Abstract
SUMMARY: A 44-year-old man developed bilateral blindness following severe periorbital cellulitis and pansinusitis. CT and MR imaging demonstrated bilateral cavernous sinus thrombosis. Diffusion-weighted imaging revealed reduced apparent diffusion coefficient in bilateral optic nerves, suggesting optic nerve ischemia caused by the cavernous sinus thrombophlebitis (CST). Following surgical debridement of pansinusitis, antimicrobial therapy, and anticoagulation, the patient recovered from the infectious episode but sustained permanent bilateral blindness. This case shows that both retinal and optic nerve ischemia can be the cause of blindness after CST. Arguments supporting an arterial-versus-venous origin for the ischemia are discussed.
Septic cavernous sinus thrombophlebitis (CST) is a rare and potentially life-threatening complication of infections involving the sinuses, face, and/or auricular region.1 It can also occur as a complication of orbital cellulitis, bacterial meningitis, subdural empyema, or epidural abscess, as well as rhinoplasty and oral surgery.1 Before efficacious antimicrobials, the mortality rate of CST was effectively 75%.2 With aggressive management, the mortality rate is now <30%,1,3 and morbidity has become the primary concern, with complete recovery being rare despite therapy.1 Sequelae include a variety of deficits, such as cranial nerve palsies, optic neuropathy, and trigeminal neuralgia. Approximately one half of patients have residual cranial nerve deficits, and one sixth of patients have some degree of visual impairment, including permanent blindness.1,3 We report a case in which imaging illustrates the pathophysiology of CST-induced blindness.
Case Report
A 44-year-old man without significant medical or surgical history was transferred to our institution for work-up and management of severe periorbital cellulitis and pansinusitis. The patient’s complaints began about 11 days before transfer. He recalled 2 days of nausea, vomiting, and fever that ensued after an insect bite to his right neck. Shortly afterward, both of his eyes became puffy and slightly sore, and he noted a pimple on his nose that produced purulent discharge. The patient was admitted to a community hospital for management of presumed bilateral orbital cellulitis. Broad-spectrum antibiotic coverage was initiated. On admission, the patient was documented as being able to appreciate hand movement and light. One day later, he reported complete loss of vision and inability to move his eyes, in association with worsening ocular pain and pressure, which motivated the patient’s transfer to our hospital.
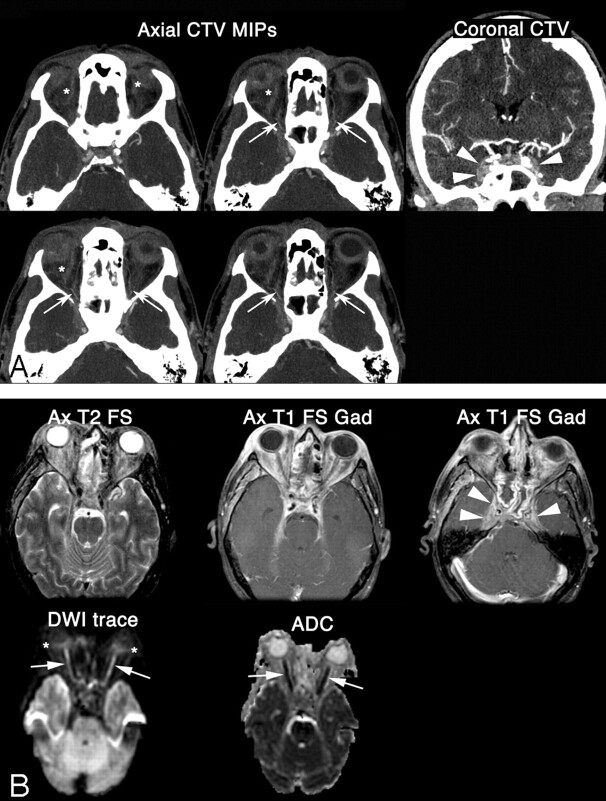
Initial imaging with CT venography of the brain demonstrated pansinusitis and severe bilateral orbital cellulitis with proptosis, associated with bilateral CST (Fig 1A). Absence of contrast-filling of the superior ophthalmic veins was interpreted as indicating bilateral thrombosis. Our CT venography protocol (16–detector row CT scanner; 120 kilovolt peak (kV[p]); 280 mAs; gantry rotation, 0.5 seconds; pitch, 1.375:1; section thickness, 0.625 mm; reconstruction interval, 0.5 mm) involves a long bolus of iodinated contrast material (4 mL/s for a total of 160 mL; duration of the injection, 40 seconds) and a delayed CT acquisition at 40 seconds after the start of the contrast injection. Consequently, the ophthalmic arteries could be assessed in addition to the venous structures. The proximal ophthalmic arteries, at the level of the orbital apices, were opacified, as well as some muscular or ciliary branches (Fig 1A).
Fig 1.
A 44-year-old man presented with headaches, chemosis, and visual loss. Clinical impression favored severe periorbital cellulitis and pansinusitis. A, Coronal CT angiography at initial presentation reveals bilateral convexity of the cavernous sinus (arrowheads), consistent with CST. Axial 5-mm-thick maximum intensity projections demonstrate an absence of contrast-filling of the superior ophthalmic veins (stars), which was interpreted as indicating bilateral thrombosis. On the contrary, the proximal ophthalmic arteries (arrows), at the level of the orbital apices, are opacified, as well as some muscular or ciliary branches. B, Follow-up T2-weighted MR image corroborates the diagnosis of severe bilateral orbital cellulitis with proptosis. The postcontrast T1-weighted images show intense enhancement within bilateral cavernous sinuses (arrowheads) and within the orbital fat bilaterally. Axial DWI and the corresponding reduced ADC map demonstrate restricted diffusion in the entirety of the optic nerves bilaterally (arrows). There is also a suggestion of reduced diffusion in the bilateral retina (stars), even if no ADC values could be measured accurately because of partial volume averaging.
Follow-up MR imaging (1.5T), including diffusion-weighted imaging (DWI) (single-shot echo-planar imaging; 1 excitation; TR/TE, 4000/114; b = 0 and b = 1000; 3 gradients; field of view, 24 cm; section thickness, 7 mm; skip, 3 mm) corroborated the diagnoses of severe bilateral orbital cellulitis with proptosis and CST (Fig 1B). The additional finding was severely reduced diffusion within both optic nerves, consistent with bilateral optic nerve ischemia (Fig 1B). The apparent diffusion coefficient (ADC) values measured within the bilateral optic nerves ranged between 168 and 744 × 10−6 mm2 × s−1 (normal optic nerves have reported ADC values of 833–1178 × 10−6 mm2 × s−1).4–6 The optic chiasm and the optic tracts were unremarkable. On the other hand, there was a suggestion of reduced diffusion in the bilateral retinas, even if no ADC values could be measured accurately because of partial volume averaging. The postcontrast T1-weighted images showed intense enhancement within the bilateral cavernous sinuses and within the orbital fat bilaterally. The evaluation of the enhancement within the optic nerves was challenged by the intense enhancement within the orbital fat and also by the fact that only T1-weighted images without fat saturation were obtained before the administration of gadolinium.
Ophthalmology examination performed immediately before the MR imaging showed diffuse bilateral chemosis, periorbital and lid edema, as well as nonreactive pupils, 7 mm on the right and 2 mm on the left. Funduscopic examination demonstrated bilateral fundal pallor and associated intraretinal and subretinal hemorrhages, with disk margins obscured by hyperemia and cotton-wool spots. Also, arterial narrowing and 4+ venous tortuosity were observed. A bilateral fluorescein eye scan revealed that the patient had sustained significant bilateral ischemic insult, likely from venous and arterial obstruction of the central retinal vessels of the right eye and from posterior ciliary artery infarction of the left eye.
The patient underwent bilateral endoscopic maxillary antrostomies, total ethmoidotomies, and sphenoidotomies. Additionally, a course of vancomycin, ceftriaxone, amphotericin B, and metronidazole was administered. Because it was not clear at the time that the patient’s vision loss was permanent, intravenous solumedrol was also initiated in the hopes of alleviating any mass effect component to the visual loss from edema and inflammation. Finally, a brief trial of anticoagulation with heparin was also provided.
The patient’s condition stabilized after the sinus debridement. He was eventually discharged with permanent bilateral blindness as his sole neurologic sequela. Subsequent work-up for possible rheumatologic etiologies revealed negative antinuclear antibody, cytoplasmic antineutrophil cytoplasmic antibody, perinuclear antineutrophil cytoplasmic antibody, and rheumatoid factors, and the patient’s presentation was thought to be consistent with sinusitis, nasal infection, and orbital cellulitis leading to CST and associated bilateral ischemic optic neuropathy. No follow-up MR imaging could be performed to assess the evolution of the imaging findings with time.
Discussion
Postulated mechanisms accounting for visual impairment and blindness in cases of CST include venous infarction of the retina and retinal ischemia caused by thrombotic occlusion of either an ophthalmic artery branch or of the central retinal artery or by mechanical pressure at the orbital apex.7–10 Corneal ulcerations secondary to a loss of the corneal reflex may be a contributing factor.7–10 These pathophysiologic hypotheses are based on theoretic and anatomic considerations rather than on scientific evidence. Our Case Report potentially sheds new light on the pathophysiology of CST-induced blindness, suggesting the involvement not only of the retinas but also of the optic nerves.
The main findings in the reported case were the presence of reduced ADC values within bilateral optic nerves, in addition to the suggestion of reduced diffusion within the retinas. ADC values in normal optic nerves typically range around 833–1178 × 10−6 mm2 × s−1 and have been reported to be elevated in a variety of conditions, including optic neuritis.4–6 In our patient with bilateral blindness secondary to CST, measured ADC values within the optic nerves were significantly lower than the normal values, ranging from 168 to 744 mm2 × s−1. The low ADC values would initially suggest an arterial rather than venous origin for the optic nerve ischemia. However, the ophthalmic arteries were opacified on the CT venogram. On the contrary, superior ophthalmic veins were thrombosed or severely compressed by the tumefaction related to the orbital cellulitis, supporting venous infarction as being mostly responsible for the optic nerve changes. The bilateral symmetric involvement also favored a venous origin.
In patients with suspected CST, MR imaging and especially DWI should be reviewed for confirming the diagnosis of CST and assessing its impact on the brain parenchyma. However, particular attention should also be paid to optic nerves for possibly lowered ADC values. In the future, better awareness of this radiologic pattern will hopefully reveal these visual complications at an earlier stage, with possible favorable impact on the patient’s outcome.
References
- 1.Ebright JR, Pace MT, Niazi AF. Septic thrombosis of the cavernous sinuses. Arch Intern Med 2001;161:2671–76 [DOI] [PubMed] [Google Scholar]
- 2.Yarington CT Jr. The prognosis and treatment of cavernous sinus thrombosis: review of 878 cases in the literature. Ann Otol Rhinol Laryngol 1961;70:263–67 [DOI] [PubMed] [Google Scholar]
- 3.Yarington CT Jr. Cavernous sinus thrombosis revisited. Proc R Soc Med 1977;70:456–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hickman SJ, Wheeler-Kingshott CA, Jones SJ, et al. Optic nerve diffusion measurement from diffusion-weighted imaging in optic neuritis. AJNR Am J Neuroradiol 2005;26:951–56 [PMC free article] [PubMed] [Google Scholar]
- 5.Iwasawa T, Matoba H, Ogi A, et al. Diffusion-weighted imaging of the human optic nerve: a new approach to evaluate optic neuritis in multiple sclerosis. Magn Reson Med 1997;38:484–91 [DOI] [PubMed] [Google Scholar]
- 6.Wheeler-Kingshott CA, Parker GJ, Symms MR, et al. ADC mapping of the human optic nerve: increased resolution, coverage, and reliability with CSF-suppressed ZOOM-EPI. Magn Reson Med 2002;47:24–31 [DOI] [PubMed] [Google Scholar]
- 7.Geggel HS, Isenberg SJ. Cavernous sinus thrombosis as a cause of unilateral blindness. Ann Ophthalmol 1982;14:569–74 [PubMed] [Google Scholar]
- 8.Friberg TR, Sogg RL. Ischemic optic neuropathy in cavernous sinus thrombosis. Arch Ophthalmol 1978;96:453–56 [DOI] [PubMed] [Google Scholar]
- 9.Coutteel C, Leys A, Fossion E, et al. Bilateral blindness in cavernous sinus thrombosis. Int Ophthalmol 1991;15:163–71 [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Jalali S, Bansal RK, et al. Anterior ischemic optic neuropathy and branch retinal artery occlusion in cavernous sinus thrombosis. J Clin Neuroophthalmol 1990;10:193–96 [PubMed] [Google Scholar]