Abstract
SUMMARY: Fetal MR imaging is an increasingly available technique used to evaluate the fetal brain and spine. This is made possible by recent advances in technology, such as rapid pulse sequences, parallel imaging, and advances in coil design. This provides a unique opportunity to evaluate processes that cannot be approached by any other current imaging technique, and it affords a unique opportunity for studying in vivo brain development and early diagnosis of congenital abnormalities inadequately visualized or undetectable by prenatal sonography. This 2-part review summarizes some of the latest developments in MR imaging of the fetal brain and spine and its application to prenatal diagnosis. The first part discussed the utility, safety, and technical aspects of fetal MR imaging; the appearance of normal fetal brain development; and the role of fetal MR imaging in the evaluation of fetal ventriculomegaly. In this second part, we focus on additional clinical applications of fetal MR imaging, including suspected abnormalities of the corpus callosum, malformations of cortical development, and spine abnormalities.
Technical advances in fetal MR imaging have made it an increasingly important diagnostic tool in the clinical evaluation of fetuses with suspected central nervous system (CNS) abnormalities. Because of its higher contrast resolution compared with prenatal sonography, fetal MR imaging allows better visualization of both normal in utero brain development as well as abnormalities therein. Moreover, its ability to detect sonographically occult structural abnormalities, such as cerebral malformations and destructive lesions, have made it an invaluable tool in cases in which an abnormality is suspected on the prenatal sonogram. It is particularly helpful in evaluating the brain in cases of sonographically diagnosed ventriculomegaly (see Part 1) as well as in suspected abnormalities of the corpus callosum and posterior fossa (see below).
Fetal MR imaging is also used in pregnancies in which the fetus is at increased risk for brain abnormalities, such as in families with a history of a prior child or fetus with CNS anomalies or a genetic disorder. In these cases, fetal MR imaging is performed even in the setting of a normal prenatal sonogram, because brain malformations can be difficult to detect with sonography. Because complications of monochorionic twinning, such as twin-twin transfusion syndrome and co-twin demise, are associated with higher risk of neurodevelopmental disabilities,1 fetal MR imaging is also used to evaluate the brain in such cases, even with a normal prenatal ultrasound of the brain. Additional indications for fetal MR imaging include maternal illness, such as maternal infection or major cardiac event. With recent advances in fetal surgical techniques, fetal MR imaging is being increasingly used before surgical intervention. In cases of Chiari II malformations, fetal MR imaging is used to identify the severity of the hindbrain malformation, the presence of associated brain and spine developmental abnormalities, and the presence of destructive lesions. In cases of twin-twin transfusion syndrome, fetal MR imaging is used to evaluate the fetal brain both before and after any surgical intervention.
Fetal MR imaging can also be useful in situations in which the fetal brain is difficult to image by sonography. These include cases of decreased amniotic fluid, large maternal body habitus, difficult position of the fetal head, and advanced gestational age, where shadowing from the calvarium can interfere with ultrasound images.
Clinical Applications of Fetal MR Imaging
Abnormalities of the Corpus Callosum
The corpus callosum is 1 of 3 major commissures that connect the cerebral hemispheres. It develops as the growth cones of developing axons navigate through the developing cerebral hemispheres and across the midline between gestational weeks 8 and 20.2 Developmental abnormalities of the corpus callosum include agenesis, hypogenesis (or partial agenesis), dysgenesis, hypoplasia, and destruction. The exact incidence of callosal agenesis is not known, though it has been estimated at 0.2%–0.7% in the general population and 3% in mentally disabled patients.3 Although callosal agenesis has been reported as an incidental finding, it is likely that most patients manifest variable neurologic symptoms, including developmental delay, cognitive impairment, and epilepsy.3–6
Callosal anomalies are highly associated with other CNS anomalies as well as specific syndromes such as Aicardi syndrome.3,7,8 On autopsy, 85% of patients with callosal agenesis have additional CNS anomalies, and 62% have additional extra-CNS anomalies.8 Brain abnormalities seen in association with callosal agenesis include Chiari II malformation, Dandy-Walker malformation, gray matter heterotopia, holoprosencephaly, schizencephaly, and encephaloceles.9 The detection of associated anomalies is significant in that these patients tend to have a higher incidence of neurodevelopmental disabilities.8,10
Abnormalities of the corpus callosum can be difficult to identify on sonography, particularly with advanced gestational age. Direct visualization of the entire corpus callosum on a midline sagittal sonographic image is very difficult. Indirect signs of callosal anomalies on sonography include lack of visualization of the cavum septi pellucidi, enlarged atria and occipital horns resulting in a teardrop configuration of the lateral ventricles, a high-riding third ventricle, and radiating medial hemispheric sulci.
Fetal MR imaging adds considerably to the evaluation of the fetal corpus callosum because it can be directly visualized in the sagittal and coronal planes after 20 weeks, and associated anomalies are more easily detected compared with sonography. Fetal MR imaging has been reported to identify an intact corpus callosum in approximately 20% of cases referred for sonographically suspected callosal abnormalities (greater specificity)11 (Fig 1). Fetal MR imaging has also been reported to have a greater sensitivity in the detection of callosal agenesis compared with prenatal sonography.12
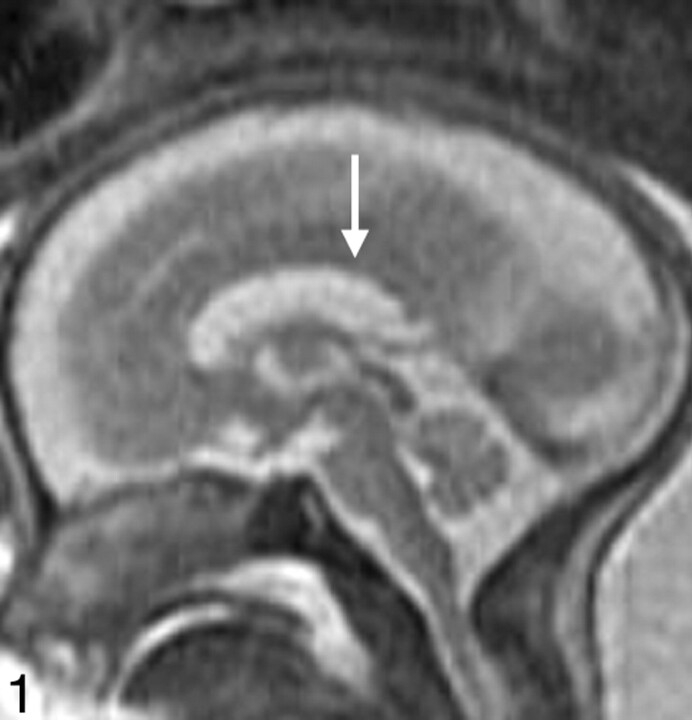
Fig 1.
Midline sagittal SS-FSE T2-weighted image demonstrates the normal appearance of the corpus callosum in a 26-gestational-week-old fetus (arrow). Obtaining a nonoblique midline sagittal image is critical for evaluating the corpus callosum. Note the uniform thickness of the corpus callosum.
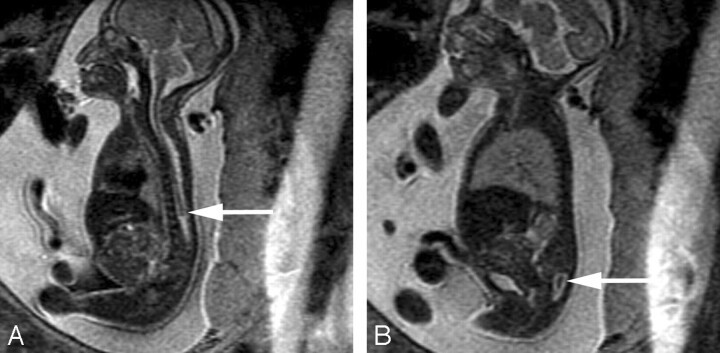
The identification of associated brain anomalies in callosal agenesis is also greater with fetal MR imaging than with prenatal sonography.11–15 Sonographically occult brain abnormalities can be detected in up to 63% of fetal MR imaging cases.11,15,16 These include anomalies of sulcation and gyration, periventricular nodular heterotopia, cerebellar abnormalities, and brain stem anomalies (Fig 2). In 25% of callosal agenesis cases, additional findings identified by fetal MR imaging can lead to the diagnosis of a specific disorder or syndrome; this has implications for the prognosis of the current pregnancy and recurrence risk in future pregnancies11 (Fig 3).
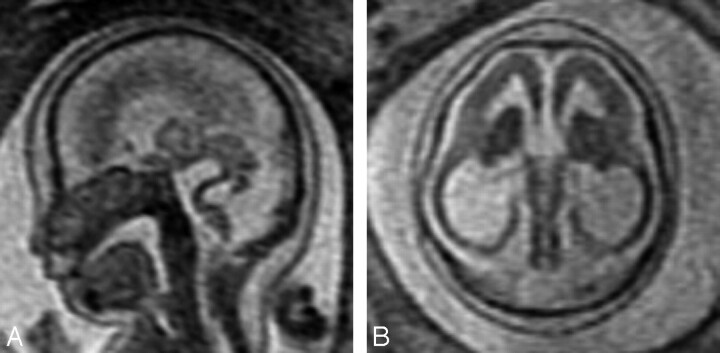
Fig 2.
A, Sagittal SS-FSE T2-weighted image in a 25-gestational-week-old fetus demonstrates agenesis of the corpus callosum as well as a small pons.
B, Axial SS-FSE T2-weighted image demonstrates abnormally shallow Sylvian fissures for gestational age.
Fig 3.
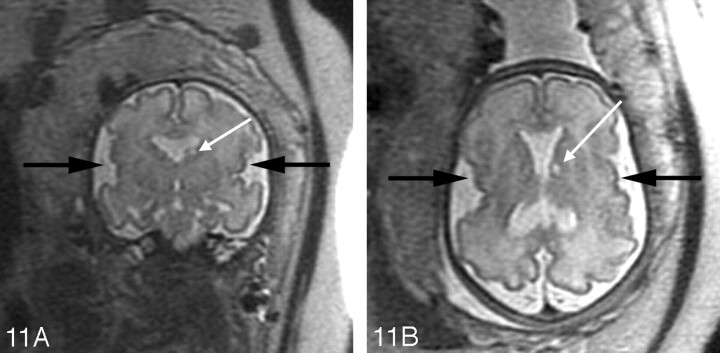
A, Axial SS-FSE T2-weighted image in a 19-gestational-week-old fetus referred for callosal agenesis demonstrates thinning of the right frontal parenchyma with several abnormal infoldings of the developing cortex (arrow) consistent with polymicrogyria.
B, Coronal SS-FSE T2-weighted image confirms multiple abnormal cortical infoldings (black arrow) and also demonstrates several areas of nodularity (white arrow) along the dilated right frontal horn consistent with periventricular nodular heterotopia. Microphthalmia of the right globe (arrowhead) is also present.
C, Axial SS-FSE T2-weighted image demonstrates abnormal morphology of the fourth ventricle with small, dysgenetic cerebellar hemispheres bilaterally. This constellation of findings led to the prenatal diagnosis of Aicardi syndrome.
Posterior Fossa Abnormalities
Posterior fossa abnormalities evaluated by fetal MR imaging include Dandy-Walker malformation, Dandy-Walker variant, mega cisterna magna, arachnoid cyst, Blake pouch cyst, cerebellar dysplasia, cerebellar hypoplasia, cerebellar hemorrhage, Walker-Warburg syndrome, and Chiari II malformation (discussed in the section below).12–14,17–20 Agenesis or hypoplasia of the cerebellar vermis in association with an enlarged posterior fossa, elevated tentorium, and cystic dilation of the fourth ventricle is diagnostic of the Dandy-Walker malformation.21 Milder forms of vermian hypoplasia with a more normal-appearing posterior fossa have been referred to as the Dandy-Walker variant, though the terms Dandy-Walker and Dandy-Walker variant have at times been confused.21 Blake pouch cyst has also been referred to as Dandy-Walker variant.22,23 Although sonography can easily identify severe Dandy-Walker malformations, it can be more limited in distinguishing mild forms of vermian hypoplasia from a mega cisterna magna or an arachnoid cyst (Fig 4). Moreover, sonographic visualization of the posterior fossa contents is limited by ossification of the skull in the third trimester. In these cases, fetal MR imaging can play an important diagnostic role.
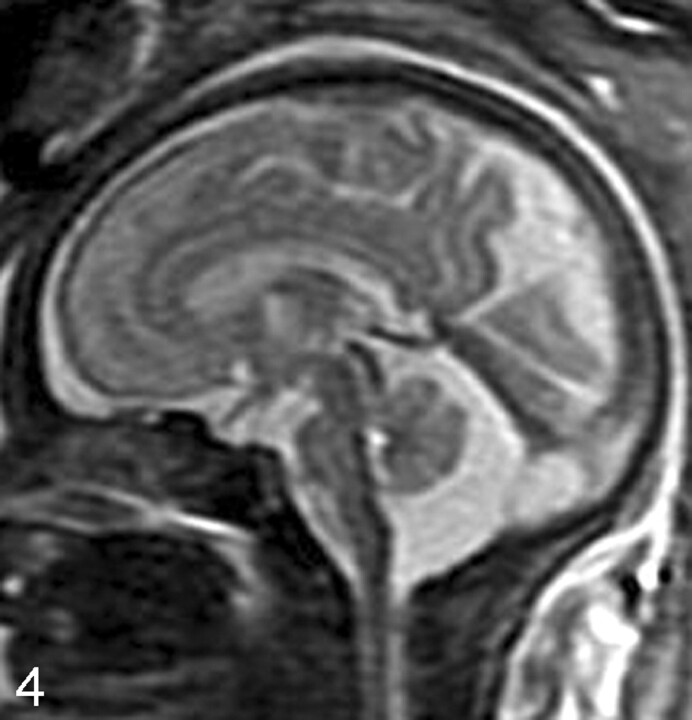
Fig 4.
Sagittal SS-FSE T2-weighted image in a 33-gestational-week-old fetus demonstrates a large cisterna magna. The vermis is identified and is normal in size, excluding the diagnosis of a Dandy-Walker malformation.
Fetal MR imaging can be helpful in determining the shape and size of the vermis in cases of sonographically suspected vermian hypoplasia. The identification of an intact vermis can help to differentiate between a Dandy-Walker variant and a mega cisterna magna. Fetal MR imaging can also be helpful in evaluating the relationship between a posterior fossa cyst and the fourth ventricle. The cerebellar hemispheres and brain stem can also be evaluated by fetal MR imaging (Fig 5). In cases in which both supratentorial and infratentorial abnormalities are suspected by prenatal sonography, fetal MR imaging can assist in better delineating the abnormalities and in assigning a diagnosis (Fig 6).
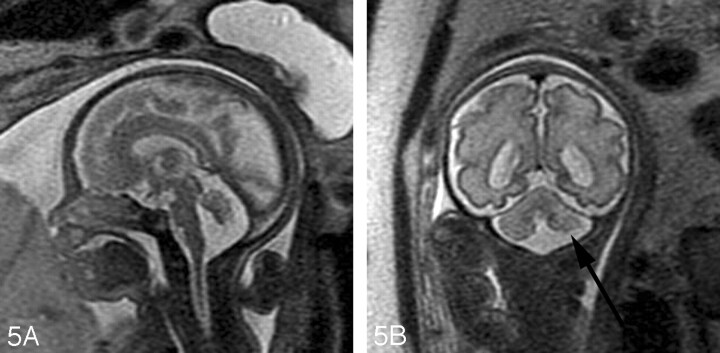
Fig 5.
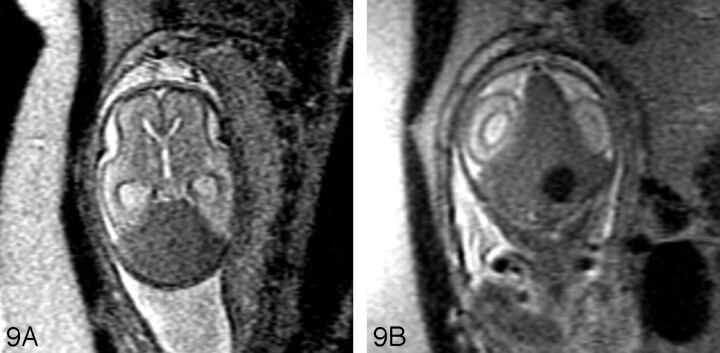
A, Sagittal SS-FSE T2-weighted image in a 29-gestational-week-old fetus demonstrates hypoplasia of the vermis.
B, Coronal SS-FSE T2-weighted image demonstrates small, slightly dysmorphic left cerebellar hemisphere (arrow).
Fig 6.
A, Sagittal SS-FSE T2-weighted image in a 23-gestational-week-old fetus demonstrates abnormally thin brain stem with kinking at pontomesencephalic junction. Callosal and vermian agenesis was detected by prenatal sonography and is also present on the MR imaging.
B, Axial SS-FSE T2-weighted image demonstrates abnormally thin and homogeneous parenchyma with absent Sylvian fissures. The deep gray nuclei appear small and dysplastic as well.
This constellation of findings led to the prenatal diagnosis of Walker-Warburg syndrome.
When a Dandy-Walker malformation is identified, fetal MR imaging can be used to detect additional CNS abnormalities, which are common in these patients and include agenesis of the corpus callosum, polymicrogyria, neuronal heterotopia, and occipital encephaloceles.24–26 Studies have shown that the presence of additional anomalies in children with Dandy-Walker malformations is associated with a worse outcome.24–26 In our experience, fetal MR imaging is particularly valuable in identifying supratentorial anomalies that can be seen in Dandy-Walker malformation and that can affect the prognosis of the fetus (Fig 7). Neurodevelopmental outcome may also be related to the degree of vermian dysplasia.27,28
Fig 7.
A, Sagittal SS-FSE T2-weighted image in 39-gestational-week-old fetus demonstrates vermian hypoplasia and enlarged posterior fossa with cyst, consistent with a Dandy-Walker malformation. An occipital meningocele is also identified.
B, Coronal SS-FSE T2-weighted image demonstrates diffusely abnormal sulcal pattern for gestational age, with areas of polymicrogyria (arrow). There is associated diminished white matter volume and ventriculomegaly.
Fetal MR imaging is also helpful in evaluating echogenic posterior fossa masses. Posterior fossa hemorrhages are typically bright on T1-weighted images, and dark on T2-weighted images (Fig 8). Fetal MR imaging can be used to identify the location of the hemorrhage (intra-axial versus extra-axial) and to assess the integrity of the cerebellar hemispheres, vermis, and brain stem. Both extra-axial posterior fossa hemorrhages and intraparenchymal cerebellar hemorrhages can be related to maternal/fetal trauma29 and to vascular malformations.30,31 Dural arteriovenous fistula can result in fetal subdural hematoma (Fig 9). Vascular causes of intraparenchymal cerebellar hemorrhage include germinal matrix hemorrhages and underlying vascular malformations, such as cavernous malformations.20 Cerebellar capillary telangiectasias can be visible on prenatal sonography and yet difficult to detect by fetal MR imaging.30 In these cases, gradient echo T2*-weighted imaging may be helpful to look for associated blood products. Although uncommon, cerebellar hemorrhage has also been reported in cases of infection with cytomegalovirus32,33 and in both immune and nonimmune hydrops fetalis,34,35 presumably from associated hematological and/or hemodynamic abnormalities. When a cerebellar hemorrhage is identified, infection should be considered, and a careful search for other abnormalities (eg, intrauterine growth retardation, microcephaly, calcifications, echogenic bowel, and hydrops fetalis) should be initiated.
Fig 8.
A, Axial SS-FSE T2-weighted image demonstrates an area of increased signal intensity with peripheral decreased signal intensity in the right cerebellar hemisphere (arrow).
B, Axial FMP-SPGR T1-weighted image at corresponding level shows areas of increased signal intensity consistent with cerebellar hemorrhage (arrow). Bilobed appearance of the hematoma on both T1- and T2-weighted images suggests possible involvement of the cerebellar vermis.
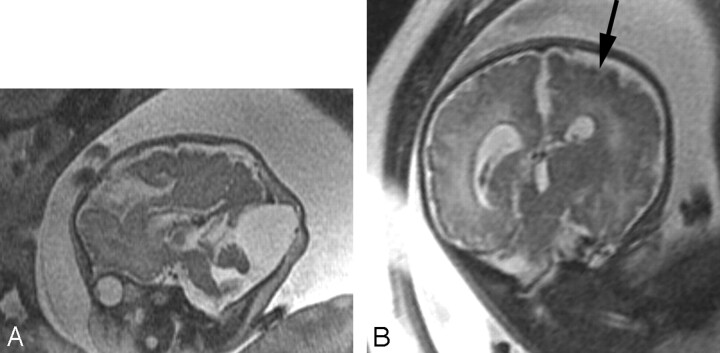
Fig 9.
SSFSE T2-weighted image of 24-gestational-week-old fetus with a large hypointense hematoma in the posterior fossa on both axial (A) and coronal (B) views. The hematoma displaces the cerebellum anteriorly and appears subdural in location. An ovoid more hypointense area is concerning for an abnormal vessel. A dural arteriovenous fistula was found at autopsy.
Malformations of Cortical Development
Because of its ability to better image certain aspects of brain development, fetal MR imaging has made a significant contribution to the prenatal detection of malformations of cortical development (MCDs). Studies have shown that fetal MR imaging can detect cortical malformations that are not identified on sonography.11,12,36,37 Fetal MR imaging was superior to sonography in identifying schizencephaly, lissencephaly, polymicrogyria, and gray matter heterotopia in a study of 20 fetuses with MCDs confirmed on either postnatal imaging or autopsy.12
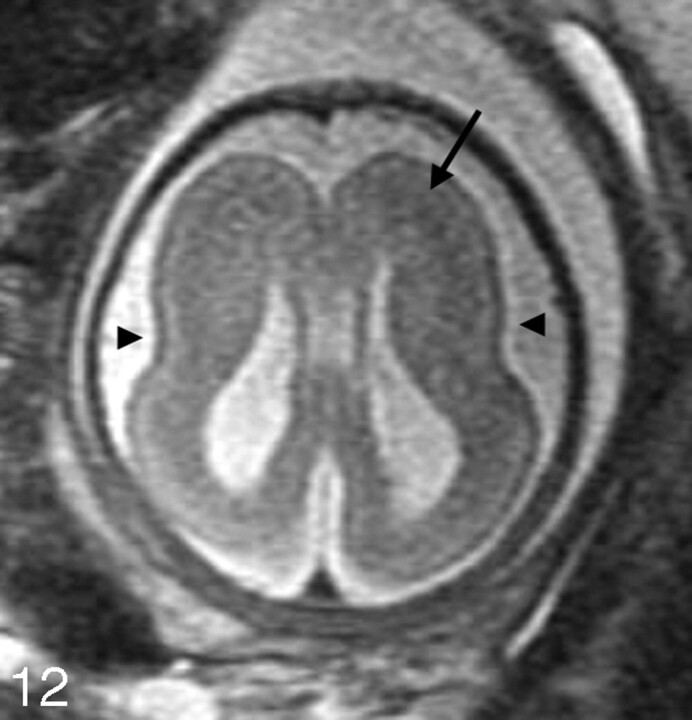
The imaging appearance of MCDs in the fetus is often similar to that in the child. Subependymal heterotopia appear as nodules that are isointense to the germinal matrix and are located along the ventricular walls. As discussed in Part 1, they cannot be reliably distinguished from subependymal nodules seen in tuberous sclerosis; therefore, it is important to search for other manifestations of tuberous sclerosis, such as cortical hamartomas (which are hypointense compared with normal unmyelinated white matter on the single-shot fast spin-echo [SS-FSE] images) and cardiac rhabdomyomas. Schizencephaly appears as a gray matter-lined cleft extending from the ventricle to the subarachnoid space (Fig 10). Polymicrogyria appears as localized and/or generalized absence of normal sulcation with multiple abnormal infoldings of the affected cortex (Fig 11). Classical lissencephaly can be identified by the shallow appearance of the Sylvian fissures, reduced number or complete absence of additional sulci for the expected gestational age of the fetus, absence of normal multilayered appearance of the brain, and, instead, a large thick band of arrested neurons within the developing white matter (Fig 12). It is important, therefore, to be familiar with the normal appearance of the fetal brain at different gestational ages to be able to identify MCDs.
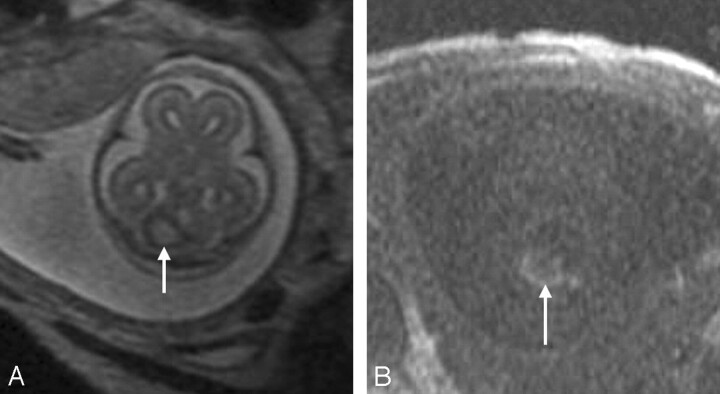
Fig 10.
Coronal SSFSE T2-weighted image in a 33-gestational-week-old fetus demonstrates bilateral open lip schizencephalic defects (arrows). The adjacent sulcal pattern is abnormal. The septum pellucidum is absent.
Fig 11.
A, Coronal SS-FSE T2-weighted image in a 30-gestational-week-old fetus demonstrates dysplastic-appearing Sylvian fissures with multiple abnormal small infoldings of the cortex (black arrows) consistent with perisylvian polymicrogyria. Absence of the septum pellucidum is present, and was noted on the prenatal sonogram. A hyperintense focus (white arrow) is seen adjacent to the left lateral ventricle in the region of the caudate head, consistent with an area of injury.
B, Axial images confirms perisylvian polymicrogyria (black arrows) and periventricular cyst (white arrow).
Fig 12.
Axial SS-FSE T2-weighted image in a 34-gestational-week-old fetus demonstrates minimal formation of the Sylvian fissures (arrowheads) consistent with classical lissencephaly. A thick band of low signal intensity (arrow) is seen in the developing fetal white matter consistent with arrested migration of neurons.
Complications of Monochorionic Twin Pregnancies
Fetal MR imaging is often used to identify parenchymal injury in monochorionic twin pregnancies, which are at increased risk for brain injury. Monochorionic twins share a common placenta, which may contain abnormal intertwin vascular connections. These abnormal vascular connections can result in abnormalities of blood flow to the fetuses; as a result, the morbidity and mortality of monochorionic twins is much higher than that of diamniotic twins.1 Twin-twin transfusion syndrome (TTTS) is characterized by abnormal blood flow from the smaller donor twin to the larger recipient twin via placental vascular connections. The recipient twin develops polyhydramnios because of volume overload, whereas the donor twin develops oligohydramnios, resulting in a “stuck twin.” The exact pathophysiology underlying TTTS is complex; however, it seems to be related to the types of intertwin vascular connections.38 The morbidity of TTTS is very high, and both twins are at risk for cerebral ischemia. Approximately half of surviving twins experience neurodevelopmental abnormalities and manifest abnormalities on neonatal head sonography.39 Because of the high morbidity and mortality of TTTS, these fetuses are often imaged to look for any areas of brain injury. Although imaging the “stuck” oligohydramniotic twin can be somewhat easy, polyhydramnios can lead to excessive motion of the recipient twin; acquiring adequate images requires patience and, sometimes, luck.
Another complication of monochorionic twinning is co-twin demise. In utero death of a co-twin is associated with increased risk of neurologic impairment in the surviving co-twin,40 perhaps because of resultant acute cerebral hypoperfusion or thromboembolic events in the surviving co-twin.40
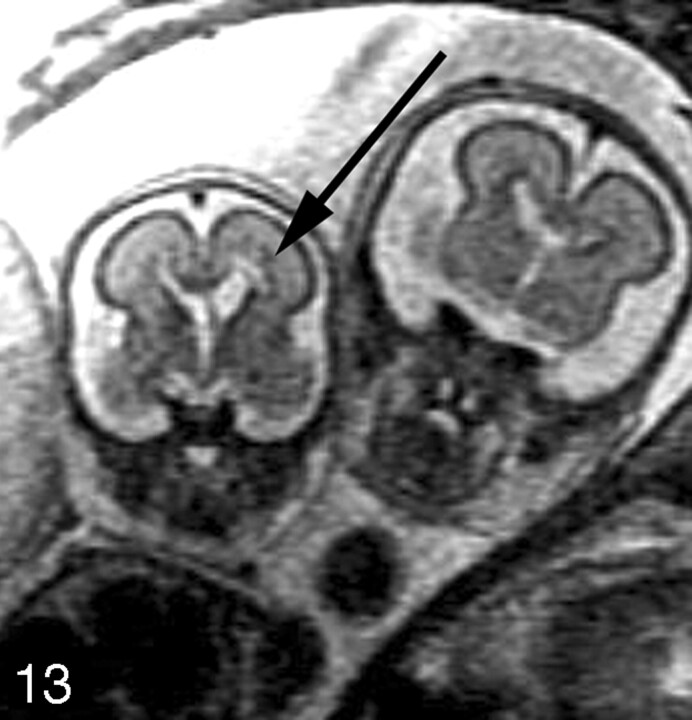
Multiple brain abnormalities have been described in monochorionic twin pregnancies complicated by TTTS and/or co-twin demise.40–54 Fetal MR imaging can detect hypoxic-ischemic parenchymal lesions such as those that may occur after co-twin demise12,13,55 or as a result of TTTS. Ischemic parenchymal injuries are seen on fetal MR imaging as focal or diffuse areas of increased T2 signal intensity in the germinal matrix, developing white matter, and/or cerebral cortex (Fig 13). Moreover, ischemic injury can lead to malformations of cortical development, such as polymicrogyria (Fig 14).53,54 We have found that these injuries are best visualized if imaging is performed at least 2 weeks after the presumed event. Future advances in fetal diffusion-weighted imaging may lead to earlier detection of parenchymal injury.
Fig 13.
Coronal SS-FSE T2-weighted image demonstrates a 24-week twin gestation complicated by twin-twin transfusion syndrome requiring in utero laser ablation of placental vessels. The smaller, donor, twin has focal area of hyperintensity (arrow) adjacent to the left frontal horn, with ex vacuo enlargement of the frontal horn. Periventricular white matter injury with coagulative necrosis was found at autopsy.
Fig 14.
A, Axial SS-FSE T2-weighted image of a 23-gestational-week-old fetus in a monochorionic twin pregnancy complicated by co-twin demise. There is a large area of encephalomalacia (arrow) involving the left frontal and parietal lobes.
B, Coronal SS-FSE T2-weighted image demonstrates several infoldings of the developing cortex (arrow) consistent with polymicrogyria. Findings were confirmed by postnatal MR imaging.
Congenital Infections
The most common cause of congenital viral infection is cytomegalovirus.56 Other viruses that can also affect the fetal brain include varicella zoster virus, parvovirus B19, rubella, and lymphocytic choriomeningitis virus.57 The parasite Toxoplasma gondii can also result in fetal brain abnormalities. Because of current practice standards in prenatal serologic screening in the United States (which often includes screening of rubella and varicella antibody status but not of cytomegalovirus or toxoplasmosis), fetal MR imaging is not commonly performed for congenital infections. When an abnormality is identified on screening prenatal sonography, however, clinical evaluation will often include maternal and/or fetal testing for congenital infections such as cytomegalovirus and toxoplasmosis. Because of different prenatal screening practices in Europe, however, congenital infection (primarily cytomegalovirus and toxoplasmosis) is a frequent indication for fetal MR imaging.57
Although sonography can identify certain abnormalities that are associated with congenital infection (eg, intrauterine growth retardation, microcephaly, intracranial calcifications, echogenic bowel, and hydrops fetalis), fetal MR imaging is helpful in identifying abnormalities of the developing brain parenchyma and cortex (Fig 15). Brain injury and malformations are more commonly seen in infections due to cytomegalovirus compared with toxoplasmosis.57 Areas of chronic parenchymal injury typically appear bright on T2-weighted images and can be focal or diffuse. Chronic injury can also manifest as volume loss and ventricular enlargement. Hemorrhages have also been observed in association with infections such as cytomegalovirus and parvovirus B19 infection. Other manifestations of congenital infection, such as cortical malformations, including lissencephaly and polymicrogyria, and delayed cortical maturation, can also be better assessed with fetal MR imaging. Acute brain injury is rarely detected by fetal MR imaging, though advances in fetal diffusion-weighted imaging may lead to the identification of areas of acute injury.
Fig 15.
Axial SS-FSE T2-weighted image of a 30-gestational-week-old fetus with congenital cytomegalovirus infection. The image shows polymicrogyria, manifested as a deep infolding of abnormal thick cortex (black arrow) in the right posterior frontal lobe and as too many, abnormally shallow sulci over both frontal convexities (white arrows). In addition, there is abnormal hyperintensity of the right frontal white matter.
Myelomeningocele and Other Spine Anomalies
One of the more common fetal spinal anomalies detected by prenatal sonography is the myelomeningocele, identified by the absence of posterior elements of the vertebral bodies at affected levels and extension of the subarachnoid space posteriorly through the bony spina bifida. Myelomeningoceles are almost always seen in combination with a small posterior fossa and herniation of cerebellar tissue into the cervical subarachnoid space; this hindbrain malformation is referred to as the Chiari II malformation. In general, Chiari II malformations and myelomeningoceles can be easily diagnosed on prenatal ultrasound. However, fetal MR imaging can be a helpful adjunct when sonography analysis is limited, such as in cases of large maternal body habitus, oligohydramnios, low position of the fetal head, or when the fetal spine is positioned posteriorly with respect to the mother.
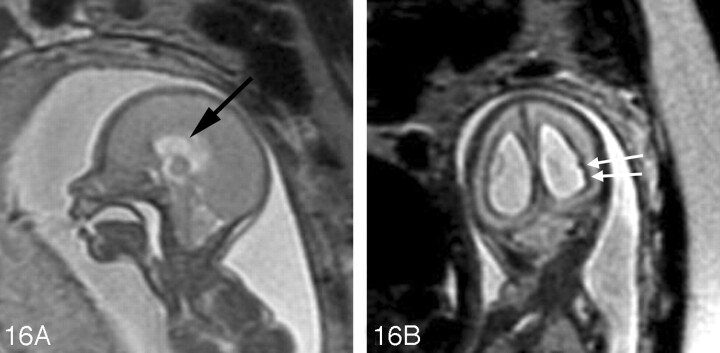
Fetal MR imaging is also useful in identifying additional CNS anomalies that are frequently associated with myelomeningoceles/Chiari II malformations, such as callosal agenesis/hypogenesis, periventricular nodular heterotopia, cerebellar dysplasia, syringohydromyelia, and diastematomyelia.58,59 The degree of hindbrain herniation and hydrocephalus can also be evaluated with fetal MR imaging (Fig 16). A multicenter National Institutes of Health trial is currently underway to evaluate the role of prenatal repair of myelomeningoceles. If fetal surgery is shown to improve long-term outcome, fetal MR imaging may become a routine examination for affected fetuses.
Fig 16.
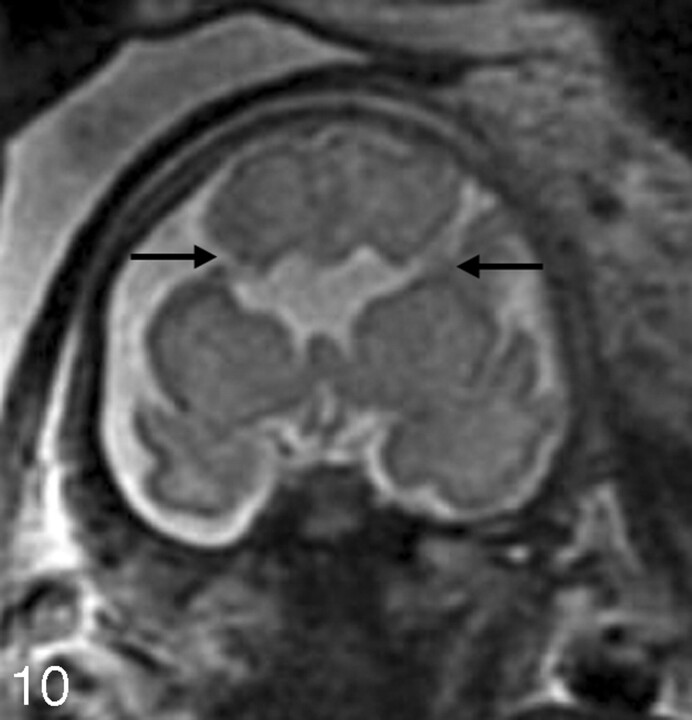
A, Sagittal SS-FSE T2-weighted image demonstrates a small posterior fossa with displacement of the cerebellar vermis inferiorly, consistent with a Chiari II malformation in a 22-gestational-week-old fetus. The corpus callosum is hypogenetic (arrow). There is diffuse effacement of the subarachnoid spaces, which is typically observed in these cases.
B, Coronal SS-FSE T2-weighted image demonstrates 2 small hypointense nodules (arrows) along the lateral margin of the atrium of the left lateral ventricle, consistent with periventricular nodular heterotopia. The ventricles are enlarged. Findings were confirmed by postnatal MR imaging.
Fetal MR imaging of the spine is also helpful in cases in which a bony spinal abnormality is detected on prenatal sonography.60 In particular, it can be used to detect underlying spinal cord anomalies, such as diastematomyelia and segmental spinal dysgenesis, which can be occult on prenatal sonography60,61 (Fig 17).
Fig 17.
Sagittal SS-FSE T2-weighted image in a 23-gestational-week-old fetus demonstrates multiple bony anomalies in the cervicothoracic region and lumbar spine. There is an abnormally high termination of the spinal cord (arrow) with marked narrowing of the bony spinal canal at the upper and midlumbar spine (A).
B, The lower lumbar bony spinal canal is more normal in caliber and contains abnormal neural tissue (arrow) in the sacral region. Findings are consistent with segmental spinal dysgenesis, and were confirmed by postnatal imaging.
Conclusions
Although significant progress in the field of fetal MR imaging has occurred with the development of ultrafast T2-weighted techniques, continued technical advances are likely to contribute to significant growth of the field within the next few years. In particular, with the recent application of diffusion-weighted imaging to the fetal brain, several aspects of brain development can now be quantified noninvasively. This has promising applications for the study of specific brain disorders, as well as for the study of normal in utero brain development. With the development of higher field strength MR scanners and improvements in spiral sampling of k-space, acquisition times will become reduced and proton spectroscopy may become easier to perform in younger fetuses instead of being limited to older fetuses whose heads are engaged in the pelvis and therefore move very little. Moreover, with continuing hardware and software improvements, such as stronger gradients, motion compensation techniques, and faster methods of interrogating k-space, additional MR images that are rapid and therefore feasible to use in clinical practice are likely to become available. These goals will be best achieved by collaborations among MR scientists, radiologists, obstetricians, child neurologists, pediatric neurosurgeons, and perinatologists.
In summary, fetal MR imaging is becoming an increasingly important tool in evaluating fetuses who have abnormalities suspected on the basis of family history or fetal sonography. With continuing improvements in technology, this will continue to be a rapidly growing field in future years.
References
- 1.Bajoria R, Wee LY, Anwar S, et al. Outcome of twin pregnancies complicated by single intrauterine death in relation to vascular anatomy of the monochorionic placenta. Hum Reprod 1999;14:2124–30 [DOI] [PubMed] [Google Scholar]
- 2.Barkovich AJ. Pediatric Neuroimaging. 4th ed. Philadelphia: Lippincott Williams & Wilkins,2005. .
- 3.Aicardi J, Chevrie JJ, Baraton J. Agenesis of the corpus callosum. In: Vinken PJ, Bruyn GW, Klawans HL, eds. Handbook of Clinical Neurology, Revised Series, Vol. 6. New York: Elsevier Science,1987. :149–73
- 4.Evangelista dos Santos AC, Midleton SR, Fonseca RL, et al. Clinical neuroimaging and cytogenetic findings in 20 patients with corpus callosum dysgenesis. Arq Neuropsiquiatr 2002;60:382–5 [PubMed] [Google Scholar]
- 5.Lacey DJ. Agenesis of the corpus callosum. Clinical features in 40 children. Am J Dis Child 1985;139:953–55 [DOI] [PubMed] [Google Scholar]
- 6.Goodyear PWA, Bannister CM, Russell S, et al. Outcome in prenatally diagnosed fetal agenesis of the corpus callosum. Fetal Diagn Ther 2000;16:139–45 [DOI] [PubMed] [Google Scholar]
- 7.Vergani P, Ghidini A, Strobelt N, et al. Prognostic indicators in the prenatal diagnosis of agenesis of corpus callosum. Am J Obstet Gynecol 1994;170:753–58 [DOI] [PubMed] [Google Scholar]
- 8.Gupta JK, Lilford RJ. Assessment and management of fetal agenesis of the corpus callosum. Prenat Diagn 1995;15:301–12 [DOI] [PubMed] [Google Scholar]
- 9.Barkovich AJ. Norman D. Anomalies of the corpus callosum: correlation with further anomalies of the brain. AJNR Am J Neuroradiol 1988;9:493–501 [DOI] [PubMed] [Google Scholar]
- 10.Blum A, Andre M, Droulle P, et al. Prenatal echographic diagnosis of corpus callosum agenesis. Genet Couns 1990;38:115–26 [PubMed] [Google Scholar]
- 11.Glenn O, Goldstein R, Li K, et al. Fetal MRI in the evaluation of fetuses referred for sonographically suspected abnormalities of the corpus callosum. J Ultrasound Med 2005;24:791–804 [DOI] [PubMed] [Google Scholar]
- 12.Sonigo PC, Rypens FF, Carteret M, et al. MR imaging of fetal cerebral anomalies. Pediatr Radiol 1998;28:212–22 [DOI] [PubMed] [Google Scholar]
- 13.Garel C, Brisse H, Sebag G, et al. Magnetic resonance imaging of the fetus. Pediatr Radiol 1998;28:201–11 [DOI] [PubMed] [Google Scholar]
- 14.Levine D, Barnes PD, Madsen JR, et al. Fetal central nervous system anomalies: MR imaging augments sonographic diagnosis. Radiology 1997;204:635–42 [DOI] [PubMed] [Google Scholar]
- 15.d’Ercole C, Girard N, Cravello L, et al. Prenatal diagnosis of fetal corpus callosum agenesis by ultrasonography and magnetic resonance imaging. Prenat Diagn 1998;18:247–53 [PubMed] [Google Scholar]
- 16.Rapp B, Perrotin F, Marret H, et al. Interet de l’IRM cerebrale foetale pour le diagnostic et le pronostic prenatal des agenesies du corps calleux. J Gynecol Obstet Biol Reprod 2002;31:173–82 [PubMed] [Google Scholar]
- 17.D’Ercole CD, Girard N, Boubli L, et al. Prenatal diagnosis of fetal cerebral abnormalities by ultrasonography and magnetic resonance imaging. Eur J Obstet Gynecol Reprod Biol 1993;50:177–84 [DOI] [PubMed] [Google Scholar]
- 18.Kubik-Huch RA, Huisman TA, Wisser J, et al. Ultrafast MR imaging of the fetus. AJR Am J Roentgenol 2000;174:1599–606 [DOI] [PubMed] [Google Scholar]
- 19.Dinh DH, Wright RM, Hanigan WC. The use of magnetic resonance imaging for the diagnosis of fetal intracranial abnormalities. Child’s Nerv Syst 1990;6:212–15 [DOI] [PubMed] [Google Scholar]
- 20.Adamsbaum C, Moutard ML, Andre C, et al. MRI of the fetal posterior fossa. Pediatr Neurol 2005;35:124–40 [DOI] [PubMed] [Google Scholar]
- 21.Barkovich AJ, Kjos BO, Norman D, et al. Revised classification of posterior fossa cysts and cystlike malformations based on the results of multiplanar MR imaging. AJR Am J Roentgenol 1989;153:1289–300 [DOI] [PubMed] [Google Scholar]
- 22.Tortori-Donati P, Fondelli MP, Rossi A, et al. Cystic malformations of the posterior cranial fossa originating from a defect of the posterior membranous area. Mega cisterna magna and persisting Blake’s pouch: two separate entities. Childs Nerv Syst 1996;12:303–08 [DOI] [PubMed] [Google Scholar]
- 23.Calabro F, Arcuri T, Jinkins JR. Blake’s pouch cyst: an entity within the Dandy-Walker continuum. Neuroradiology 2000;42:290–95 [DOI] [PubMed] [Google Scholar]
- 24.Golden JA, Rorke LB, Bruce DA. Dandy-Walker syndrome and associated anomalies. Pediat Neurosci 1987;13:38–44 [DOI] [PubMed] [Google Scholar]
- 25.Maria BL, Zinreich SJ, Carson BC, et al. Dandy-Walker syndrome revisited. Pediat Neurosci 1987;13:45–51 [DOI] [PubMed] [Google Scholar]
- 26.Bindal AK, Storrs BB, McLone DG. Management of Dandy-Walker syndrome. Pediat Neurosurg 1990;16:163–69 [DOI] [PubMed] [Google Scholar]
- 27.Boddaert N, Klein O, Ferguson N, et al. Intellectual prognosis of the Dandy-Walker malformation in children: the importance of vermian lobulation. Neuroradiology 2003;45:320–24 [DOI] [PubMed] [Google Scholar]
- 28.Klein O, Pierre-Kahn A, Boddaert N, et al. Dandy-Walker malformation: prenatal diagnosis and prognosis. Childs Nerv Syst 2003;19:484–89 [DOI] [PubMed] [Google Scholar]
- 29.Breysem L, Cossey V, Mussen E, et al. Fetal trauma: brain imaging in four neonates. Eur Radiol 2004;14:1609–14 [DOI] [PubMed] [Google Scholar]
- 30.Guibaud L, Garel C, Annie B, et al. Prenatal diagnosis of capillary telangiectasia of the cerebellum–ultrasound and MRI features. Prenat Diagn 2003;23:791–96 [DOI] [PubMed] [Google Scholar]
- 31.Sharony R, Kidron D, Aviram R, et al. Prenatal diagnosis of fetal cerebellar lesions: a case report and review of the literature. Prenat Diagn 1999;19:1077–80 [PubMed] [Google Scholar]
- 32.Ortiz JU, Ostermayer E, Fischer T, et al. Severe fetal cytomegalovirus infection associated with cerebellar hemorrhage. Ultrasound Obstet Gynecol 2004;23:402–06 [DOI] [PubMed] [Google Scholar]
- 33.Moinuddin A, McKinstry RC, Martin KA, et al. Intracranial hemorrhage progressing to porencephaly as a result of congenitally acquired cytomegalovirus infection–an illustrative report. Prenat Diagn 2003;23:797–800 [DOI] [PubMed] [Google Scholar]
- 34.Glenn OA, Callen PW, Parer JT, et al. Cerebellar hemorrhage in fetal parvovirus infection. Poster presented at the 43rd Annual Meeting of the American Society of Neuroradiology; May 21–27, 2005; Toronto, Ontario, Canada.
- 35.Ghi T, Brondelli L, Simonazzi G, et al. Sonographic demonstration of brain injury in fetuses with severe red blood cell alloimmunization undergoing intrauterine transfusions. Ultrasound Obstet Gynecol 2004;23:428–31 [DOI] [PubMed] [Google Scholar]
- 36.Levine D, Barnes D, Madsen JR, et al. Central nervous system abnormalities assessed with prenatal magnetic resonance imaging. Obstet Gynecol 1999;94:1011–19 [DOI] [PubMed] [Google Scholar]
- 37.Simon EM, Goldstein RB, Coakley FV, et al. Fast MR imaging of fetal CNS anomalies in utero. AJNR Am J Neuroradiol 2000;21:1688–98 [PMC free article] [PubMed] [Google Scholar]
- 38.Feldstein VA. Understanding twin-twin transfusion syndrome: role of Doppler ultrasound. Ultrasound Q 2002;18:247–54 [DOI] [PubMed] [Google Scholar]
- 39.Haverkamp F, Lex C, Hanisch C, et al. Neurodevelopmental risks in twin-to-twin transfusion syndrome: preliminary findings. Eur J Paediatr Neurol 2001;5:21–27 [DOI] [PubMed] [Google Scholar]
- 40.van Heteren CF, Nijhuis JG, Semmekrot BA, et al. Risk for surviving twin after fetal death of co-twin in twin-twin transfusion syndrome. Obstet Gynecol 1998;92:215–19 [DOI] [PubMed] [Google Scholar]
- 41.Norman MG. Bilateral encephaloclastic lesions in a 26 week gestation fetus: effect on neuroblast migration. Can J Neurol Sci 1980;7:191–94 [DOI] [PubMed] [Google Scholar]
- 42.Barth PG, van der Harten JJ. Parabiotic twin syndrome with topical isocortical disruption and gastroschisis. Acta Neuropathol (Berl) 1985;67:345–49 [DOI] [PubMed] [Google Scholar]
- 43.Szymonowicz W, Preston H, Yu VY. The surviving monozygotic twin. Arch Dis Child 1986;61:454–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson RL, Golbus MS, Curry CJ. Central nervous system damage and other anomalies in surviving fetus following second trimester antenatal death of co-twin. Report of four cases and literature review. Prenat Diagn 1990;10:513–18 [DOI] [PubMed] [Google Scholar]
- 45.Fusi L, McParland P, Fisk N, et al. Acute twin-twin transfusion: a possible mechanism for brain-damaged survivors after intrauterine death of a monochorionic twin. Obstet Gynecol 1991;78(3 Pt 2):517–20 [PubMed] [Google Scholar]
- 46.Larroche JC, Droulle P, Delezoide AL, et al. Brain damage in monozygous twins. Biol Neonate 1990;57:261–78 [DOI] [PubMed] [Google Scholar]
- 47.Larroche JC, Girard N, Narcy F, et al. Abnormal cortical plate (polymicrogyria), heterotopias and brain damage in monozygous twins. Biol Neonate 1994;65:343–52 [DOI] [PubMed] [Google Scholar]
- 48.Fusi L, Gordon H. Twin pregnancy complicated by single intrauterine death. Problems outcome with conservative management. Br J Obstet Gynaecol 1990;97:511–16 [DOI] [PubMed] [Google Scholar]
- 49.Sugama S. Kusano K. Monozygous twin with polymicrogyria and normal co-twin. Pediatr Neurol 1994;11:62–63 [DOI] [PubMed] [Google Scholar]
- 50.Weig SG, Marshall PC, Abroms IF, et al. Patterns of cerebral injury and clinical presentation in the vascular disruptive syndrome of monozygotic twins. Pediatr Neurol 1995;13:279–85 [DOI] [PubMed] [Google Scholar]
- 51.Shafrir Y, Latimer M, France M. Multifocal neuronal migration disorder as a probable result of a well-documented ischemic event at 18 weeks gestation. Ann Neurol 1996;40:296 [Google Scholar]
- 52.Van Bogaert P, Donner C, David P, et al. Congenital bilateral perisylvian syndrome in a monozygotic twin with intra-uterine death of the co-twin. Dev Med Child Neurol 1996;38:166–70 [DOI] [PubMed] [Google Scholar]
- 53.Righini A, Salmona S, Bianchini E, et al. Prenatal magnetic resonance imaging evaluation of ischemic brain lesions in the survivors of monochorionic twin pregnancies: report of 3 cases. J Comput Assist Tomogr 2004;28:87–92 [DOI] [PubMed] [Google Scholar]
- 54.Glenn O, Norton M, Goldstein RB, et al. Prenatal diagnosis of polymicrogyria by fetal magnetic resonance imaging in monochorionic cotwin death. J Ultrasound Med 2005;24:711–16 [DOI] [PubMed] [Google Scholar]
- 55.de Laveaucoupet J, Audibert F, Guis F, et al. Fetal magnetic resonance imaging (MRI) of ischemic brain injury. Prenat Diagn 2001;21:729–36 [DOI] [PubMed] [Google Scholar]
- 56.Hollier LM, Grissom H. Human herpes viruses in pregnancy: cytomegalovirus, Epstein-Barr virus, and varicella zoster virus. Clin Perinatol 2005;32:671–96 [DOI] [PubMed] [Google Scholar]
- 57.Barkovich AJ, Girard N. Fetal brain infections. Childs Nerv Syst 2003;19:501–07 [DOI] [PubMed] [Google Scholar]
- 58.Gilbert JN, Jones KL, Rorke LB, et al. Central nervous system anomalies associated with meningomyelocele, hydrocephalus and the Arnold-Chiari malformation: reappraisal of theories regarding the pathogenesis of posterior neural tube closure defects. Neurosurgery 1986;18:559–64 [DOI] [PubMed] [Google Scholar]
- 59.Wolpert S, Anderson M, Scott R, et al. Chiari II malformation: MR imaging evaluation. AJR Am J Roentgenol 1987;149:1033–42 [DOI] [PubMed] [Google Scholar]
- 60.von Koch CS, Glenn OA, Goldstein RB, et al. Fetal magnetic resonance imaging enhances detection of spinal cord anomalies in patients with sonographically detected bony anomalies of the spine. J Ultrasound Med 2005;24:781–89 [DOI] [PubMed] [Google Scholar]
- 61.Wagenvoort AM, Bekker MN, Go AT, et al. Ultrafast scan magnetic resonance in prenatal diagnosis. Fetal Diagn Ther 2000;15:364–72 [DOI] [PubMed] [Google Scholar]