Abstract
BACKGROUND AND PURPOSE: The dural venous sinuses in neonates differ from those in adults or older children in that the caliber of venous sinuses is smaller and there is skull molding. The aim of this retrospective study is to evaluate the presence of flow gaps in venous sinuses in neonates on 2D time-of-flight (TOF) MR venography (MRV).
METHODS: Fifty-one neonates underwent coronal 2D TOF MRV. Nine also had CT venography (CTV) for comparison. In 1 neonate, a further 2D TOF MRV was performed in the sagittal plane; in another neonate, images were captured in the axial plane; and in another, a further coronal TOF MRV with shorter echo time was performed.
RESULTS: Flow gap was seen in the posterior aspect of the superior sagittal sinus in 35 of 51 (69%). Focal narrowing of the superior sagittal sinus, in the region of convergence of lambdoid sutures, was detected in 7 of 51 (14%). The right and left transverse sinuses demonstrated flow gap in 13 of 51 (25%) and 32 of 51 (63%) respectively. There was normal filling of contrast on CTV in the superior sagittal sinus, transverse sinus and sigmoid sinus in those cases with flow gap detected on coronal 2D TOF MRV. Right, left, and codominance of the transverse sinuses are as follows: 32 of 51 (63%), 5 of 51 (10%), and 14 of 51 (27%), respectively. The right and left sigmoid sinuses demonstrated flow gap in 7 of 51 (14%) and 8 of 51 (16%), respectively, and the left sigmoid sinus was absent in 1 of 51 (2%).
CONCLUSION: The high proportion of flow gap in the venous sinuses of neonates, particularly of the superior sagittal sinus, could be attributed to the smaller caliber venous sinuses, slower venous flow, and skull molding.
D time-of-flight (TOF) MR venography (MRV) is a technique commonly used to assess the cerebral venous sinuses because it is easy to perform and does not require contrast administration. The normal anatomy, variants, and pitfalls of 2D TOF MRV in the pediatric population have been recently described.1–3 There is very little information available, however, on the appearance of the cerebral venous sinuses of the neonates on 2D TOF MRV. The caliber of the dural venous sinuses in neonates is smaller than that in adults. The skull is molded in neonates. Therefore, it is possible that the appearance of dural venous sinuses in neonates as seen on 2D TOF MRV is different from that in adults or older children. Rollins et al3 have found problems with saturation effects with 2D TOF MRV, especially in infants. Therefore, it is likely that flow gaps are more likely to be seen in neonates than adults or older children. The aim of this retrospective study was to evaluate the incidence of flow gaps in the major dural venous sinuses in neonates as seen on 2D TOF MRV.
Methods
This retrospective study has the approval of the local research ethics board. The neonates were identified from the MR database. Between 2002 and June 2005, 51 neonates with no thrombus identified on either 2D TOF MRV or structural MR were recruited into the study. All except 2 neonates were born at term (ie, 38 weeks or later). Two neonates were born premature, 1 at gestational week 31 and the other at week 33. The TOF MRV was performed between 1 and 28 days after birth (mean, 6.6 days). Neonates with venous sinus thrombosis detected on 2D TOF MRV and CT venography (CTV) were excluded from the study. Brain imaging was performed for a variety of reasons, including seizures (23 cases), hypoxic-ischemic encephalopathy (16 cases), hypoglycemia (3 cases), and hypotonia secondary to metabolic disorder or hypoglycemia (3 cases). Brain MR was performed to exclude ischemic changes in the brain parenchyma secondary to congenital heart disease in 4 cases. Of the 23 patients with seizures, 9 cases were secondary to metabolic disorder, hypoglycemia, or sepsis. Eight of these 23 patients were secondary to hypoxic-ischemic encephalopathy. Three patients are included in both groups as they had multiple causes for seizures, and in 9 of the 23, no cause was found. All procedures were performed with the patients under sedation.
MR imaging was performed on a 1.5T scanner (GE Medical Systems, Milwaukee, Wis) in the supine position in all cases. MR imaging consisted of diffusion-weighted imaging in 3 planes, axial T1, axial fast spin-echo (FSE) T2, coronal FSE T2, and sagittal T1. Other sequences were performed based on clinical indication and MR findings. Coronal 2D TOF MRV was performed with an inferior saturation band to eliminate signal intensity from arterial structures. The parameters include echo time (TE), 5–6 ms; repetition time (TR), 50 ms; flip angle, 60°; section thickness, 1.5 mm; matrix, 256 by 160; field of view, 20 cm. In 1 patient, the coronal 2D TOF MRV was repeated using different TE (first with TE of 5.5 ms and the second examination with TE of 4.9 ms). Sagittal 2D TOF was performed in 1 case and axial 2D TOF was performed in another case using similar imaging parameters. Maximum intensity projections (MIP) were constructed from the source images, and multiple oblique and orthogonal projections were viewed on the PACS workstation (GE Medical Systems). Images were reviewed by 2 pediatric neuroradiologists (E.W. and M.S.).
Nine patients had CTV in addition to the 2D TOF MRV. CTV was performed using 8-row multidetector CT scan (LightSpeed Ultra; GE Medical Systems) in the supine position. An unenhanced CT brain scan was performed before the contrast injection. A bolus of 2.5 mL/kg iohexol USP 65% (Omnipaque 300; GE Healthcare, Oakville, Canada) was given intravenously by hand injection. Imaging was performed immediately after the bolus of contrast. The axial unenhanced and enhanced images were acquired parallel to the orbitomeatal line, and the same coverage and same imaging parameters were used. The imaging parameters include section thickness of 5 mm, x-ray tube of 120 kV, x-ray current of 80 mA, 2 images per rotation, and a field of view of 22 cm. The axial enhanced images were then reconstructed at 1.25 mm, and sagittal and coronal reformatted images were acquired. The axial reconstructed images and the sagittal and coronal reformatted images were reviewed. CTVs were performed between 3 days before and 2 days after 2D TOF MRV (mean, 0.4 days). The venous sinuses that were assessed on TOF MRV were also assessed on CTV.
The venous structures assessed included the superior sagittal sinus, transverse sinuses, sigmoid sinuses, internal jugular veins, and occipital sinuses. These venous structures were assessed for the presence of narrowing, flow gap, or aplasia. Narrowing was defined as a short segment narrowing of the vein that measured less than 5 mm long, and there was still flow seen in the sinus. Flow gap on the MIP or source images was defined as lack of high signal intensity. A transverse sinus was considered absent if the sinus could not be identified on the MIP and source images of the TOF MRV, from the torcular to the sigmoid sinus. Transverse sinus dominance was also assessed.
χ2 analysis was performed to determine whether there was a relationship between hypoxic-ischemic encephalopathy and flow gap in the superior sagittal sinus and transverse sinuses.
Results
On TOF MRV, flow gap was seen in the superior sagittal sinus in 35 (69%) of 51 on the MIP images, predominantly in the posterior third of the superior sagittal sinus. There was discrepancy in the conspicuity of the posterior third of the superior sagittal sinus on the source and MIP images. On the source images, flow gap was seen in 18 (35%) of 51 and the flow gap was shorter than on the MIP images. Focal narrowing of the superior sagittal sinus, in the region where the lambdoid sutures converge, was detected in 7 (14%) of 51. The right transverse sinus demonstrated flow gap in 13 (25%) of 51 on the MIP images and 8 (16%) of 51 on the source images. The left transverse sinus demonstrated flow gap in 32 (63%) of 51 on the MIP images in 17 (33%) of 51 on the source images. The flow gap detected in the transverse sinuses occurred at the medial portion of the sinuses. The right transverse sinus was absent in 1 (2%) of 51 and the left transverse sinus was absent in 1 (2%) of 51. In 32 (63%) of 51 examinations, dominance of the right transverse sinus was found; in 5 (10%) of 51 examinations, dominance of the left transverse sinus was found. In 14 (27%) of 51 examinations, the transverse sinuses were codominant. The right sigmoid sinus demonstrated flow gap at the distal sigmoid sinus in 7 of 51(14%) on the MIP images and in 4 (8%) of 51 on the source images. The left sigmoid sinus was absent in 1 (2%) of 51. The left sigmoid sinus showed flow gap at the distal sigmoid sinus in 8 (16%) of 51 on the MIP images and in 4 (8%) of 51 on the source images. The occipital sinus was detected in 9 (18%) of 51. The internal jugular veins were present in all cases with no flow gap visualized on either side.
Nine neonates had CTV and 2D TOF MRV and the findings of both examinations were presented in Table 1. On the CTV, 2 of 9 demonstrated narrowing of the superior sagittal sinus, in the region where the lambdoid sutures converge. There was normal filling of contrast on CTV in the superior sagittal sinus, transverse sinus and sigmoid sinus in those cases with flow gap detected on coronal 2D TOF MRV (Fig 1). In 1 of these cases, the right transverse and sigmoid sinuses appeared prominent on the source images despite the loss of signal intensity (Fig 2) on the source and MIP images. Structural T1 and T2 MR demonstrated loss of the normal low signal intensity in the right transverse and sigmoid sinuses. However, CTV showed that the venous sinuses were patent and demonstrated no thrombus.
Flow void, stenosis, or absence of venous sinuses detected in 9 neonates who have had coronal 2D time-of-flight MR venography (TOF MRV) and CT venography (CTV)
| Coronal 2D TOF MRV ON MIP Images | Coronal 2D TOF MRV on Source Images | CTV | ||
|---|---|---|---|---|
| Superior sagittal sinus | Narrowing | 2 | 2 | 2 |
| Flow gap | 6 | 3 | 0 | |
| Right transverse sinus | Flow gap | 3 | 2 | 0 |
| Absent | 1 | 1 | 1 | |
| Left transverse sinus | Flow gap | 6 | 4 | 0 |
| Right sigmoid sinus | Flow gap | 3 | 2 | 0 |
| Left sigmoid sinus | Flow gap | 2 | 1 | 0 |
| Right internal jugular vein | Flow gap | 0 | 0 | 0 |
| Left internal jugular vein | Flow gap | 0 | 0 | 0 |
Note:—MIP indicates maximum intensity projections.
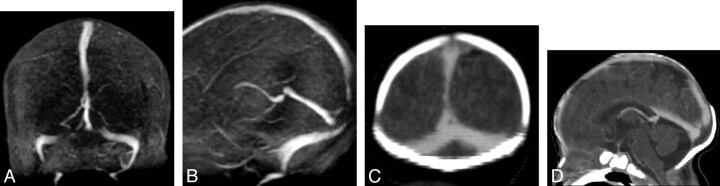
Fig 1.
Coronal (A) and sagittal (B) views from maximum intensity projection (MIP) of 2D TOF MRV demonstrate signal intensity void in the posterior aspect of superior sagittal sinus and medial aspect of transverse sinuses. On the coronal (C) and sagittal (D) reformatted images of the CTV, there is normal flow seen in the superior sagittal sinus and transverse sinuses, which confirms that the signal intensity void seen in the superior sagittal and transverse sinuses is artifactual.
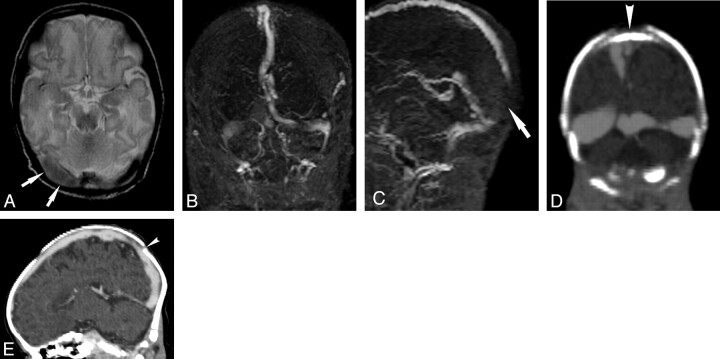
Fig 2.
A, Axial T2 shows loss of the normal flow void in the right transverse sinus (arrows). B, Coronal view of MIP from 2D TOF MRV shows loss of signal intensity in the right and left transverse sinuses. C, Sagittal view of MIP from 2D TOF MRV shows loss of signal intensity in the posterior aspect of superior sagittal sinus (arrow), which is clearly demonstrated despite image degradation by motion. The appearance is suspicious of artifactual flow gap in the transverse sinuses and superior sagittal sinus caused by in-plane saturation, possibly exacerbated by skull molding. Coronal (D) and sagittal (E) reformatted images of CTV show that the transverse and superior sagittal sinuses are patent. This confirms that the flow gaps seen in the venous sinuses on TOF MRV are artifactual. There is, however, persistent narrowing of the superior sagittal sinus in the region where the lambdoid sutures converge (arrowhead), which suggests that skull molding may play a role in narrowing the caliber of the superior sagittal sinus, thereby increasing the likelihood of demonstrating flow gaps in the superior sagittal sinus on 2D TOF MRV in neonates.
In 1 neonate, coronal 2D TOF MRV demonstrated flow gap in the posterior third of superior sagittal sinus. Axial 2D TOF MRV confirmed the presence of flow within the posterior aspect of the superior sagittal sinus but showed persistent focal narrowing in the region of the convergence of lambdoid sutures (Fig 3). In another case, coronal 2D TOF MRV showed loss of high signal intensity in the right transverse sinus. The sagittal 2D TOF MRV demonstrated the presence of flow in the right transverse sinus. In 1 neonate, the coronal 2D TOF MRV performed with TE of 5.5 ms demonstrated flow gap in the posterior aspect of the superior sagittal sinus, left transverse sinus medially, and the left sigmoid sinus distally. In contrast, a repeat coronal 2D TOF MRV performed with shorter TE (4.9 ms) demonstrated focal narrowing of the superior sagittal sinus in the region where the lambdoid sutures meet. The transverse and sigmoid sinuses were patent, though the left transverse and sigmoid sinuses were of smaller caliber compared with the right side (Fig 4).
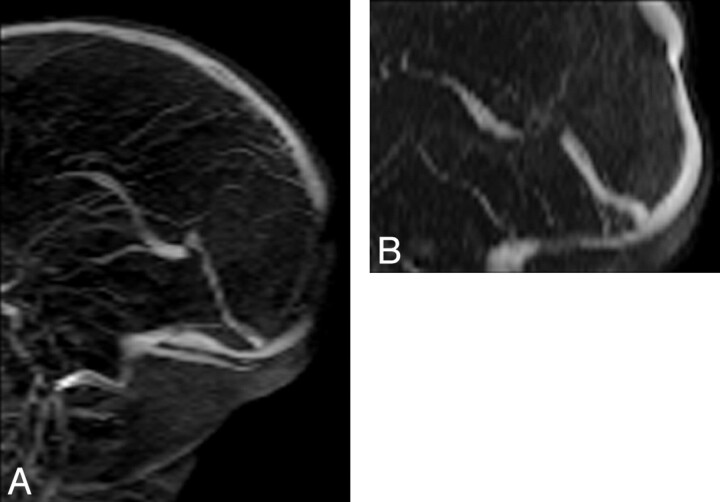
Fig 3.
A, Flow gap is seen in the posterior aspect of superior sagittal sinus on the coronal 2D TOF MRV. This flow gap is artifactual as a result of in-plane saturation of the imaging plane. This effect is negated by changing the plane of imaging. B, On the axial 2D TOF MRV, there is flow in the superior sagittal sinus. There is short segment stenosis of the superior sagittal sinus on axial 2D TOF MRV, in the region of the convergence of lambdoid suture, possibly as a result of skull molding and pressure effect in the supine position.
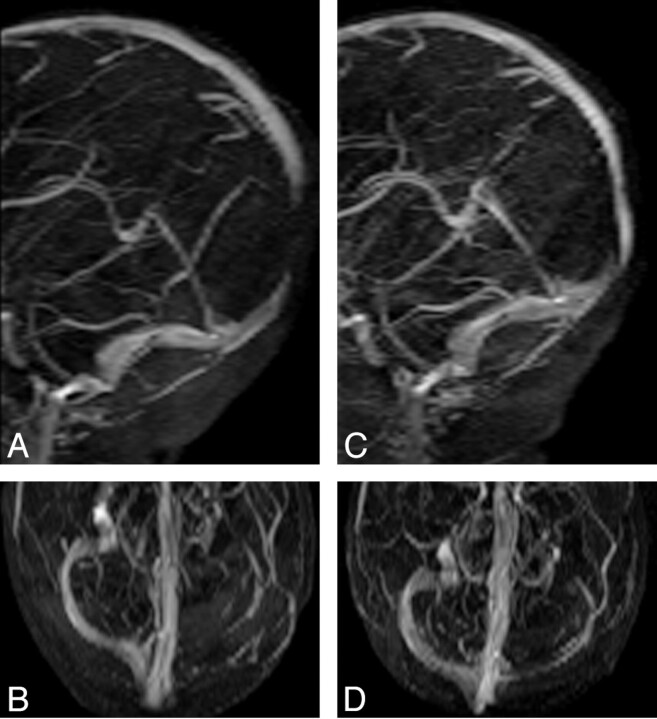
Fig 4.
A and B, MIP images of coronal 2D TOF MRV acquired with TE of 5.5 ms demonstrate flow gap in the superior sagittal sinus and left transverse sinus. The flow gap is artifactual as a result of in-plane saturation. C and D, MIP images of coronal 2D TOF MRV acquired with TE of 4.9 ms. Flow gap due to in-plane saturation is minimized by using shorter TE (4.9 ms) and thereby improves visualization of superior sagittal and transverse sinus. The left transverse sinus is smaller compared with the right transverse sinus but patent, possibly due to congenitally smaller caliber left transverse sinus.
There was no association between hypoxic-ischemic encephalopathy and flow void in the superior sagittal sinus (χ2 = 1.50, P > .05), right transverse sinus (χ2 = 0.07, P > .05), and left transverse sinus (χ2 = 0.03, P > .05).
Discussion
Cerebral venous sinus thrombosis in the neonatal period is probably more common than previously thought. The incidence of neonatal venous sinus thrombosis was estimated as 40.7 per 100,000 live births per year.4 Clinical signs are nonspecific, particularly in neonates. Most neonates presented with seizures (72%) and other nonspecific signs, including jitteriness and lethargy (59%).5–7 Therefore, the diagnosis of cerebral venous sinus thrombosis relies heavily on imaging. There have been several recent reports on the appearance of normal dural venous sinuses on MR venography in the pediatric population.1–3 The venous system in the pediatric population differs from that of the adult population. In this study, we present the first series of dural venous sinus study of TOF MRV in neonates.
Most flow gaps on 2D TOF MRV in the posterior aspect of superior sagittal sinus and transverse sinuses were likely to be related to saturation of in-flow or intravoxel dephasing rather than a true anatomic hypoplasia of the venous sinuses. Blood signal intensity is higher when the imaging plane is perpendicular to the direction of the blood flow. Saturation is induced if blood flow is parallel to the imaging plane. Because the bulk of the intracranial venous flow is in the anteroposterior direction, section acquisition in the coronal plane 2D TOF MRV allows better visualization of most of the cerebral venous sinuses. However, signal intensity loss can potentially become a problem in the region of the torcular Herophili, posterior aspect of the superior sagittal sinus, and transverse sinus, in that these segments are coplanar with the imaging plane. The flow gap seen on coronal TOF MRV was not present in the 2 cases when the imaging plane was altered to the axial or sagittal plane. In addition to the flow gap in the transverse sinuses, we have also found a large proportion of neonates with flow gap detected in the posterior third of the superior sagittal sinus (67%). Although flow gap in the posterior third of the superior sagittal sinus is a recognized potential problem of TOF MRV, this has not been reported in previous studies of TOF MRV in the pediatric population1,3 and has only been reported in the adult population.8
CTV was performed in 9 neonates. CTV is a rapid method to depict the intracranial venous circulation with consistent high quality.9 Compared with digital subtraction angiography, CTV has been found to be a reliable method to depict the cerebral venous structures.10 The advantages of CTV are that it is quick to perform, it is easier to monitor the sick neonates in the CT scanner, and it does not suffer from in-plane saturation of TOF MRV. The disadvantage of the CT is the radiation to the neonates. However, the CTV protocol that we have used was acquired from the postcontrast head CT. Therefore, no additional scan was performed to obtain the CTV, because we reconstructed the postcontrast head CT to thin axial sections and reformatted to the sagittal and coronal planes. We have found CTV to be a reliable method of assessing the venous sinuses in neonates, though formal studies are required to directly compare CTV and MRV in neonates. We have not used contrast-enhanced MR venography in neonates because we have found the technique to be ineffective. Although contrast-enhanced MRV is a reliable method of assessing the venous sinuses in children, we have not been able to achieve reliable contrast enhanced MRV in neonates, possibly because of the small volume of contrast used, inability to use a power injector, and poor signal-to-noise ratio as a result of small head size.
Of the 9 cases that had TOF MRV and CTV, the transverse and superior sagittal sinuses that demonstrated flow gaps on the TOF MRV were patent on the CTV. It is important to recognize that the flow gap in the posterior third of the superior sagittal sinus was due to in-plane saturation and to avoid mistaking this artifact for sinus thrombosis. In contrast to the flow gap in the superior sagittal sinus on coronal TOF MRV that reversed on the CTV, the focal narrowing of the superior sagittal sinus seen on coronal TOF MRV was also present on the axial TOF MRV and CTV. The focal narrowing seen in the region of the convergence of lambdoid suture could be due to skull molding and pressure effect of the occipital bone on the superior sagittal sinus in the supine position. In neonates, the smaller caliber venous sinuses and slower venous flow may have contributed to the high proportion of flow gap in the superior sagittal sinus. Molding of the occipital region of the skull from the dependent supine position in the head coil may compress the posterior portion of the superior sagittal sinus, thereby giving rise to focal narrowing in the region of the convergence of lambdoid suture. The compression of the superior sagittal sinus posteriorly results in increased heterogeneity and turbulent flow with resultant intravoxel dephasing and flow gap in the posterior aspect of superior sagittal sinus.
Alteration in the intracranial pressure is known to be associated with a change in the caliber of the dural venous sinuses. Disturbance of flow in the transverse sinuses has been found in idiopathic intracranial hypertension.11 Lateral sinus flow gap was more frequently seen in those with idiopathic intracranial hypertension compared with control subjects.12 In contrast, prominent venous dilation was more likely to be seen in those with spontaneous intracranial hypotension.13 Severe skull molding in infants may result in alteration of the venous sinus caliber. Low-birth-weight infants on positive pressure ventilation have been found to have an increased incidence of intracerebral hemorrhages, possibly as a result of altered venous drainage secondary to the strap used for mask attachment.14 In our study, straps were not used on the neonates during the MRV or CTV examination. Both the MRV and CTV were performed in the supine position. The supine position of the neonates may have exacerbated the skull molding, thereby causing the occipital bone to exert pressure on the superior sagittal sinus. The skull molding may have contributed to a reduction in the caliber and hence the flow of the superior sagittal sinus, resulting in increased flow gap.
All the MR and CT examinations were performed under sedation, therefore it is not possible to determine whether sedation has a confounding factor by altering the physiologic parameter of venous flow, thereby increasing the likelihood of detecting flow gap. A relatively large number of cases were imaged as a result of hypoxic-ischemic encephalopathy. In these cases, it is possible that global reduction of cerebral blood flow could lead to reduction of the venous blood flow in the dural venous sinuses and could therefore be a possible explanation of the observed flow gap in the posterior aspect of the superior sagittal sinus and transverse sinuses. However, analysis of this group has not supported the hypothesis that hypoxic-ischemic encephalopathy contributed to flow gap within the dural venous sinuses.
The physics of TOF MR angiography has been well described previously.15 2D TOF imaging is sensitive to slow flow. Nevertheless, there is a minimum threshold below which sufficient signal intensity from flowing blood cannot be obtained. To overcome the limitations of slow flow, the section thickness should be set as small as possible.16 Signal intensity loss can also result from intravoxel spin phase dispersion because of a wide spectrum of flow velocity in the voxel, higher orders of motion, and heterogeneity of the magnetic field. Intravoxel dephasing can be minimized using smaller voxels, shorter TE, and flow compensation.17 In 1 of the neonates, coronal 2D TOF MRV was repeated with a shorter TE that resulted in reversal of the flow gap in the superior sagittal sinus and transverse sinus. In this case, even a small reduction in TE of less than 1 ms resulted in considerable reduction in flow gap in the venous sinuses. Although it is generally recognized that shorter TE should be used to reduce intravoxel dephasing, the optimum TE for TOF MRV has not been defined. Inferring from data available on the effect of TE on carotid stenosis, we can assume that the shorter the TE, the lesser the artifact. TOF sequences with shorter TEs have reduced duration of spatial encoding sequence gradients and are therefore less sensitive to high-order motion that leads to better assessment of stenoses.18 Smith et al19 have found that TOF MR angiography with TE of 5.1 ms improved signal intensity recovery compared with TE of 6.5 ms. This effect was more pronounced in children than in adults. Therefore, these authors have recommended the use of a shorter TE TOF MR angiography specifically in children to improve signal intensity recovery and diagnostic evaluation.
The MIP ray-tracing algorithm can underestimate vessel caliber and overestimate stenosis. This is an inherent limitation of the MIP algorithm, in that lower intensity features of the vessels may be lost, leading to overestimation of flow gaps. In approximately half of the cases in which flow gap was noted in the superior sagittal sinus or transverse sinuses on the MIP images, the flow gap was less conspicuous or shorter on the source images. Rollins et al3 have found similar findings in that the discrepancy in the conspicuity of the transverse sinuses on MIP and source images were detected in slightly more than half of those with flow gaps in the transverse sinuses, and this was most pronounced in those less than 6 months of age. Therefore, the MIP images should be reviewed in conjunction with the source images.
With regard to phase contrast MRV, we have not been successful in using this effectively in neonates. Phase contrast MRV suffers from potential lack of sensitivity to slow flow if selected velocity encoding is incorrect. The velocity of blood flow in the dural sinuses in neonates differs from those of older children and adults. Color Doppler studies in neonates have shown that venous flow rates in the superior sagittal sinus are 9.2 mL/s,20 compared with the 20 cm3/s velocity encoding used for phase contrast MRV in adults. Another study by Fenton et al21 in neonates showed a wide range of velocities in the vein of Galen ranging from 2.3 to 9.5 cm/s. In addition, phase contrast MRV also suffers from gradient imperfections, eddy currents, and long acquisition times. This is more so in neonates, in that vendors often do not optimize such sequences for neonatal MR. It is feasible that with newer MR machines, and improved dedicated head coils for neonates, phase contrast MRV would be more effective in neonates.
There are major ethical limitations to the study of normal neonates using MR, in part due to the need to sedate or anesthetize the neonates to perform MR imaging. In this study, neonates with a variety of nonspecific symptoms have been imaged. It is possible that some of the symptoms could be attributed to venous sinus thrombosis. However, in cases with available comparative imaging data using CTV or modifications of the 2D TOF MRV technique, either by altering the plane of imaging or reducing the TE, the flow gaps seen on the TOF MRV were found to be technique-related and to occur in predictable locations. These flow gaps were much more commonly seen in neonates than in children or adults because of the unique anatomy and blood flow of the cerebral venous sinuses in neonates.
In this study, we have found that there was a high proportion of flow gap in the transverse sinuses, in 62% of cases in the left transverse sinus, and 25% of cases in the right transverse sinus. Flow gaps were seen in the transverse sinus in 31% of normal adults and were common in the nondominant transverse sinus.8 In children younger than 2 years of age, Rollins et al3 found signal intensity loss in the nondominant transverse sinus in 24 (63%) of 38 on the MIP images. The flow gap detected in the transverse sinuses in this series occurred at the medial portion of the sinuses. We have found that dominance of the right transverse sinus occurred in 32 (63%) of 51, dominance of the left transverse sinus in 5 (10%) of 51, and codominant transverse sinuses in 14 (27%) of 51. Our results were similar to those reported by Ayanzen et al.16 They have found that in their cohort of patients ranging in age from 9 days to 83 years, the right transverse sinuses were dominant in 59%, the left transverse sinuses were dominant in 25%, and the sinuses were codominant in 16%. In contrast, Rollins et al3 have found that a greater proportion of their patients younger than 2 years have codominance of the transverse sinuses.
The occipital sinuses were detected in 18% of cases in this series. Widjaja et al1 have found that the occipital sinuses were present in 18% of children on the TOF MRV; 56% of those were in children younger than 2 years. Rollins et al3 have found that persistent occipital sinuses were present in 13% of their cohort <25 months of age but only 2% of children older than 5 years. The apparent age-related involution of the occipital sinus could be attributed to alteration in the posterior fossa venous flow from the supine to the erect position.1
Conclusion
We have found that two thirds of cases demonstrated flow gap in the posterior aspect of superior sagittal sinus and in the medial aspect of transverse sinus. Although flow gap in the venous sinuses is a recognized potential problem of 2D TOF MRV due to in-plane saturation, flow gap in the posterior aspect of superior sagittal sinus is less well described, particularly in the neonatal population. The proportion of flow gap in the venous sinuses of neonates was much higher than those reported in the pediatric or adult population. This could be attributed to the smaller caliber venous sinuses and slower venous flow, possibly exacerbated by molding of the skull.
Footnotes
This work was funded by the Department of Medical Imaging research award, University of Toronto.
References
- 1.Widjaja E, Griffiths PD. Intracranial MR venography in children: normal anatomy and variations. AJNR Am J Neuroradiol 2004;25:1557–62 [PMC free article] [PubMed] [Google Scholar]
- 2.Rollins N, Ison C, Reyes T, et al. Cerebral MR venography in children: comparison of 2D time-of-flight and gadolinium-enhanced 3D gradient-echo techniques. Radiology 2005;235:1011–17 [DOI] [PubMed] [Google Scholar]
- 3.Rollins N, Ison C, Booth T, et al. MR venography in the pediatric patient. AJNR Am J Neuroradiol 2005;26:50–55 [PMC free article] [PubMed] [Google Scholar]
- 4.Shroff M, deVeber G. Sinovenous thrombosis in children. Neuroimaging Clin N Am 2003;13:115–38 [DOI] [PubMed] [Google Scholar]
- 5.deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med 2001;345:417–23 [DOI] [PubMed] [Google Scholar]
- 6.Rivkin MJ, Anderson ML, Kaye EM. Neonatal idiopathic cerebral venous thrombosis: an unrecognized cause of transient seizures or lethargy. Ann Neurol 1992;32:51–56 [DOI] [PubMed] [Google Scholar]
- 7.Shevell MI, Silver K, O’Gorman AM, et al. Neonatal dural sinus thrombosis. Pediatr Neurol 1989;5:161–65 [DOI] [PubMed] [Google Scholar]
- 8.Lewin JS, Masaryk TJ, Smith AS, et al. TOF intracranial MRV: evaluation of the sequential oblique section technique. AJNR Am J Neuroradiol 1994;15:1657–64 [PMC free article] [PubMed] [Google Scholar]
- 9.Casey SO, Alberico RA, Patel M, et al. Cerebral CT venography. Radiology 1996;198:163–70 [DOI] [PubMed] [Google Scholar]
- 10.Wetzel SG, Kirsch E, Stock KW, et al. Cerebral veins: comparative study of CT venography with intraarterial digital subtraction angiography. AJNR Am J Neuroradiol 1999;20:249–55 [PMC free article] [PubMed] [Google Scholar]
- 11.Fera F, Bono F, Messina D, et al. Comparison of different MR venography techniques for detecting transverse sinus stenosis in idiopathic intracranial hypertension. J Neurol 2005;252:1021–25 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JN, Gillard JH, Owler BK, et al. MR venography in idiopathic intracranial hypertension: unappreciated and misunderstood. J Neurol Neurosurg Pshychiatry 2004;75:621–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roll JD, Larson TC 3rd, Soriano MM, et al. Cerebral angiographic findings of spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2003;24:707–08 [PMC free article] [PubMed] [Google Scholar]
- 14.Pape KE, Armstrong DL, Fitzhardinge PM. Central nervous system pathology associated with mask ventilation in the very low birthweight infant: a new etiology for intracerebellar hemorrhages. Pediatrics 1976;58:473–83 [PubMed] [Google Scholar]
- 15.Saloner D. The AAPM/RSNA physics tutorial for residents. An introduction to MR angiography. Radiographics 1995;15:453–64 [DOI] [PubMed] [Google Scholar]
- 16.Ayanzen RH, Bird CR, Keller PJ, et al. Cerebral MR venography: normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol 2000;21:74–78 [PMC free article] [PubMed] [Google Scholar]
- 17.Liauw L, van Buchem MA, Spilt A, et al. MR angiography of the intracranial venous system. Radiology 2000;214:678–82 [DOI] [PubMed] [Google Scholar]
- 18.Lev MH, Romero JM, Gonzalez RG. Flow voids in time-of-flight MR angiography of carotid artery stenosis. It depends on the TE! AJNR Am J Neuroradiol 2003;24:2120. [PMC free article] [PubMed] [Google Scholar]
- 19.Smith AS, Haacke EM, Lin W, et al. Short versus long echo time for cranial MR angiography in children & adults. AJNR Am J Neuroradiol 1994;15:1557–64 [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor GA. Intracranial venous sytem in the new born: evaluation of normal anatomy and flow characteristics with color Doppler US. Radiology 1992;183:449–52 [DOI] [PubMed] [Google Scholar]
- 21.Fenton AC, Papathoma E, Evans DH, et al. Neonatal cerebral venous flow velocity measurement using a color flow Doppler system. J Clin Ultrasound 1991;19:69–72 [DOI] [PubMed] [Google Scholar]