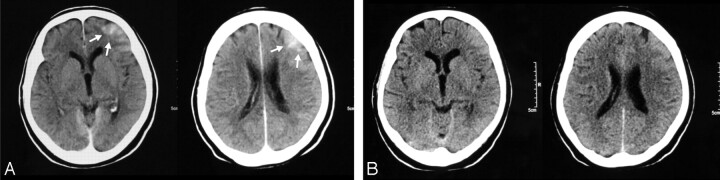
Focal increased attenuation was seen in the cortex immediately after the endovascular aneurysm treatment of some patients (Fig 1). The presence of this finding in relation to several parameters has been evaluated in this study to enlighten the possible etiologic and pathogenetic denominators and their clinical significance.
Fig 1.
Small right MCA aneurysm treated with balloon assistance (patient 51).
A, CT obtained 80 minutes after the treatment reveals increased cortical attenuation (arrows).
B–C, Follow-up CT examinations with a 4-hour interval; each shows partial (B) and total (C) resolution of the cortical increased attenuation.
D, DWI image of the MR image obtained immediately after the first CT (A) shows no corresponding abnormality.
This study included 93 consecutive patients with a total of 100 aneurysms, which were evaluated prospectively. This study was approved by the institutional review board, and informed consent was obtained from the patients. The protocol included brain CT examinations before and just after endovascular treatments. If the post-treatment CT showed any new abnormal finding, an additional MR imaging examination immediately after and a repeat CT examination 4–6 hours after the initial posttreatment CT were performed.
The patients were treated with either simple coiling or balloon-assisted techniques, including coils and/or Onyx. The patients who were treated with parent artery occlusion, with stent only, with stent and coils (without balloon assistance), with any endovascular methods preceding intra- or extracranial bypass surgery or who had intraprocedural apparent aneurysm perforation, thrombus formation, or distal emboli and major catheter-induced vasospasm were excluded from the study. The patients who had acute or subacute infarct in the relevant territory were also excluded. If the initial CT examination or the control CT, in the patients with abnormal findings, could not be obtained for any reason (ie, the patient refused the examination, financial constraints, poor medical condition, or insufficient puncture site control), these patients were not included. These exclusion criteria were initiated to avoid any bias associated with a 1-time very brief balloon inflation, such as in-stent placement with balloon expandable stents, and to eliminate the effects of any apparent adverse event on the contrast enhancement, as well as any factor that might have obscured the statistical analysis.
Each patient’s age, sex, body surface area (BSA), aneurysm size and location, corrected heparin and contrast amount, activated clotting time (ACT) levels, size and duration of the microcatheter used, and blood pressure were recorded.
Sixty-one patients were women and 32 were men. Their ages ranged from 18 to 83 years with a mean of 52.2 years. Eighty-three of the 100 aneurysms were treated with coils. The remaining aneurysms were treated with either stents and coils (n = 3) by using stent balloons for remodeling, by Onyx alone (n = 8), by stents and Onyx (n = 4), or by coil and Onyx (n = 2). Seventy-four aneurysms were treated with balloon assistance, and 26 aneurysms were treated without balloon assistance.
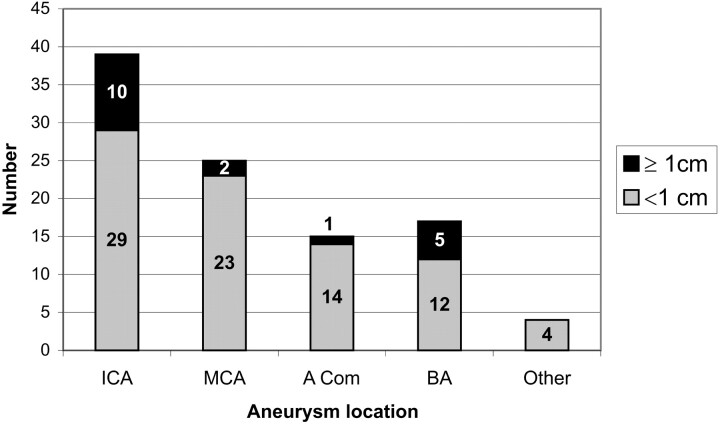
According to the relevant parent artery territory, the aneurysms were separated into 5 groups: internal carotid artery (ICA) (n = 39), middle cerebral artery (MCA) (n = 25), basilar artery (n = 17), anterior communicating artery (AcomA) (n = 15), and others (n = 4; 1 anterior cerebral artery, 2 posterior inferior cerebellar arteries, and 1 vertebral artery). The aneurysms were between 2–30 mm in diameter. Two of the aneurysms were giant (≥2.5 cm), 16 of them were large (1–2.5 cm), and 82 of them were small (≤1 cm). Figure 2 shows the distribution of the aneurysms based on their location and size.
Fig 2.
Graph shows the distribution of aneurysms according to their location and size. ICA indicates internal carotid artery; MCA, middle cerebral artery; Acom, anterior communicating artery; BA, basilar artery.
Thirty-eight patients were treated in the acute stage of subarachnoid hemorrhage (SAH) and 20 of them had angiographically demonstrated vasospasm. The other presentations included headache, diplopia, and other mass-effect symptoms. Some aneurysms were asymptomatic and were discovered incidentally during the evaluation for unrelated symptoms.
The presence of AcomA and posterior communicating arteries (PcomA) was noted for the evaluation of collateral circulation. In the patients who presented with SAH, the presence of vasospasm was also noted for possible cerebral blood flow compromise.
All patients underwent a complete physical and neurologic examination before and immediately after endovascular treatment. The endovascular aneurysm treatments were performed with the patient under general anesthesia. All patients were systemically anticoagulated with intravenously administered doses of heparin to maintain an ACT of 2–2.5 times that of the baseline value. The amount of heparin and contrast material was taken into consideration on the basis of administered doses per kilogram of weight.
Systolic and mean arterial blood pressures were monitored and recorded during the procedures through arterial lines. Mean arterial pressure (MAP) was our main parameter when evaluating hemodynamic effects. Patients were categorized as those having MAP values of <50 mm Hg during the procedure, MAP values of 50–100 mm Hg, and MAP values of >100 mm Hg. Systolic blood pressures were also monitored and changes ≥25% from baseline values were grouped separately because of the possibility of significant effect on hemodynamic status.
The microcatheters used for the catheterization of the aneurysms were separated into 3 groups according to their distal lumen outer diameters: 2.5F, 1.9F, and 1.7F. During the treatment of the aneurysms with the balloon-remodeling technique, HyperGlide (Micro Therapeutics) and HyperForm (Micro Therapeutics) balloons were used. The balloons of the stents were also used for remodeling, when necessary, in the patients in whom an adjunctive balloon-expandable stent was placed before coil/liquid polymer filling.
In the patients who were treated with the balloon remodeling technique, the size of the balloon, total balloon inflation period, the longest duration of balloon inflation at 1 time, and number of balloon inflations were recorded.
All patients underwent preprocedural and immediate postprocedural brain CT scans. MR imaging examinations were performed immediately after the abnormal initial CT examinations in 27 patients. In the remaining patients with abnormal findings on initial examinations, control MR images could not be obtained because of limitations in their general condition and financial constraints. CT examinations were performed in 2 different scanners (Tomoscan AVE1, Philips Medical Systems, Rotterdam, the Netherlands; and Somatom Volume Zoom, Siemens, Erlangen, Germany). MR imaging examinations were performed with a 3T unit (Allegra, Siemens). A neuroradiologist (I.S) and a radiologist (A.O.), together, evaluated these examinations.
The pretreatment CT examinations were evaluated for hydrocephalus, SAH, ischemia, or infarcts relevant to the territory of the parent artery and any other findings such as atrophy, cysts, and so forth. On postporcedural CT scans, the presence of cortical hyperattenuation was noted. This finding was categorized according to the territory of the parent artery harboring the aneurysm (partial or total territorial involvement) and resolution in the repeat CT was noted.
MR imaging examinations included transverse fluid-attenuated inversion recovery (FLAIR) and gradient-echo T2-weighted sequences, as well as diffusion-weighted MR imaging (DWI) (single-shot spin-echo echo-planar sequence with TR/TE, 3400/94 ms; b of 0, 500, and 1000 seconds/mm2 in 3 axes; matrix, 240 × 256; field of view, 230 mm) and apparent diffusion coefficient (ADC) maps.
Statistical analyses were performed by using a Student t test, chi-square test, logistic regression analysis, Pearson correlation tests, and the classification and regression tree (C&RT) method, as necessary (P < .05).
Results
The Table shows patients’ data, including demographics, presence of SAH with or without vasospasm, collateral circulation, and procedural details in regard to the presence of the cortical hyperattenuation findings.
Patient data including patient demographics, presence of subarachnoid hemorrhage (SAH) with or without vasospasm, collateral circulation, and procedural details in regard to presence of the cortical hyperdensity finding
| Patient No./Sex/Age (y) | Aneurysm Location | Aneurysm Size | SAH | Vasospasm | Balloon Remodeling | Contrast Material (cc/kg) | Heparin (u/kg) | Total Microballoon Inflation Time (s) | No. of Balloon Inflations | Maximum Microballoon Inflation Time (s) | Size of Micro-catheter (F) | Micro-catheter Time (min) | Elapsed Time Until CT Performed (min) | Collateral |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcomA | PcomA | ||||||||||||||
| 1/M/51 | RICA | Small | + | − | + | 2.6 | 158 | 440 | 3 | 158 | 2.5 | 45 | 80 | + | + |
| RICA | Small | + | − | + | 2.6 | 158 | 360 | 4 | 135 | 2.5 | 50 | 85 | + | + | |
| 2/F/50 | RMCA | Small | + | − | + | 4.4 | 143 | 900 | 8 | 87 | 1.7 | 53 | 97 | + | + |
| 3/F/83 | LICA | Small | + | + | + | 6.7 | 183 | 300 | 5 | 90 | 2.5 | 60 | 60 | + | + |
| 4/F/44 | RICA | Small | − | − | + | 3.9 | 104 | 588 | 8 | 187 | 1.9 | 90 | 30 | + | + |
| 5/F/40 | RMCA | Small | − | − | + | 3.9 | 93,7 | 521 | 9 | 89 | 2.5 | 62 | 59 | + | + |
| 6/F/30 | RMCA | Small | + | + | + | 4.6 | 170 | 292 | 5 | 90 | 1.7 | 105 | 40 | + | + |
| 7/F/59 | RMCA | Small | + | + | − | 5.2 | 250 | 1.7 | 55 | 80 | + | + | |||
| 8/F/50 | RICA | Large | − | − | + | 4.4 | 160 | 606 | 14 | 95 | 2.5 | 120 | 45 | + | − |
| 9/F/74 | RMCA | Small | + | + | − | 2.0 | 80,6 | 2.5 | 30 | 68 | − | + | |||
| 10/F/38 | Basilar tip | Large | + | + | − | 4.6 | 140 | 2.5 | 75 | 70 | − | − | |||
| 11/M/46 | LICA | Small | − | − | + | 3.5 | 156 | 476 | 6 | 110 | 2.5 | 55 | 66 | + | + |
| 12/M/48 | LICA | Small | − | − | + | 2.9 | 125 | 820 | 8 | 150 | 2.5 | 70 | 72 | + | + |
| 13/F/34 | RMCA | Small | + | − | + | 4.2 | 154 | 260 | 3 | 115 | 2.5 | 40 | 46 | + | + |
| 14/M/42 | RMCA | Small | − | − | + | 3.6 | 118 | 597 | 7 | 120 | 2.5 | 48 | 81 | + | + |
| 15/F/63 | AcomA | Small | − | − | − | 3.3 | 139 | 2.5 | 50 | 80 | + | − | |||
| 16/F/60 | AcomA | Small | + | + | − | 2.9 | 167 | 2.5 | 60 | 60 | + | + | |||
| 17/M/62 | RMCA | Small | + | − | − | 3.3 | 139 | 1.7 | 65 | 80 | + | + | |||
| 18/F/65 | RMCA | Small | + | + | − | 3.1 | 125 | 1.7 | 20 | 50 | − | + | |||
| 19/M/27 | RICA | Small | − | − | + | 1.4 | 139 | 825 | 5 | 295 | 1.9 | 50 | 45 | + | + |
| 20/F/52 | Basilar fenest | Small | − | − | + | 2.8 | 139 | 187 | 3 | 95 | 1.7 | 45 | 70 | + | − |
| 21/F/60 | LMCA | Small | + | − | − | 4 | 200 | 2.5 | 28 | 38 | − | − | |||
| 22/M/54 | Basilar tip | Small | − | − | + | 1.7 | 107 | 150 | 2 | 90 | 1.7 | 15 | 70 | − | − |
| 23/F/48 | Basilar tip | Small | + | + | − | 1.5 | 149 | 2.5 | 40 | 75 | − | + | |||
| 24/F/67 | LICA | Giant | − | − | + | 4.5 | 224 | 1552 | 17 | 141 | 2.5 | 120 | 64 | + | − |
| 25/F/63 | RPcomA | Small | − | − | − | 2.4 | 105 | 1.7 | 45 | 40 | − | + | |||
| 26/M/52 | Basilar tip | Small | − | − | + | 3.3 | 208 | 802 | 10 | 120 | 1.7 | 60 | 90 | − | − |
| 27/M/52 | Basilar tip | Small | + | + | + | 1.2 | 149 | 440 | 10 | 50 | 1.7 | 70 | 45 | + | + |
| 28/F/50 | LICA | Giant | − | − | + | 3.8 | 125 | 1488 | 7 | 323 | 1.9 | 60 | 40 | + | + |
| 29/M/60 | AcomA | Small | + | + | − | 5.3 | 200 | 1.9 | 180 | 90 | + | + | |||
| 30/F/53 | LICA | Small | − | − | + | 4.6 | 192 | 1287 | 15 | 210 | 1.7 | 115 | 45 | + | + |
| 31/F/46 | RMCA | Small | − | − | − | 2.4 | 165 | 1.7 | 64 | 50 | − | + | |||
| 32/F/65 | RICA | Large | + | + | + | 4.6 | 115 | 999 | 13 | 174 | 1.7 | 150 | 56 | + | + |
| 33/F/67 | RICA | Small | − | − | + | 2.8 | 117 | 795 | 7 | 245 | 1.7 | 90 | 89 | − | − |
| 34/M/70 | RPcomA | Small | + | − | + | 4.1 | 123 | 1322 | 15 | 139 | 1.7 | 120 | 70 | − | + |
| 35/M/48 | RMCA | Small | − | − | + | 4.7 | 88,2 | 318 | 3 | 169 | 3 | 70 | 50 | − | + |
| 36/F/70 | AcomA | Small | + | + | − | 2.3 | 100 | 1.7 | 35 | 120 | + | + | |||
| 37/M/52 | LICA | Large | − | − | + | 3.3 | 175 | 2282 | 6 | 779 | 1.9 | 90 | 67 | + | + |
| 38/M/32 | RICA | Small | − | − | + | 4.3 | 143 | 1235 | 5 | 417 | 1.9 | 56 | 135 | + | + |
| 39/F/49 | AcomA | Small | − | − | + | 5 | 167 | 592 | 9 | 95 | 1.7 | 89 | 90 | − | + |
| 40/F/59 | AcomA | Small | − | − | + | 2 | 125 | 300 | 4 | 106 | 1.7 | 60 | 58 | + | + |
| 41/F/57 | RICA | Large | − | − | + | 3.8 | 93.7 | 711 | 6 | 172 | 1.7 | 43 | 90 | + | − |
| 42/F/65 | LICA | Small | − | − | + | 4 | 152 | 1350 | 7 | 635 | 1.9 | 150 | 40 | + | + |
| 43/F/52 | LICA | Small | − | − | + | 2 | 100 | 1705 | 10 | 301 | 1.9 | 80 | 70 | + | + |
| 44/F/60 | LICA | Small | − | − | + | 3.4 | 269 | 612 | 7 | 160 | 1.9 | 68 | 75 | + | + |
| 45/F/50 | LMCA | Large | − | − | + | 4 | 183 | 301 | 8 | 69 | 3 | 40 | 45 | − | − |
| 46/F/41 | RICA | Small | − | − | + | 5 | 258 | 641 | 3 | 323 | 1.9 | 85 | 110 | + | + |
| 47/F/68 | RICA | Small | − | − | + | 3 | 156 | 529 | 2 | 470 | 3 | 20 | 110 | + | − |
| 48/F/70 | AcomA | Small | − | − | + | 4 | 24 | 504 | 6 | 131 | 1.7 | 90 | 70 | + | + |
| 49/F/46 | LPICA | Small | + | − | − | 2.5 | 154 | 1.9 | 40 | 47 | − | − | |||
| 50/M/57 | LMCA | Small | − | − | + | 2 | 159 | 680 | 7 | 170 | 1.7 | 45 | 85 | − | + |
| 51/M/34 | RMCA | Small | − | − | + | 5.4 | 239 | 881 | 10 | 125 | 1.9 | 60 | 80 | − | + |
| 52/M/68 | Basilar tip | Small | − | − | + | 3.5 | 165 | 3 | 15 | 180 | + | + | |||
| AcomA | Small | − | − | + | 3.5 | 165 | 323 | 6 | 77 | 1.7 | 60 | 125 | + | + | |
| 53/M/49 | RACA | Small | + | − | − | 3.8 | 93.8 | 3 | 65 | 200 | + | + | |||
| 54/M/44 | LPcomA | Small | − | − | + | 6.1 | 296 | 1643 | 19 | 199 | 1.7 | 225 | 45 | + | + |
| 55/M/18 | AcomA | Small | + | − | + | 3.6 | 242 | 498 | 4 | 121 | 1.7 | 45 | 68 | + | + |
| 56/F/40 | LPICA | Small | + | − | − | 3.8 | 125 | 1.7 | 45 | 138 | + | + | |||
| 57/F/41 | RMCA | Small | + | + | − | 3.3 | 250 | 1.7 | 105 | 70 | + | + | |||
| 58/F/52 | L Sup Cer | Small | + | + | − | 3.8 | 119 | 1.7 | 70 | 90 | − | − | |||
| RMCA | Small | + | − | + | 3.8 | 119 | 355 | 6 | 117 | 3 | 40 | 90 | − | − | |
| 59/F/52 | Basilar tip | Small | − | − | + | 3.6 | 250 | 566 | 12 | 69 | 1.7 | 45 | 60 | + | + |
| 60/F/45 | RMCA | Small | − | − | + | 5.9 | 192 | 302 | 5 | 85 | 1.7 | 30 | 53 | + | + |
| 61/F/55 | RPCA | Large | + | − | − | 6.4 | 179 | 1.7 | 30 | 70 | − | + | |||
| 62/M/23 | Midbasilar | Large | − | − | + | 3.9 | 212 | 820 | 8 | 176 | 1.7 | 45 | 180 | − | + |
| 63/M/33 | RMCA | Small | + | − | + | 4.2 | 183 | 115 | 2 | 69 | 1.7 | 45 | 90 | + | + |
| 64/F/54 | LICA | Small | − | − | + | 4.8 | 218 | 1652 | 7 | 300 | 1.9 | 70 | 148 | + | + |
| 65/M/51 | RPCA | Small | − | − | − | 1.4 | 159 | 1.7 | 35 | 65 | − | + | |||
| 66/F/43 | LMCA | Small | + | + | + | 6.9 | 254 | 276 | 3 | 150 | 1.7 | 40 | 120 | + | + |
| RMCA | Small | + | + | + | 6.9 | 254 | 229 | 3 | 96 | 1.7 | 15 | 120 | + | + | |
| 67/F/61 | LICA | Small | − | − | − | 6 | 500 | 1.7 | 100 | 160 | − | + | |||
| 68/M/59 | RICA | Small | + | − | + | 7.9 | 283 | 471 | 6 | 169 | 3 | 195 | 49 | − | + |
| 69/M/25 | RPCA | Large | − | − | − | 5 | 228 | 3 | 150 | 63 | − | + | |||
| 70/F/43 | RICA | Small | − | − | + | 5 | 179 | 769 | 8 | 164 | 1.7 | 60 | 110 | + | − |
| 71/M/52 | Basilar tip | Large | − | − | + | 5.1 | 179 | 723 | 8 | 150 | 3 | 130 | 90 | − | − |
| 72/F/39 | RICA | Small | − | − | + | 6.7 | 117 | 547 | 7 | 114 | 3 | 45 | 37 | + | + |
| 73/M/32 | AcomA | Small | − | − | + | 3.8 | 153 | 870 | 11 | 130 | 1.7 | 90 | 63 | + | + |
| 74/F/51 | LICA | Small | − | − | + | 3.8 | 244 | 1912 | 6 | 530 | 1.9 | 60 | 90 | + | + |
| 75/F/65 | R Sup Cer | Small | + | + | − | 6.2 | 149 | − | − | − | 1.7 | 45 | 110 | − | + |
| 76/F/52 | RICA | Small | + | − | + | 5 | 188 | 587 | 6 | 110 | 1.7 | 47 | 63 | + | + |
| 77/F/42 | RMCA | Small | + | − | + | 4.1 | 164 | 366 | 8 | 105 | 1.7 | 60 | 100 | + | − |
| 78/F/49 | RICA | Large | − | − | + | 4 | 327 | 303 | 8 | 49 | 3 | 50 | 180 | + | − |
| 79/F/32 | LICA | Small | − | − | + | 5.4 | 278 | 1189 | 5 | 434 | 1.9 | 76 | 50 | + | + |
| 80/F/59 | RICA | Giant | + | + | + | 6.3 | 188 | 1298 | 10 | 181 | 3 | 195 | 84 | + | + |
| 82/F/63 | Basilar tip | Small | - | - | + | 4.7 | 133 | 557 | 8 | 183 | 1.7 | 35 | 140 | - | + |
| RMCA | Small | − | − | + | 4.7 | 133 | 259 | 6 | 55 | 1.7 | 35 | 70 | − | − | |
| 83/F/65 | RVA | Small | + | + | − | 2.8 | 139 | 1.7 | 40 | 130 | − | − | |||
| 84/F/50 | LSupCer | Small | − | − | + | 3.5 | 104 | 214 | 3 | 95 | 1.7 | 20 | 225 | + | + |
| LICA | Small | − | − | + | 3.5 | 104 | 673 | 4 | 490 | 1.9 | 40 | 164 | + | + | |
| 85/M/57 | LMCA | Small | − | − | + | 4 | 91,5 | 472 | 7 | 135 | 1.7 | 35 | 49 | + | + |
| 86/M/50 | ACom | Small | − | − | + | 3.6 | 137 | 691 | 10 | 95 | 1.7 | 60 | 81 | + | − |
| 87/M/60 | AcomA | Small | + | − | + | 7.4 | 219 | 975 | 16 | 133 | 1.7 | 145 | 130 | + | + |
| 88/F/81 | AcomA | Large | + | + | − | 3 | 185 | 1.7 | 30 | 82 | − | + | |||
| 89/M/63 | AcomA | Small | − | − | + | 3 | 122 | 686 | 8 | 148 | 1.7 | 60 | 60 | + | + |
| 90/M/66 | LICA | Large | − | − | + | 4.3 | 185 | 486 | 10 | 80 | 1.7 | 40 | 78 | + | + |
| RICA | Small | − | − | + | 4.3 | 185 | 516 | 5 | 165 | 1.7 | 15 | 60 | + | − | |
| 91/F/70 | LICA | Small | + | − | + | 6 | 182 | 2047 | 10 | 420 | 1.9 | 50 | 42 | + | + |
| 92/M/32 | AcomA | Small | + | − | + | 2.6 | 165 | 346 | 7 | 103 | 1.7 | 50 | 85 | + | − |
| 93/F/55 | LMCA | Large | − | − | + | 3.1 | 147 | 801 | 11 | 105 | 3 | 65 | 75 | − | + |
Note:—L indicates left; R, right; ICA, internal carotid artery; MCA, middle cerebral artery; AcomA, anterior communicating artery; PcomA, posterior communicating artery; PICA, posterior inferior cerebellar artery; ACA, anterior cerebral artery; PCA, posterior cerebral artery; VA, vertebral artery; Sup Cer, superior cerebellar artery; Basilar Fenest, basilary fenestration; SAH, subarachnoid hemorrhage. *All patients in whom Onyx was used in the treatment (Onyx, Onyx + coil, Onyx + stent).
On the postprocedural CT images, focal cortical hyperattenuation was noted following the endovascular treatment of 49 of 100 (49%) aneurysms (Figs 1 and 3–6). This finding was seen in CT examinations of 40 of 74 (54%) aneurysms that were treated with balloon assistance (Figs 1, 3, 5, and 6) and 9 of 26 (34.6%) aneurysms that were treated without balloon assistance (Fig 4). Increased cortical attenuation was confined to the territory of the parent artery harboring the aneurysm, partially involving the territory in 44 cases and entirely, in 5. MR imaging examinations did not reveal any relevant lesions in any of the patients (Figs 1 and 5), and the cortical hyperattenuation resolved totally or partially in the CT scan that was repeated 4–6 hours later (Figs 1 and 3–6). All patients were asymptomatic.
Fig 3.
Posttreatment angiogram (A) shows the completely occluded small aneurysm (arrows) at the vertebrobasilar junction of the fenestrated basilar artery (patient 20). Posttreatment CT image (B) reveals increased attenuation in the posterior cerebral artery territory bilaterally (arrow). Control CT scan (C) obtained 5 hours after the first one shows complete resolution of the finding.
Fig 6.
Early (A) and late (B) postembolization CT scans of the patient who had a large ICA aneurysm (patient 8). CT scan obtained 45 minutes after the procedure (A) shows very prominent hyperattenuation in the ipsilateral cortex, resolving totally in the control CT scan obtained after 6 hours (B).
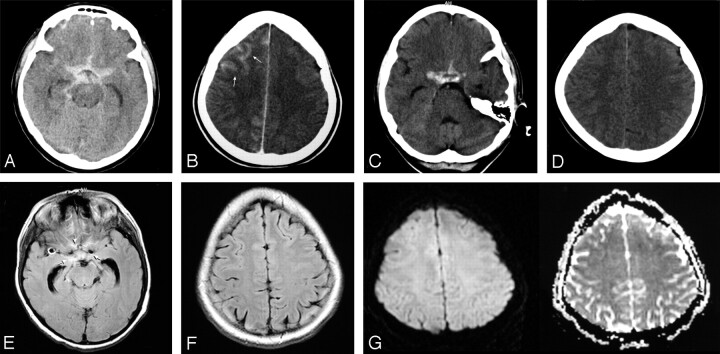
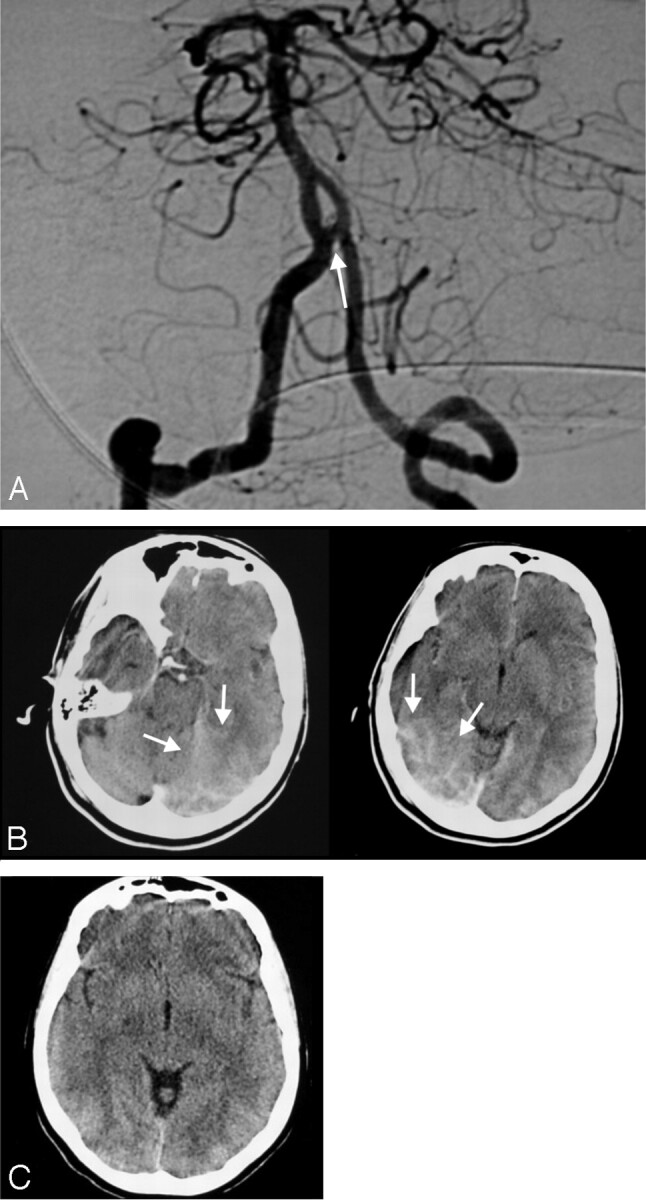
Fig 5.
A–B, CT examinations performed 53 minutes after the endovascular treatment of the right MCA aneurysm by using a remodeling technique. Patient 60 shows blood within the suprasellar cistern (A) in addition to increased cortical attenuation (arrows) in the right frontal region (B). C–D, Follow-up CT scans obtained after 6 hours demonstrated the persistence of blood appearance within the suprasellar cistern (C) and complete resolution of the cortical hyperattenuation (D). E–G, FLAIR (E and F) and DWI (G) of MR images obtained after the first CT examination confirm the presence of blood (arrows) within the cisterns (E) with no MR imaging abnormality in the region of cortical hyperattenuation (F and G).
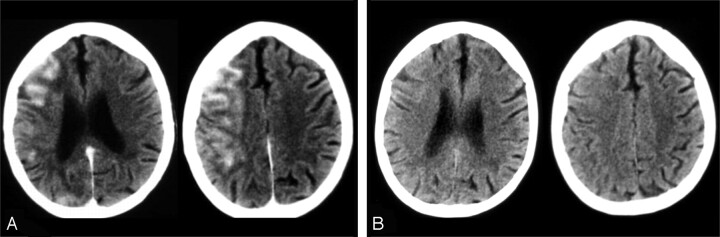
Fig 4.
Small MCA aneurysm treated with no balloon assistance (patient 21). A, CT images obtained 38 minutes after the treatment reveal increased cortical attenuation in the left frontal region (arrows). Control CT examination (B) shows resolution of the finding.
In 1 particular patient (1%), cortical increased attenuation coexisted with hemorrhage within the suprasellar cistern after an uneventful procedure, despite the normal findings on the patient’s neurologic examination (Fig 5). MR imaging confirmed the presence of the hemorrhage in the suprasellar cistern (Fig 5E) but did not show any corresponding abnormality in the region of cortical hyperattenuation (Fig 5F, -G). In the control CT, the cortical hyperattenuation showed resolution, but the appearance of cisternal SAH persisted (Fig 5C, -D).
During the procedure, nonionic water-soluble iodinated contrast material (300 mg/dL) ranging between 1.2 and 7.9 mL/kg was used (mean, 4 mL/kg). ACT levels were maintained at levels 2–2.5 times those (mean, 2.17) of the basal measurement, both during and after the procedures. The amount of heparin used during the procedure was between 80.7 and 500 U/kg (mean, 170.5 U/kg). The patients’ BSAs were calculated with Mostello’s formula and ranged between 1.47 and 2.41 m2 (mean, 1.86 m2). There was no statistically significant relationship between blood pressure changes and the presence of focal cortical hyperattenuation findings (P > .05).
There was no statistically significant relationship between the presence of cortical hyperattenuation and age, sex, presence of SAH and vasospasm, aneurysm location (Fig 3) and size, and microcatheter size. However, the occurrence of cortical hyperattenuation in the patients in whom the microcatheters with largest outer diameter (2.5F) were used was slightly more common than in the patients in whom other catheters (1.9F and 1.7F) were used (57.1% versus 45%).
The effect of AcomA presence on internal carotid and AcomA aneurysm groups and of PcomA presence on basilar artery aneurysm groups was investigated; however, the presence or absence of communicating arteries had no significant effect on the occurrence of cortical hyperattenuation. The frequency of cortical hyperattenuation was higher in the patients in whom balloon remodeling was used (54% with balloon remodeling versus 34.6% without balloon assistance). We found a statistically significant relationship between the parameters listed here and the presence of cortical hyperattenuation:
BSA.
The occurrence of cortical hyperattenuation increased when BSA decreased (Student t test, P < .01).
Total Amount of Contrast Material per Kilogram of Body Weight.
(cutoff value, 4.37 mL/kg; C&RT method). The amount of contrast per kilogram of body weight did not significantly differ among the groups of patients treated with and without balloon remodeling (Student t test, P > .05). It did not show a statistically significant relationship with the microcatheter time in each group (Pearson correlation test, P > .05). The amount of contrast did not show a statistically significant difference in relation to the number of balloon inflations (Pearson correlation test, P > .05).
Microcatheter Time.
The average microcatheter time was 68.9 minutes in the group of patients treated with balloon remodeling and 58.3 minutes in the group with no balloon use. However, this difference between the 2 was not statistically significant (Student t test). The occurrence of cortical hyperattenuation increased when microcatheter time increased (P < .001). If microcatheter time was longer than 110 minutes, the likelihood of cortical hyperattenuation occurrence increased (C&RT method).
Total Balloon Inflation Time and Number of Balloon Inflations (When Balloon Remodeling Was Used).
The occurrence of cortical hyperattenuation increased with the increase in the number of balloon inflations and total balloon inflation time. This finding was present in 45.2% of the patients in whom the balloon was inflated less than 7 times, whereas in the group in which the balloon was inflated 7 or more times and total balloon inflation time was less than 353 seconds, cortical hyperattenuation occurred at a rate of 65%. However, no statistically significant relationship was found between the balloon inflation duration each time the balloon was inflated and the occurrence of cortical hyperattenuation.
Elapsed Time Until CT Was Performed.
The shorter the time that elapsed between the endovascular procedure and the postprocedure CT examination, the more likely was the occurrence of cortical hyperattenuation (Fig 6).
Discussion
Endovascular treatment of intracranial aneurysms has been found to be associated with significantly lower morbidity and mortality rates compared with those of surgery and has gained wide acceptance in recent years.18,23–26 However, endovascular treatment of intracranial aneurysms is still associated with potential risks, not only due to the disease itself, when ruptured, but also related to the treatment technique.18,19,26,27
Thromboembolic events associated with endovascular treatment, despite their being asymptomatic in most cases, are one of the most serious and frequently seen complications during and after the treatment.20–22,28–31 Anticoagulant/antiaggregant therapy may be recommended to prevent these complications. Yet, anticoagulant/antiaggregant therapy has the potential risk of intracerebral and systemic bleeding. Moreover, one of the contraindications for anticoagulant/antiaggregant therapy is procedure-related intracerebral hemorrhage due to aneurysm rupture or perforation. Rates of intraprocedural aneurysmal rupture are 1.9%–16% for ruptured aneurysms and 0%–1.3% for unruptured aneurysms.24,27,32–34 Minor hemorrhages may also occur during the treatment with no apparent clinical or angiographic evidence, and it is essential to rule out any hemorrhagic event, whether clinically apparent or silent, to administer the necessary medication affecting the coagulation processes.
Few studies have been published regarding systematic imaging of patients following endovascular aneurysm treatment in which symptomatic or asymptomatic findings were reported.20,22,29,35 In this prospective study, increased cortical attenuation (Figs 1 and 3–6) confined to the territory of the parent artery harboring the aneurysm was found in the immediate postembolization CT scans of patients with intracranial aneurysms after uneventful endovascular therapy. This attenuation was observed in 49% of the patients in this series. Subsequent MR images did not reveal any corresponding abnormality, and cortical hyperattenuation resolved partially or totally in the control CT scans, obtained 4–6 hours after the postprocedure CT scans. All of those patients were clinically asymptomatic, but this finding still bears clinical significance due to its differential diagnosis from“silent” subarachnoid/intraparenchymal hemorrhage because it will influence the medical management of the patient.
To explain the possible underlying mechanism and related pathophysiologic factors of the observed increased cortical hyperattenuation, we investigated several parameters. Statistically significant relationships were observed in the entire group between the presence of the cortical hyperattenuation and increased contrast material used per kilogram of body weight, increased microcatheter time, decreased BSA, and decreased time elapsed until the CT scan was obtained. In the group of patients who were treated with balloon assistance, an increase in both the number of balloon inflations and total balloon inflation time increased the occurrence of cortical hyperattenuation.
The blood-brain barrier (BBB) is impermeable to radiographic contrast material under normal conditions. Transfer of contrast material increases if BBB is disrupted or if contrast material is overdosed or applied intra-arterially.36–40
Incomplete continuous cerebral ischemia causes changes in BBB permeability.41 Following a temporary period of focal cerebral ischemia, a transient opening of the BBB occurs. This opening recovers with time and is associated with hyperemic cerebral blood flow. There are 2 phases of BBB opening during reperfusion. The first phase occurs in the immediate period of reperfusion and is due to a sudden increase in intraluminal hydrostatic pressure, which results from the restoration of cerebral blood flow to a vasculature that is maximally dilated and lacks tone or autoregulation. The latter phase coincides with cellular lysis and the release of by-products, which increase permeability.42
Reperfusion, hyperperfusion, and luxury perfusion are the terms used to define the status of cerebral blood flow following ischemia.43 Blood flow returns to the ischemic brain tissue in reperfusion, and cerebral blood flow does not exceed that of the contralateral hemisphere.44 In the early stage of reperfusion, extravasation of contrast material may occur as a result of BBB permeability changes. Hyperperfusion is an increase in cerebral blood flow relative to the control values. Early hyperperfusion of brain tissue after ischemic stroke is reported to be harmless and, in most cases, is followed by complete and rapid clinical recovery, with a lack of gross infarction on histopathologic examination.43 Niels Lassen45 defines classic “luxury perfusion syndrome” as “an excessive cerebral blood flow relative to the metabolic needs of the brain tissue with abnormally small cerebral arteriovenous difference of oxygen.” After a period of vascular arrest, the blood flow is temporarily increased above the resting level in all tissues. A few minutes of hypoxia are followed by reactive hyperemia lasting hours, and the hyperemia lasts much longer than would be needed to re-oxygenate the brain.45 Early reperfusion may prevent infarct growth but also may aggravate edema formation and hemorrhage, resulting in further neuronal damage, so-called reperfusion injury.43
In our study, the immediate occurrence of cortical hyperattenuation and the absence of both clinical symptoms and fast resolution were different from enhancement patterns that were seen in the ischemia. Moreover, MR imaging, DWI in particular, did not show any corresponding acute ischemic lesion.
Statistically significant parameters (ie, increase in the number of balloon inflations and total balloon inflation time in patients who were treated with balloon assistance and increase in the microcatheter time) suggested that cortical hyperattenuation may have resulted from the temporary compromise of the cerebral blood flow. Following this intraprocedural ischemia, blood flow might show reactive increase and cause secondary BBB changes with subsequent contrast extravasation. Despite the fact that it occurred more often in the group treated with balloon remodeling (54% with balloon remodeling versus 34.6% without balloon remodeling), this study did not statistically reveal a direct relationship between the balloon remodeling technique and this finding. This may have been due to the difference in the number of patients treated with and without balloon remodeling technique. Additionally, microcatheterization, only, may have caused incomplete but continuous cerebral ischemia during the treatment in the group with no balloon assistance.
There was also a statistically significant relationship between the amount of contrast material used per kilogram of body weight and the occurrence of cortical hyperattenuation. Another fact was that this finding was confined to the arterial territory of the selective microcatheterization, in which selective contrast injections were made. These observations were in agreement with the knowledge that intra-arterial injection or contrast overdose results in BBB disruption and subsequent contrast extravasation.38,39 An inverse relationship with BSA was noted in this study, and it was most likely related to the increased use of contrast material per kilogram of body weight. Moreover, a significant inverse relationship was found between the occurrence of this finding and the elapsed time until the initial posttreatment CT was obtained, so that the more time elapsed, the less frequent the finding occurred. This implied the progressive washout of excessive iodine from the parenchyma, which was also evidenced by the finding being resolved in the control CT 4–6 hours later.
Evaluating the BBB changes following temporary and permanent ischemia in an experimental model, Kastrup et al46 showed gadolinium enhancement in the ischemic parenchyma and nearby ventricular wall, as well as ischemic changes on MR imaging with DWI and reperfusion findings on perfusion MR imaging, following 1 hour of MCA occlusion in rats. Michel et al47 reported contrast enhancement in the subarachnoid spaces or pial surfaces in the patients who underwent balloon test occlusions of the ICA. These patients also showed abnormal perfusion maps indicating subclinical ischemia with no corresponding DWI abnormalities.47 This enhancement was hypothesized to account for contrast extravasation into the CSF, leakage across damaged endothelial cells of pial vessels following the ischemic events.47 In our study, MR imaging examinations were noncontrast studies and did not include perfusion series. However, when considering the direct relation between the number of balloon inflations, total duration of balloon inflation, microcatheter time, and the occurrence of cortical hyperattenuation, we may still hypothesize that subclinical ischemia may have occurred, which resulted in BBB changes with subsequent contrast enhancement of the cortex on CT.
SAH is another factor that may affect BBBs in the patients who had intracranial aneurysms treated with endovascular means. A retrospective study reported pathologic contrast enhancement in nearly two fifths of patients with acute SAH on contrast-enhanced cranial CT.48 It was also shown that this enhancement may have resulted not only from hyperemia but also from extravasation of contrast material due to BBB disruption. Vasospasm after SAH may also cause ischemia.49 Yet, we could not find a statistically significant relationship between cortical hyperattenuation and the presence of SAH or vasospasm in our study group.
Aging is associated with changes in BBB,50 including loss of capillary endothelial changes, elongation of remaining endothelial cells, and decreased capillary diameter. In our study, we did not find a significant relationship between patient age and the occurrence of cortical hyperattenuation.
BBB permeability is increased in spontaneous hypertension as a result of transient ischemia.51 In our study, all patients were treated under general anesthesia, and their systolic and mean arterial blood pressures were monitored. All patients, even those with prior hypertension, remained normotensive during the procedure, and no relationship was found between the presence of cortical hyperattenuation and blood pressure changes.
Intraventricular and parenchymal contrast enhancement have been reported after conventional angiography.37–39,52 Overdose of contrast material may cause BBB disruption, resulting in diffuse cortical hyperattenuation and increased attenuation of intraventricular CSF, which may mimic SAH.39,40,52 CSF and brain parenchyma enhancement associated with transient cortical blindness have been reported as complications of angiography, which were attributed to the neurotoxic effects of contrast material on BBB.37,53 In our study, all of the patients were asymptomatic, and MR imaging, including DWI series, did not show any abnormality. Therefore, we considered that contrast-related neurotoxicity was unrelated to the cortical hyperattenuation we observed.
Wilkinson et al54 reported abnormal leptomeningeal enhancement in the ipsilateral cerebral hemisphere on MR imaging after successful carotid stent placement in 11 of 12 asymptomatic patients. Following the intervention, with the increase in the flow, leptomeningeal collateral vessels dilated in the absence of compensatory autoregulation, causing contrast enhancement. This series differs from ours in that there was obvious flow compromise as the baseline status in their group of patients.
In a very recent case report, long after the completion of our data collection, a very similar observation was reported in a case of ruptured AcomA aneurysm by Uchiyama et al.55 Their single case showed increased attenuation in the ipsilateral ICA territory, in which the guiding catheter was placed, both in the cortex and basal ganglia. No balloon assistance was used. Their case differs from ours in that their patient had neurologic deficit, though transient. They measured increased iodine concentration in the CSF with spectrophotometry.
Conclusion
To summarize, we report a transient finding of cortical hyperattenuation seen after apparently uneventful endovascular treatment of intracranial aneurysms, most likely resulting from some degree of BBB disruption that resulted in accumulation of contrast medium. Significant relationship was found between the occurrence of this finding and the amount of contrast material used per kilogram body weight, the microcatheter time, the number of balloon inflations, the total time period of balloon inflation, and an inverse relation with the time elapsed until the CT was performed. Relatively increased amounts of contrast material injected intra-arterially in a selective territory and transient changes in brain hemodynamics (ie, flow compromise with subsequent reperfusion during the endosaccular packing) may have resulted in BBB disruption. Although no symptoms occurred in association with the observed cortical hyperattenuation, we believe that it is important to recognize this condition because it can mimic SAH (Fig 6), which may cause unnecessary intimidation and delay in anticoagulant/antiaggregant treatment.
Acknowledgments
The authors thank Pınar Ozdemir Geyik for providing statistical analysis.
References
- 1.Guglielmi G, Vinuela F, Dion J, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg 1991;75:8–14 [DOI] [PubMed] [Google Scholar]
- 2.Brilstra EH, Rinkel GJE, van der Graaf Y, et al. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke 1999;30:470–76 [DOI] [PubMed] [Google Scholar]
- 3.Murayama Y, Nien YL, Duckwiler G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg 2003;98:959–66 [DOI] [PubMed] [Google Scholar]
- 4.Moret J, Cognard C, Weill A, et al. Reconstruction technic in the treatment of wide-neck intracranial aneurysms: long-term angiographic and clinical results—apropos of 56 cases [in French]. J Neuroradiol 1997;24:30–44 [PubMed] [Google Scholar]
- 5.Lefkowitz MA, Gobin YP, Akiba Y, et al. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysms. Part II. Clinical results. Neurosurgery 1999;45:531–38 [DOI] [PubMed] [Google Scholar]
- 6.Aletich VA, Debrun GM, Mısra M, et al. The remodeling technique of balloon-assisted Guglielmi detachable coil placement in wide-necked aneurysms: experience at the University of Illinois at Chicago. J Neurosurg 2000;93:388–96 [DOI] [PubMed] [Google Scholar]
- 7.Tong FC, Cloft HJ, Dion JE. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils: emphasis on new techniques. J Clin Neurosci 2000;7:244–53 [DOI] [PubMed] [Google Scholar]
- 8.Vallee JN, Pierot L, Bonafe A, et al. Endovascular treatment of intracranial wide-necked aneurysms using three-dimensional coils: predictors of immediate anatomic and clinical results. AJNR Am J Neuroradiol 2004;25:298–306 [PMC free article] [PubMed] [Google Scholar]
- 9.Raymond J, Gilbert F, Roy D. Neck-bridge device for endovascular treatment of wide-neck bifurcation aneurysms: initial experience. Radiology 2001;221:318–26 [DOI] [PubMed] [Google Scholar]
- 10.Molyneux AJ, Cekirge S, Saatci I, et al. Cerebral aneurysm multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol 2004;25:39–51 [PMC free article] [PubMed] [Google Scholar]
- 11.Mawad ME, Cekirge S, Ciceri E, et al. Endovascular treatment of giant and large intracranial aneurysms by using a combination of stent placement and liquid polymer injection. J Neurosurg 2002;96:474–82 [DOI] [PubMed] [Google Scholar]
- 12.Cekirge HS, Saatci I, Ozturk MH, et al. Late angiographic and clinical follow-up results of first 100 consecutive aneurysms treated with ONYX reconstruction: largest single center experience. Neuroradiology 2006;48:113–26. Epub 2006 Jan 4 [DOI] [PubMed] [Google Scholar]
- 13.Vanninen R, Manninem H, Ronkainen A. Broad-based intracranial aneurysms: thrombosis induced by stent placement. AJNR Am J Neuroradiol 2003;24:263–66 [PMC free article] [PubMed] [Google Scholar]
- 14.Benitez RP, Silva MT, Klem J, et al. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery 2004;54:1359–68 [DOI] [PubMed] [Google Scholar]
- 15.dos Santos Souza MP, Agid R, Willinsky RA, et al. Microstent-assisted coiling for wide-necked intracranial aneurysms. Can J Neurol Sci 2005;32:71–81 [DOI] [PubMed] [Google Scholar]
- 16.Saatci I, Cekirge HS, Ozturk MH, et al. Treatment of internal carotid artery aneurysms with a covered stent: experience in 24 patients with mid-term follow-up results. AJNR Am J Neuroradiol 2004;25:1742–49 [PMC free article] [PubMed] [Google Scholar]
- 17.Katz JM, Tsiouris AJ, Biondi A, et al. Advances in endovascular aneurysm treatment: are we making a difference? Neuroradiology 2005;47:695–701. Epub 2005 Jul 19 [DOI] [PubMed] [Google Scholar]
- 18.Vinuela F, Duckwiller G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–82 [DOI] [PubMed] [Google Scholar]
- 19.Cloft HJ, Kallmes DF. Cerebral aneurysm perforations complicating therapy with Guglielmi detachable coils: a meta analysis. AJNR Am J Neuroradiol 2002;23:1706–09 [PMC free article] [PubMed] [Google Scholar]
- 20.Soeda A, Sakai N, Sakai H, et al. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2003;24:127–32 [PMC free article] [PubMed] [Google Scholar]
- 21.Soeda A, Sakai N, Murao K, et al. Thromboembolic events associated with Guglielmi detachable coil embolization with use of diffusion-weighted MR imaging. Part II. Detection of the microemboli proximal to cerebral aneurysm. AJNR Am J Neuroradiol 2003;24:2035–38 [PMC free article] [PubMed] [Google Scholar]
- 22.Rordorf G, Bellon RJ, Budzik RE Jr, et al. Silent thromboembolic events associated with the treatment of unruptured cerebral aneurysms by use of Guglielmi detachable coils: prospective study applying diffusion-weighted imaging. AJNR Am J Neuroradiol 2001;22:5–10 [PMC free article] [PubMed] [Google Scholar]
- 23.Ng P, Khangure MS, Phatouros CC, et al. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils: analysis of midterm angiographic and clinical outcomes. Stroke 2002;33:210–17 [DOI] [PubMed] [Google Scholar]
- 24.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–74 [DOI] [PubMed] [Google Scholar]
- 25.Berg van den R, Rinkel GJE, Vandertop WP. Treatment of ruptured intracranial aneurysms: implications of the ISAT on clipping versus coiling. Eur J Radiol 2003;46:172–77 [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez N, Murayama Y, Nien YL, et al. Treatment of unruptured aneurysms with GDCs: clinical experience with 247 aneurysms. AJNR Am J Neuroradiol 2004;25:577–83 [PMC free article] [PubMed] [Google Scholar]
- 27.Park HK, Horowitz M, Jungreis C, et al. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol 2005;26:506–14 [PMC free article] [PubMed] [Google Scholar]
- 28.Quereshi AI, Luft AR, Sharma M, et al. Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures. Part II. Clinical aspects and recommendations. Neurosurgery 2000;46:1360–76 [DOI] [PubMed] [Google Scholar]
- 29.Albayram S, Selcuk H, Kara B, et al. Thromboembolic events associated with balloon-assisted coil embolization: evaluation with diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2004;25:1768–77 [PMC free article] [PubMed] [Google Scholar]
- 30.Studley MT, Robinson DH, Howe JF. Delayed thromboembolic events 9 weeks after endovascular treatment of an anterior communicating aneurysm: case report. AJNR Am J Neuroradiol 2002;23:975–77 [PMC free article] [PubMed] [Google Scholar]
- 31.Lenthall RK, McConachie NS, Jaspan T. Delayed reconfiguration of a Guglielmi detachable coil mass associated with late occlusion of an adjacent aneurysm and parent vessel wall. AJNR Am J Neuroradiol 2000;21:1908–10 [PMC free article] [PubMed] [Google Scholar]
- 32.Sluzewski M, Bosch JA, van Rooij WJ, et al. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg 2001;94:238–40 [DOI] [PubMed] [Google Scholar]
- 33.Byrne JV, Sohn MJ, Molyneux AJ. Five year experience in using coil embolization for unruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656–63 [DOI] [PubMed] [Google Scholar]
- 34.Kremer C, Groden C, Lammers G, et al. Outcome after endovascular therapy of ruptured intracranial aneurysms: morbidity and impact of rebleeding. Neuroradiology 2002;44:942–45. Epub 2002 Sep 18 [DOI] [PubMed] [Google Scholar]
- 35.Saatci I, Cekirge HS, Ciceri EFM, et al. CT and MR findings and their implications in the follow-up of patients with intracranial aneurysms with endosaccular occlusion with Onyx. AJNR Am J Neuroradiol 2003;24:567–78 [PMC free article] [PubMed] [Google Scholar]
- 36.Sage MR, Wilson AJ. The blood-brain barrier: an important concept in neuroimaging. AJNR Am J Neuroradiol 1994;15:601–22 [PMC free article] [PubMed] [Google Scholar]
- 37.DeWispelarer JF, Trigaux JP, Van Beers B, et al. Cortical and CSF hyperdensity after iodinated contrast medium overdose: CT findings. J Comput Assist Tomogr 1992;16:998–1003 [DOI] [PubMed] [Google Scholar]
- 38.Okazaki H, Tanaka K, Shishido T, et al. Disruption of the blood-brain barrier caused by nonionic contrast medium used for abdominal angiography: CT demonstration. J Comput Asist Tomogr 1989;13:893–95 [DOI] [PubMed] [Google Scholar]
- 39.Numaguchi Y, Fleming MS, Hasuo K, et al. Blood-brain barrier disruption due to cerebral arteriography. J Comput Asist Tomogr 1984;8:936–39 [DOI] [PubMed] [Google Scholar]
- 40.Kuhn MJ, Burk TJ, Powell FC. Unilateral cerebral cortical and basal ganglia enhancement following overdosage of nonionic contrast media. Comput Med Imag Graph 1995;19:307–11 [DOI] [PubMed] [Google Scholar]
- 41.Sampaolo S, Nakagawa Y, Iannotti F, et al. Blood-brain barrier permeability to micromolecules and edema formation in the early phase of incomplete continuous ischemia. Acta Neuropathol (Berl) 1991;82:107–11 [DOI] [PubMed] [Google Scholar]
- 42.Kuroiwa T, Ting P, Martinez H, et al. The biphasic opening of the blood-brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol (Berl) 1985;68:122–29 [DOI] [PubMed] [Google Scholar]
- 43.Marchal G, Young AR, Baron JC. Early postischemic hyperperfusion: pathophysiologic insights from positron emission tomography. J Cerebral Blood Flow Metab 1999;19:467–82 [DOI] [PubMed] [Google Scholar]
- 44.Young AR, Sette G, Touzani O, et al. Relationships between high oxygen extraction fraction in the acute stage and final infarction in reversible middle cerebral artery occlusion: an investigation in anesthetized baboons with positron emission tomography. J Cereb Blood Flow Metab 1996;16:1176–78 [DOI] [PubMed] [Google Scholar]
- 45.Lassen NA. The luxury perfusion syndrome and its possible relation to acute metabolic acidosis localised within the brain. Lancet 1966;2:1113–15 [DOI] [PubMed] [Google Scholar]
- 46.Kastrup A, Engelhorn T, Beaulieu C, et al. Dynamics of cerebral injury, perfusion, and blood-brain barrier changes after temporary and permanent middle cerebral artery occlusion in the rat. J Neurol Sci 1999;166:91–99 [DOI] [PubMed] [Google Scholar]
- 47.Michel E, Liu H, Remley KB, et al. Perfusion MR in patients undergoing balloon test occlusion of the internal carotid artery. AJNR Am J Neuroradiol 2001;22:1590–96 [PMC free article] [PubMed] [Google Scholar]
- 48.Doczi T. The pathogenetic and prognostic significance of blood-brain barrier damage at the acute stage of aneurysmal subarachnoid hemorrhage: clinical and experimental studies. Acta Neurochir (Wien) 1985;77:110–32 [DOI] [PubMed] [Google Scholar]
- 49.Weir B, Macdonald L, Stoodley M. Etiology of cerebral vasospasm. Acta Neurochir Suppl 1999;72:27–46 [DOI] [PubMed] [Google Scholar]
- 50.Mooradian AD. Effect of aging on the blood-brain barrier. Neurobiol Aging 1988;9:31–39 [DOI] [PubMed] [Google Scholar]
- 51.Abraham CS, Harada N, Deli MA, et al. Transient forebrain ischemia increases the blood-brain barrier permeability for albumin in stroke–prone spontaneously hypertensive rats. Cell Mol Neurobiol 2002;22:455–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckel TS, Breiter SN, Monsein LH. Subarachnoid contrast enhancement after spinal angiography mimicking diffuse subarachnoid hemorrhage. AJR Am J Roentgenol 1998;170:503–05 [DOI] [PubMed] [Google Scholar]
- 53.Velden J, Milz P, Winkler F, et al. Nonionic contrast neurotoxicity after coronary angiography mimicking subarachnoid hemorrhage. Eur Neurol 2003;49:249–51 [DOI] [PubMed] [Google Scholar]
- 54.Wilkinson ID, Griffiths PD, Hoggard N, et al. Unilateral leptomeningeal enhancement after carotid stent insertion detected by magnetic resonance imaging. Stroke 2000;31:848–51 [DOI] [PubMed] [Google Scholar]
- 55.Uchiyama Y, Abe T, Hirohata M, et al. Blood brain-barrier disruption of nonionic iodinated contrast medium following coil embolization of a ruptured intracerebral aneurysm. AJNR Am J Neuroradiol 2004;25:1783–86 [PMC free article] [PubMed] [Google Scholar]