Abstract
SUMMARY: Intracranial arteriovenous malformations (AVM) are a rare feature of Bannayan-Riley-Ruvalcaba syndrome (BRRS). Palencia et al reported a case of intracranial arteriovenous malformation in a child with BRRS in a Spanish journal in 1986. However, the occurrence of dural AVM in a patient with BRRS has not since been addressed in the literature. Advancements in imaging and therapeutic embolization, and the ability now to screen for phosphatase and tensin homologue (PTEN) mutations allow us to detect and manage these patients sooner. Early detection of intracranial AVMs is necessary because of the risk for progression to venous ischemia and resultant neurologic damage. We present the case of a child with headaches and periorbital venous congestion due to a dural AVM with bilateral venous outflow occlusion who was treated with multiple embolizations, now with interval remission of headache symptoms.
Bannayan-Riley-Ruvalcaba syndrome (BRRS) is a rare autosomal-dominant overgrowth syndrome that is frequently associated with phosphatase and tensin homologue (PTEN) mutations.1 We submit the case of a child with BRRS who presented with headaches and periorbital venous congestion due to a dural arteriovenous malformation (AVM) with bilateral venous outflow occlusion. Early detection and treatment of large symptomatic intracranial AVMs is optimal because of the risk for progression to venous ischemia and resultant neurologic damage. The presence of congenital macrocephaly in this child may have contributed to the late diagnosis of this intracranial vascular malformation.
Case Report
The patient was a 9-year-old boy who was the product of a 34-week triplet pregnancy complicated by gestational diabetes, maternal cardiomegaly, pulmonary compromise, and preterm labor. He was macrocephalic at birth and had perinatal seizures. Early childhood history was remarkable for developmental (motor, language, and cognitive) delay. He has a 15-year-old sister with speech difficulties. His triplet sister and brother do not have macrocephaly or significant medical or developmental problems.
The patient was referred to the genetics service at age 4. He was in the 95th percentile for both height and weight, much larger than his triplet siblings, and had macrocephaly and dolichocephaly, with slight frontal bossing and a reticular staining pattern prominent on the face, thighs, and lower abdomen, consistent with cutis marmorata. At age 7, penile lentigines were noted, and the diagnosis of BRRS was considered. PTEN mutation testing revealed a single base pair insertion in exon 8. This would result in a frameshift mutation of the PTEN tumor-suppressor gene, thus confirming the diagnosis of BRRS at the molecular level. Family history was negative for thyroid, breast, or cervical cancer.
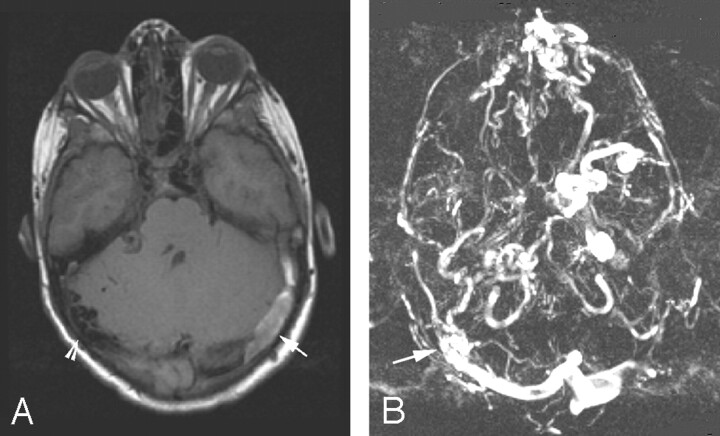
Persistent severe headaches associated with prominent periorbital veins prompted his referral to the Vascular Anomalies Clinic at 9 years of age. Cerebral MR imaging revealed mild ventriculomegaly and vascular abnormalities consistent with a right posterior dural AVM with bilateral transverse sinus occlusion. Thrombus was evident in the left sigmoid sinus, suggesting recent occlusion (Fig 1A, -B).
Fig 1.
A, Cerebral T1-weighted MR image demonstrates a clot in an occluded left transverse sinus (arrow). Prominent flow voids are noted on the right (arrowhead).
B, MR venogram of the brain demonstrates the site of the dural AVM (arrow). Also noted are sigmoid sinus occlusion and prominent collateral veins to the left orbital vein.
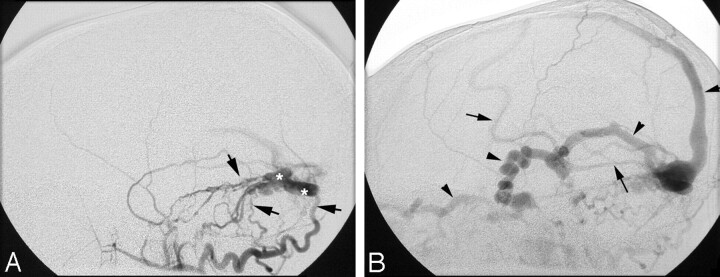
Diagnostic angiography confirmed the presence of a right occipital dural arteriovenous fistula within a segment of the right sigmoid sinus, with supply from meningeal branches of the right internal and external carotid arteries, the vertebrobasilar system, and the left internal carotid artery (Fig 2A). The fistula drained through the basal vein of Rosenthal into the left superior orbital vein, with additional reflux into a right cortical vein to the superior sagittal sinus (Fig 2B). Bilateral sigmoid sinus occlusion was confirmed, and most of the cerebral veins drained anteriorly, through collaterals to the orbital veins. Multiple feeding branches to the AVM were embolized on 4 separate occasions. Currently, the patient denies headaches and his periorbital venous congestion is reduced.
Fig 2.
A, Right external carotid artery angiogram shows meningeal and accessory meningeal arteries (arrows) communicating with a segment of the occluded left sigmoid sinus (asterisk).
B, Venous phase image of the right external carotid artery angiogram shows drainage of the AVM through the tentorial sinus, basal vein of Rosenthal, and orbital veins. Note the reflux into the cortical veins (arrows) and the superior sagittal sinus (arrowheads).
Discussion
BRRS is an autosomal-dominant inherited syndrome closely associated with Cowden syndrome.1 BRRS is characterized by overgrowth, including macrocephaly, penile lentigines, developmental and language delay, and cognitive impairment. Individuals with BRRS are generally macrocephalic and have weight and height above the 95th percentile. Other associations include ectodermal dysplasia, cutis marmorata or cutaneous vascular malformations, lipomatous masses, and intestinal hamartomatous polyps. Extremity AVMs in association with BRRS have been reported in the literature, and there was 1 report of an intracranial AVM by Palencia et al in 1986.2,3
In 1997, the association between BRRS and PTEN mutations was elucidated. Mutations in PTEN have also been associated with Cowden syndrome and familial polyposis. According to a study by Zhou et al,4 germline intragenic mutations in PTEN are present in 80% of patients with Cowden syndrome and 60% of patients with BRRS. PTEN is a tumor-suppressor gene located on chromosome 10q, which operates on a 2-hit hypothesis, akin to that of the retinoblastoma gene. One mutation in PTEN is present from birth and the second is acquired; when both mutations are present, they confer an increased susceptibility to certain forms of cancer. Patients with mutations of the PTEN gene and BRRS have an approximately 3%–10% lifetime risk of thyroid cancer and an approximately 30%–50% risk of breast or cervical cancer.1 The breast cancer risk is lower in men. There is also increased risk for nonhamartomatous polyps of the large bowel. Cancer is more likely to develop in adults, but yearly thyroid sonographic examinations are recommended in addition to breast self-examinations beginning in the teenage years. Urinalysis is recommended in the teen years, and colonoscopies should begin at age 50. Women with PTEN mutations should begin mammography and screening for cervical cancer yearly, beginning at 30 years of age.5
The association of AVMs with PTEN mutations has been documented in numerous case reports in the literature. Hamada et al6 recently described the involvement of the PTEN gene in normal vascular development. A mutation in PTEN would, therefore, result in abnormal angiogenesis, thus explaining the incidence of AVMs. Central nervous system manifestations are not limited to dural AVMs. Lok et al7 reported the occurrence of cerebral venous and cavernous malformations in Cowden syndrome, which is highly associated with PTEN mutations.
An AVM represents anomalous vascular development in which arteries connect directly to veins. Dural AVMs account for 10%–15% of all intracranial AVMs. Intracranial AVMs are generally classified on the basis of their location. Pial AVMs are the most common intracranial AVMs and consist of shunts between cortical branches of the internal carotid or vertebral circulation and the cortical veins. Dural AVMs, on the other hand, are shunts within the dural sinuses, supplied by the meningeal branches of the external carotid, internal carotid, and vertebrobasilar circulation. Deep cerebral AVMs include the vein-of-Galen aneurysmal malformation, in which limbic (choroidal) arteries connect with the deep cerebral veins, including the embryonic precursor of the vein of Galen.
Dural AVMs often are presumed to be acquired in adult patients, sometimes following postsurgical dural sinus thrombosis. Dural AVMs may be present at birth, and pediatric lesions may be associated with progressive dural sinus outflow occlusion, believed to be caused by a myointimal reaction to high pressure and flow in the dural sinuses. Not all dural AVMs are dangerous or require treatment. Those with outflow occlusion and especially cortical venous drainage, however, must be treated as soon as possible to prevent the sequelae of progressive venous ischemia caused by venous hypertension. Clinically, affected infants may present with a high output cardiac state, hydrocephalus, or pulsatile mass, whereas older patients often present with bruit, headache, and tinnitus. Most pediatric dural AVMs are generally posterior in location, involving the transverse or sigmoid sinus. Venous outflow obstruction, as in the case of our patient, can be present at birth but more often develops later in infancy or childhood. In the absence of good collateral drainage, venous hypertension occurs and is associated with the development of hydrocephalus, seizures, developmental regression, and progressive neurologic decline. The AVM reported in a child with BRRS by Palencia et al2 also represented a dural AVM involving a transverse sinus.
Cognard et al8 described a classification system for dural AVMs in 1995. Dural AVMs were classified into 5 types on the basis of the venous drainage. Our patient had a type II lesion, which has reflux into cortical veins and is associated with hemorrhage in 40% of cases.
The mainstay of treatment for pediatric dural AVMs is embolization by using permanent ablative agents such as n-butyl 2-cyanoacrylate or absolute alcohol. Transvenous embolization of the affected sinus is the most efficient method but may be impossible in the presence of outflow occlusion. Treatment should be initiated early due to the risk for neurologic damage with progressive venous ischemia.
Both dural AVMs and BRRS can present with macrocephaly independently. If BRRS is diagnosed first, the possibility for missing the diagnosis of an asymptomatic dural AVM exists. Our patient’s macrocephaly as a feature of his syndrome may have excluded the clinical consideration of dural AVM. If it was not for the development of periorbital venous prominence and headaches, the diagnosis may have been delayed further. Because of the risk of deleterious consequences from untreated progressive dural AVMs and the association between AVMs and BRRS, early screening in these patients may be beneficial. This could include MR imaging/MR angiography of the brain. The lack of radiation exposure makes this screening ideal and potentially lifesaving if these patients are lost to follow-up.
Conclusion
High-flow vascular malformations, including dural AVM, may exist in patients with BRRS. Macrocephaly without hydrocephalus is common in this condition, so the presence of intracranial AVM could be missed without specific symptoms or careful imaging. Early MR imaging/MR angiography in patients with BRRS may be warranted, to avoid delay in diagnosis and treatment, leading to venous ischemia and neurologic injury.
References
- 1.Marsh DJ, Kum JB, Lunetta KL, et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet 1999;8:1461–72 [DOI] [PubMed] [Google Scholar]
- 2.Palencia R, Ardura J. Bannayan syndrome with intracranial arteriovenous malformations [in Spanish]. An Esp Pediatr 1986;25:462–66 [PubMed] [Google Scholar]
- 3.Naidich JJ, Rofsky NM, Rosen R, et al. Arteriovenous malformation in a patient with Bannayan-Zonana syndrome. Clin Imaging 2001;25:130–32 [DOI] [PubMed] [Google Scholar]
- 4.Zhou XP, Waite KA, Pilarski R, et al. Germline PTEN promoter mutations and deletions in Cowden/Bannayan-Riley-Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. Am J Hum Genet 2003;73:404–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian (Clinical practice guidelines in oncology, Verision 1.2006), pages 12–24. Available at: http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf
- 6.Hamada K, Sasaki T, Koni PA, et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev 2005;19:2054–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lok C, Viseux V, Avril MF, et al. Brain magnetic resonance imaging in patients with Cowden syndrome. Medicine (Baltimore) 2005;84:129–36 [DOI] [PubMed] [Google Scholar]
- 8.Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995;194:671–80 [DOI] [PubMed] [Google Scholar]