Abstract
Purpose
To assess current use and acute safety profiles of gadolinium-based contrast agents (GBCAs) in cardiac MRI given recent suspensions of GBCA approval.
Materials and Methods
Patients were retrospectively included from the multinational multicenter European Society of Cardiovascular Radiology (ESCR) MR/CT Registry collected between January 2013 and October 2019. GBCA-associated acute adverse events (AAEs) were classified as mild (self-limiting), moderate (pronounced AAE requiring medical management), and severe (life threatening). Multivariable generalized linear mixed-effect models were used to assess AAE likelihood.
Results
A total of 154 779 patients (average age, 53 years ± 19 [standard deviation]; 99 106 men) who underwent cardiac MRI were included, the majority of whom underwent administration of GBCAs (94.2% [n = 145 855]). While linear GBCAs were used in 15.2% of examinations through 2011, their use decreased to less than 1% in 2018 and 2019. Overall, 0.36% (n = 556) of AAEs were documented (mild, 0.12% [n = 178]; moderate, 0.21% [n = 331]; severe, 0.03% [n = 47]). For nonenhanced cardiac MRI, examination-related events were reported in 2.59% (231 of 8924) of cases, the majority of which were anxiety (0.98% [n = 87]) and dyspnea (0.93% [n = 83]). AAE rates varied significantly by pharmacologic stressor, GBCA molecular structure (macrocyclic vs linear GBCA: multivariable odds ratio, 0.634; 95% confidence interval: 0.452, 0.888; P = .008), GBCA subtype, and imaging indication.
Conclusion
Gadolinium-based contrast agent administration changed according to recent regulatory decisions, with use of macrocyclic agents almost exclusively in 2018 and 2019; these agents also demonstrated a favorable acute safety profile.
Supplemental material is available for this article.
© RSNA, 2020
Summary
Gadolinium-based contrast agents used in cardiac MRI have an acute adverse event rate of 0.38%; in Europe, macrocyclic contrast agents are primarily used and demonstrate a favorable safety profile when compared with linear agents.
Key Points
■ Use of linear MRI gadolinium-based contrast agents for cardiac MRI declined from 15.2% of examinations in 2011 to less than 1% in 2018 and 2019.
■ Acute adverse events with contrast-enhanced cardiac MRI are rare (0.38% [556 of 145 855]).
■ With nonenhanced cardiac MRI (n = 8924), examination-related events were reported in 2.59% (n = 231) of patients, with anxiety (0.98% [n = 87]) and dyspnea (0.93% [n = 83]) accounting for the majority of such events.
Introduction
Cardiac MRI is a well-established cardiac imaging modality and is considered the reference standard for several diseases (1). According to international registries, intravenous gadolinium-based contrast agents (GBCAs) are used in most cardiac MRI examinations for indications ranging from myocarditis and cardiomyopathy imaging to myocardial viability assessment (2,3). GBCAs can be considered safe for the referred population in general and the cardiac MRI population in particular, with reported acute adverse event (AAE) rates between 0.04% and 2.2% (4–11).
Because of the toxicity of free gadolinium3+ ions, ligands are used to create gadolinium chelates for safe use of GBCAs in humans. Based on the type of ligand, GBCAs can be generally classified as linear or macrocyclic (12). There have been growing concerns about cerebral gadolinium depositions after repeated GBCA administration, with some studies suggesting that the molecular GBCA structure affected these depositions, with higher likelihood for deposit with linear compounds compared with macrocyclic GBCAs (13,14). As a preventive measure, in late 2017, the European Medicines Agency (EMA) suspended the marketing authorization of linear GBCA types (except for hepatic imaging), and this suspension was ratified by the European Commission (15). To our knowledge, there are currently no data on the effects of these regulatory changes on case numbers or on the cardiac MRI safety profile.
This study assesses the contemporary effect of the EMA regulatory decision on use of GBCAs in cardiac MRI and the associated safety profile of GBCAs with regard to AAEs using a multinational registry.
Materials and Methods
Study Design
This retrospective study was approved by the institutional review board (Leipzig University, No 131/17-ek) and was conducted according to the Declaration of Helsinki. Patient consent was waived by the institutional review board owing to analyses of anonymized multicenter data.
The data source for this analysis was the European Society of Cardiovascular Radiology (ESCR) MR/CT Registry, a multinational multicenter database on cardiac CT and MRI. Participation of individual centers in the registry is voluntary and is not restricted to ESCR members. In some countries, submission of cases to the MR/CT Registry is part of the national certification and accreditation process in cardiovascular radiology. All participating centers underwent initial audit by the ESCR office in Vienna, Austria, to ensure data quality. The ESCR MR/CT Registry uses a standardized online questionnaire to acquire mandatory information on patient characteristics, indication, diagnosis, imaging technique, contrast media application, and occurrence of AAEs (reported as the most severe event for each patient) from the submitting physician. Integrated plausibility checks provided immediate feedback to submitting physicians and ensured data consistency. The ESCR MR/CT Registry received unrestricted educational funding from Siemens Healthcare (Erlangen, Germany), Bayer HealthCare (Leverkusen, Germany), Philips Healthcare (Franklin, Tenn), and Bracco Imaging (Milan, Italy).
The MR/CT Registry of the ESCR was queried for cardiac MRI studies submitted between January 2013 and October 2019. Patients were included if data were submitted to the MR/CT Registry at one of the participating centers. Patient inclusion was irrespective of patient demographics, cardiac MRI protocol, and imaging indication. Patients were excluded if they were reported to have “back pain” or “other/unspecified” AAEs, as these were not classifiable according to the American College of Radiology (ACR).
A total of 72 839 included patients were reported in an earlier study that focused on AAE patterns across different GBCA subtypes and pharmacologic stressors (4). The study reported herein adds data from 3 years, with an additional 81 940 patients.
Cardiac MRI and GBCA Variables
The molecular structure of each GBCA was classified as macrocyclic or linear, its ionic properties were classified as ionic or nonionic, and its thermodynamic chelate stability was classified using the logarithmic of the thermodynamic stability constant (log Ktherm) (16). Further variables evaluated were administration and type of pharmacologic stressor, the main indication for cardiac MRI, GBCA volume and concentration, cardiac MRI scan time, and patient demographics. Submitting physicians rated cardiac MRI quality as very good, good, adequate, poor, or very poor.
Outcomes
The use of GBCA was descriptively assessed as the frequency of contrast-enhanced cardiac MRI after the EMA’s decision in July 2017 to suspend approval of linear GBCAs for cardiac imaging (15).
We categorized AAEs according to the ACR Manual on Contrast Media, version 10.3 (17). AAEs were categorized as allergic-like or physiologic and were classified as mild (self-limiting AAE), moderate (pronounced AAE requiring medical management), and severe (life-threatening AAE). Hypersensitivity AAEs comprised urticaria and hives, as well as AAEs categorized as “hypersensitive” without further detail by the submitting physician. Anxiety AAEs included claustrophobic events, if listed separately. For statistical analyses, occurrence of any AAE was considered the primary outcome. Unspecific “anxiety” was conservatively included as an AAE for our analyses, although no post hoc discrimination between claustrophobic reactions and true adverse reactions to GBCA was possible. Multivariable analyses should remain unbiased, assuming claustrophobic events were evenly distributed across subgroups.
Statistical Analysis
For descriptive analyses, continuous variables are provided as median and interquartile range, and categorical variables are provided as frequency and percentage.
Multivariable logistic regression models were used to assess predictors of any AAE, with variable selection based on univariate significance and clinical knowledge. When using a generalized linear mixed-effect model, the submitting institution was considered a random effect to account for institutional differences in patient population and reporting patterns. A priori, a test for multiplicative interaction between the pharmacologic stressor and GBCA was planned. The Clapper-Pearson method was used to calculate 95% confidence intervals (CIs) for AAE rates. All statistics were performed using R, version 3.3.2, and R Studio, version 1.0.44 (R Foundation for Statistical Computing, Vienna, Austria) (18,19). All P values provided are two-sided. An α level of .05 was chosen for statistical significance.
Results
Baseline Characteristics
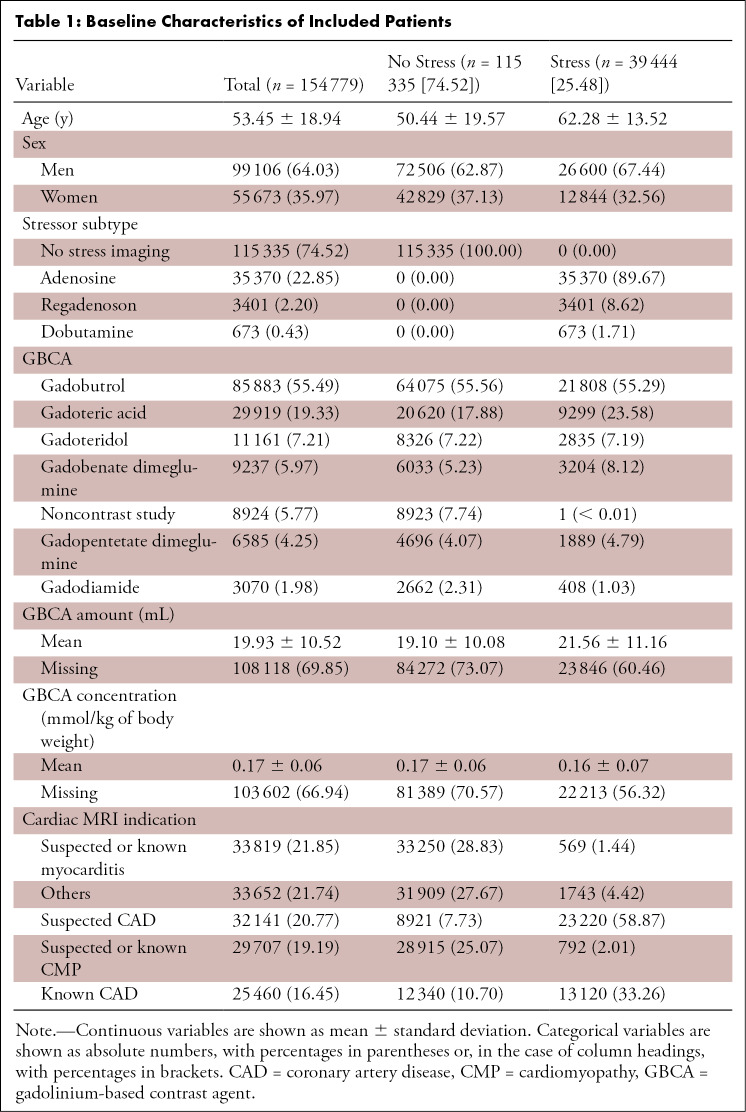
This study included 154 779 patients (average age, 53 years ± 19 [standard deviation]; 99 106 men), while 26 patients with “other” AAEs and nine with “back pain” during cardiac MRI were excluded. Academic centers submitted 67 218 cardiac MRI cases (43.43%), while nonacademic centers submitted 87 561 cases (56.57%).
Most cardiac MRI examinations were conducted without use of a pharmacologic stressor (74.52% [n = 115 335]); pharmacologic stressors were predominantly used in older men with known or suspected coronary artery disease, as shown in Table 1. The median cardiac MRI scan time was 38 minutes (interquartile range, 28–45 minutes). Data on cardiac MRI quality were available for 118 621 examinations and were rated as very good in 29.96% (n = 35 533) of patients, good in 60.13% (n = 71 321), adequate in 7.9% (n = 9376), poor in 1.8% (n = 2130), and very poor in 0.3% (n = 351).
Table 1:
Baseline Characteristics of Included Patients
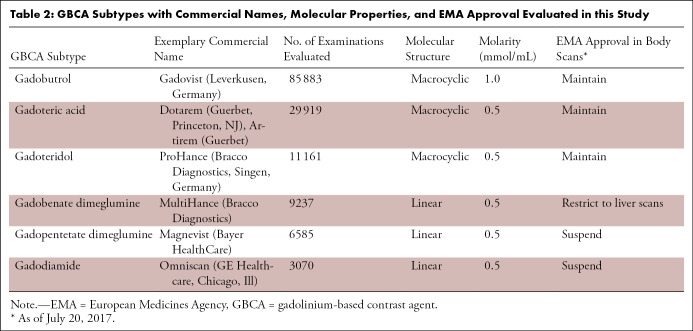
Most patients who underwent cardiac MRI underwent intravenous GBCA administration (94.2% [n = 145 855]). As summarized in Table 1, the most frequently used GBCA subtype was gadobutrol (55.5% [n = 85 883]), followed by gadoteric acid (19.3% [n = 29 919]) (Table 2). A total of 8924 patients (5.8%) underwent cardiac MRI without administration of a GBCA; none of these patients received pharmacologic stressors.
Table 2:
GBCA Subtypes with Commercial Names, Molecular Properties, and EMA Approval Evaluated in this Study
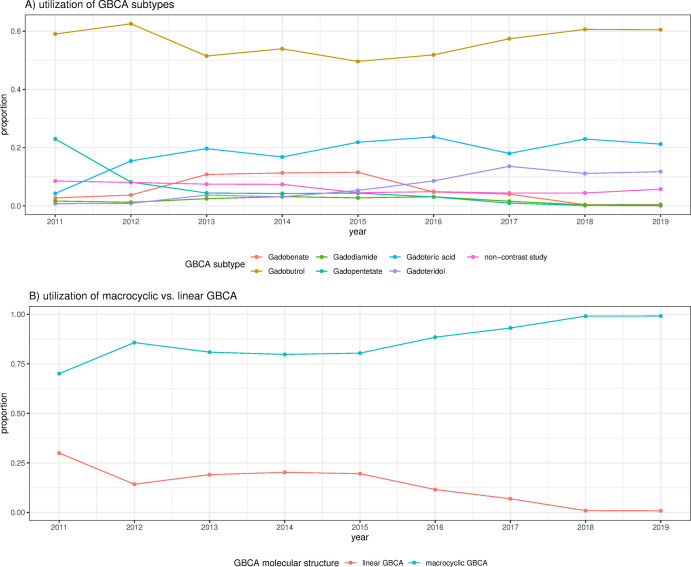
As shown in the Figure, the use of GBCA subtypes changed over the years, with linear GBCA use decreasing from 15.2% in 2011 and before to less than 1% in 2018 and 2019. Although the number of annually submitted cardiac MRI cases varied, no declining trend in GBCA use in cardiac MRI was noted in 2018 and 2019 after the EMA’s decision on GBCA restrictions.
Figure:
Proportion of gadolinium-based contrast agent (GBCA) subtypes and molecular structure used for cardiac MRI in the European Society of Cardiovascular Radiology MR/CT Registry. A, Use of GBCA subtypes. B, Use of macrocyclic versus linear GBCA.
AAE Overview
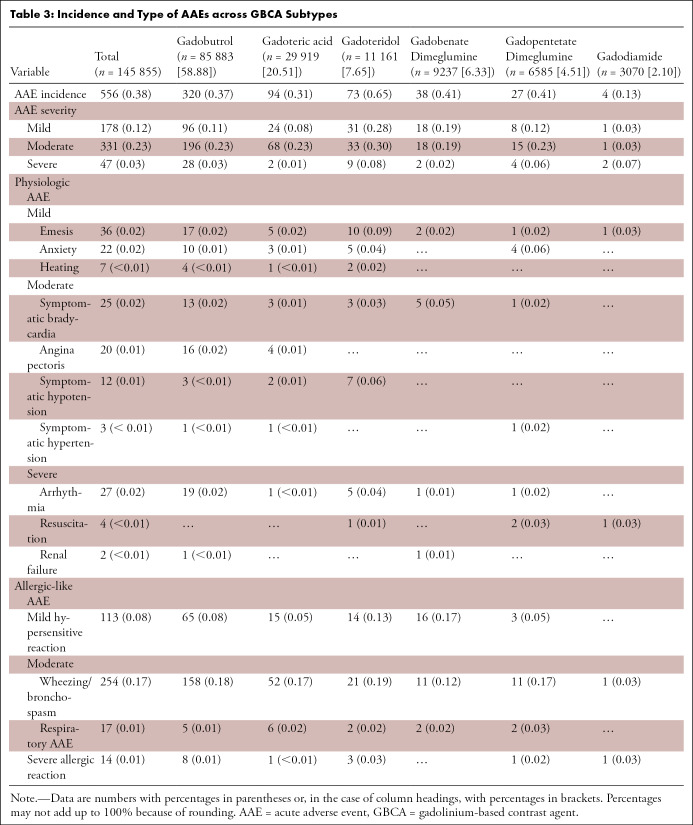
Overall, of the 154 779 patients, 0.36% (n = 556; 95% CI: 0.33%, 0.39%) had documented AAEs. Assessing each submitting institution separately, the institutional AAE rates ranged from 0.01% to 10.5%, with an interquartile range of 0.2%–0.8%. The majority of AAEs were classified as moderate (0.21% [n = 331]; 95% CI: 0.19%, 0.24%; relative, 59.53% [331 of 556]) or mild (0.12% [n = 178]; 95% CI: 0.1%, 0.13%; relative, 32.01% [178 of 556]). Only a minority of AAEs were categorized as severe (0.03% [n = 47]; 95% CI: 0.02%, 0.04%; relative, 8.45% [47 of 556]). Allergic-like AAEs were more common than physiologic AAEs (71.58% [398 of 556] vs 28.42% [158 of 556]). Among patients undergoing GBCA-enhanced MRI (n = 145 855), the most common AAEs were wheezing and bronchospasm (0.17% [n = 254]; relative, 45.68% [254 of 556]), hypersensitive reaction (0.08% [n = 113]; relative, 20.32% [113 of 556]), and emesis (0.02% [n = 36]; relative, 6.47% [36 of 556]), as detailed in Table 3.
Table 3:
Incidence and Type of AAEs across GBCA Subtypes
Among patients who did not undergo administration of a GBCA (n = 8924), a total of 231 (2.59%; 95% CI: 2.27%, 2.94%) acute examination-related events were reported. The majority of these events were anxiety (0.98% [n = 87]) or dyspnea (0.93% [n = 83]). Among patients who received a GBCA, the AAE rate was 0.38% (556 of 145 855; 95% CI: 0.35%, 0.41%).
AAEs were also more frequent after administration of pharmacologic stressors (0.62% [244 of 39 444]) compared with nonstress cardiac MRI examinations (0.18% [213 of 115 335]). Across pharmacologic stressors, AAEs were more common after administration of dobutamine (1.78% [12 of 673]) and regadenoson (1.26% [43 of 3401]) than after administration of adenosine (0.53% [189 of 35 370]). AAEs also varied by GBCA subtype, with the highest overall AAE incidence reported for patients who received gadoteridol (0.65% [73 of 11 161]). In general, cardiac MRI scan quality was lower in patients with AAEs (very poor, 4.6%; poor, 16.1%) compared with cases without AAEs (very poor, 0.3%; poor, 1.7%; P < .001).
AAE Predictors
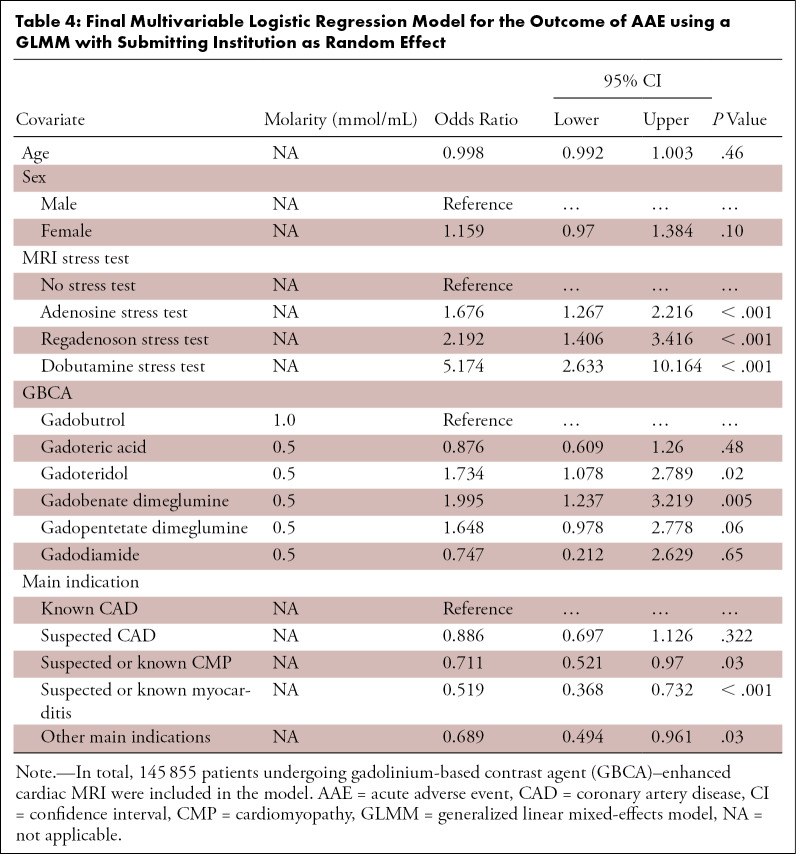
For statistical modeling of AAE probability, only patients who underwent GBCA-enhanced cardiac MRI (n = 145 855) were included to avoid bias resulting from early termination in nonenhanced studies. As shown in Table 4, the final multivariable model included patient age and sex as covariates despite lack of statistical significance, as these variables are reported as confounders in the literature.
Table 4:
Final Multivariable Logistic Regression Model for the Outcome of AAE using a GLMM with Submitting Institution as Random Effect
After multivariable adjustment, AAEs were more common after administration of any pharmacologic stressor. AAEs were more likely after administration of dobutamine (odds ratio [OR], 3.087; 95% CI: 1.607, 5.932; P < .001) and regadenoson (OR, 1.31; 95% CI: 0.84, 2.35; P = .23) than after administration of adenosine.
AAE incidence also correlated with GBCA molecular properties, with lower AAE likelihood with macrocyclic GBCAs than with linear GBCAs (multivariable OR, 0.634; 95% CI: 0.452, 0.888; P = .008). Neither GBCA chelate stability nor ionic properties had a significant effect on AAEs (chelate stability per one-unit log Ktherm increase [OR, 0.989; 95% CI: 0.916, 1.068; P = .78]; ionic vs nonionic GBCAs [OR, 1.18; 95% CI: 0.895, 1.547; P = .24]).
In further analyses, AAE rates varied with GBCA subtype. When compared with cardiac MRI performed with gadobutrol, AAEs were more likely if cardiac MRI was performed with gadobenate dimeglumine (OR, 1.995; 95% CI: 1.237, 3.219; P = .005) or gadoteridol (OR, 1.734; 95% CI: 1.078, 2.789; P = .02). There was marginal evidence that AAEs were more likely after administration of gadopentetate dimeglumine versus gadobutrol (OR, 1.648; 95% CI: 0.978, 2.778; P = .06).
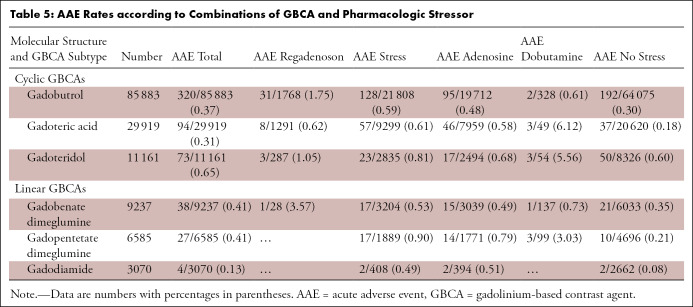
AAE incidence also varied by main imaging indication, where the highest AAE rates were reported in patients with known coronary artery disease. In separate analyses, there was a significant interaction between GBCA subtype and pharmacologic stressor (15 degrees of freedom, P = .002). As shown in Table 5, certain combinations of GBCA subtype and pharmacologic stressor were associated with higher AAE rates.
Table 5:
AAE Rates according to Combinations of GBCA and Pharmacologic Stressor
Subgroup Analyses
For 21.44% (33 178 of 154 779) of patients, information on GBCA volume (measured in milliliters) and molal concentration (measured in millimoles per kilogram of body weight) was documented. In univariate generalized linear mixed-effect models, there was no influence of GBCA volume (per 1 mL increase, OR, 0.999; 95% CI: 0.978, 1.020; P = .93) or molal concentration (OR, 0.579; 95% CI: 0.025, 13.196; P = .732) on AAE incidence.
On subgroup analyses that separately evaluated physiologic and allergic-like AAEs, direction and magnitude of point estimates were comparable to the full cohort, except for a significantly higher allergic-like AAE probability in younger women (Tables E1–E5 [supplement]). For further separate subgroup analyses of patients with mild, moderate, or severe AAE, findings were comparable to those in the full cohort (Tables E1–E5 [supplement]).
Discussion
Cardiac MRI is routinely used in clinical practice to diagnose and manage various cardiac diseases. After recent studies on cerebral gadolinium depositions after repeated GBCA administration, the EMA decided to suspend the marketing authorization of linear GBCAs. In this study, we used data from the multinational multicenter ESCR MR/CT Registry and demonstrated the regulatory decisions of the EMA were translated into clinical practice with a rapid decline of linear GBCA administrations for cardiac MRI performed after 2017. Despite the regulatory restrictions, neither the overall nor the relative number of cardiac MRI cases with GBCA reported to the MR/CT Registry declined after 2017. Our results further indicate that cardiac MRI is broadly available not only in the academic setting but also in nonacademic centers and in private practice, which accounted for 57% of all submitted cases.
The high number of cardiac MRI cases submitted from nonacademic centers probably reflects the growing expertise of radiologists in cardiac MRI assessment and the clinical relevance of this modality. Although the registry includes academic centers, nonacademic centers, and private practices, the data reported may not be fully representative, as participation in the ESCR MR/CT Registry was voluntary.
Only a minority of cardiac MRI examinations were performed without GBCA administration. Interestingly, the number of examination-related events in these patients was significantly higher compared with AAEs in contrast-enhanced cardiac MRI (2.59% vs 0.38%; P < .001). Most events in the nonenhanced cardiac MRI subgroup were categorized as anxiety (0.98%). One might speculate that these cardiac MRI examinations were preemptively aborted prior to GBCA administration owing to either claustrophobic events or factors related to the patients’ underlying diseases, such as dyspnea in the setting of congestive heart failure (20).
Long cardiac MRI scanning times might further aggravate these symptoms, which seems to provide additional confirmation that cardiac diseases should be considered risk factors for all MRI examinations, not just cardiac MRI examinations. Event rates in the nonenhanced subgroup also offer a new perspective on the incidence of AAEs after GBCA administration, as some reactions might be attributable to a patient’s underlying cardiac disease (eg, dyspnea, angina pectoris) or general constitution (eg, anxiety) rather than the result of GBCA administration. To our knowledge, to date there is no literature on examination-related events in nonenhanced cardiac MRI.
In this study, the overall AAE incidence in GBCA-enhanced cardiac MRI was 0.38%, which is in line with earlier results both in the MR/CT Registry and in other large data sets (range, 0.12%–0.36%) (4,9,21). The large variability of institutional AAE rates might be attributable to distinct patient cohorts and potential reporting bias. Most AAEs in GBCA-enhanced cardiac MRI were categorized as either moderate (0.21%) or mild (0.12%), with only a minority categorized as severe (0.03%). In multivariable analyses accounting for potential bias by submitting centers, the AAE incidence varied based on pharmacologic stressors, GBCA molecular structure, GBCA subtypes, and imaging indications. AAEs were more likely in patients undergoing stress cardiac MRI, with the highest AAE rates in those administered dobutamine (1.78%) and regadenoson (1.26%) and the lowest rates in those receiving adenosine (0.53%). These differences might be attributable to the pharmacologic properties, with a longer half-life and different modes of action of regadenoson and dobutamine compared with adenosine, or owing to so-called nonresponder patients lacking sufficient hemodynamic response to adenosine stress (22,23). The majority of regadenoson-associated AAEs were respiratory events, which is in agreement with the literature (24,25). These results conflict with the European Cardiovascular Magnetic Resonance Registry, which reported lower adverse event rates in stress imaging (although confounders were not accounted for) (9,21).
In this study, AAE rates were lower for macrocyclic GBCAs than for linear GBCAs (OR, 0.638; 95% CI: 0.455, 0.894; P = .009), while GBCA chelate stability and ionic properties did not significantly affect AAE incidence. In further analyses, AAE incidence varied by specific GBCA subtype. For example, compared with gadobutrol as the most frequently used GBCA, AAEs were significantly more likely to occur after administration of gadobenate dimeglumine and gadoteridol. These results proved robust upon several subgroup analyses. The fact that a few predictors showed no statistical significance might well be attributable to small sample sizes, reducing statistical power. It remains debatable whether AAE differences resulted from a combination of GBCA properties, such as molecular structure, ionicity, and chelate stability, or truly varied across specific GBCA subtypes, both of which have been described in the literature (7,16,26,27).
Further, AAE differences were evident across specific GBCA and pharmacologic stressor combinations, which corroborates earlier results from the MR/CT Registry, although the underlying pathomechanism has yet to be investigated (4).
This study had limitations. First, the ESCR MR/CT Registry only covered AAEs and lacked information on late-onset events, such as nephrogenic fibrosis (28). Second, owing to the design of the registry, institutions may not have submitted consecutive imaging cases, which could lead to underreporting of AAEs in this cohort. Third, bias is possible, in that selection of specific pharmacologic stressors might have been based on patient factors related to occurrence of AAEs. Finally, there were no data regarding renal function and previous history of adverse reactions to contrast media, nor was there detailed information on patients’ underlying disease. Although well-established, the ACR classification of AAEs as physiologic or allergic-like is the subject of current scientific debate (29).
Further, the term anxiety is not specific, and anxiety may well be attributable to the scanning procedure rather than GBCA administration, especially given the incidence of anxiety in nonenhanced cardiac MRI. Still, we see no possibility of differentiating between anxiety resulting from scanning technique and anxiety associated with a GBCA. The ACR Manual on Contrast Media, version 10.3, lists anxiety as a potential GBCA AAE; thus, anxiety was conservatively included in our analyses. However, results of our multivariable analyses comparing different GBCA subtypes should be unbiased by this method since it can be assumed that scanning-related anxiety is evenly distributed across different GBCA subtypes.
In conclusion, GBCA administration changed according to recent regulatory decisions, with almost exclusive use of macrocyclic agents for cardiac MRI in 2018 and 2019. Macrocyclic agents demonstrated a favorable acute safety profile compared with linear agents. The high rate of examination-related events in nonenhanced cardiac MRI may result from patients’ underlying conditions or the mental strain of MRI examination and provides additional perspective on the overall low AAE rate of GBCA-enhanced cardiac MRI.
SUPPLEMENTAL TABLES
Acknowledgments
Acknowledgments
We thank the European Society of Cardiovascular Radiology office, Vienna, Austria, and LoeScap Technology, Berlin, Germany, for administrative and technical support.
Disclosures of Conflicts of Interest: J.U. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received a speaker fee from Bayer HealthCare. Other relationships: disclosed no relevant relationships. O.A. disclosed no relevant relationships. R.S. disclosed no relevant relationships. M.F. disclosed no relevant relationships. R.V. disclosed no relevant relationships. J.B. disclosed no relevant relationships. J.L. disclosed no relevant relationships. M.G. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received a speaker honorarium and travel support from Bayer HealthCare, Bracco Imaging, Siemens HealthCare, Philips Healthcare, GE Healthcare, and Circle Health. Other relationships: disclosed no relevant relationships.
The European Society of Cardiovascular Radiology MR/CT Registry received unrestricted educational funding from Siemens Healthcare, Bayer HealthCare, Philips Healthcare, and Bracco Diagnostics.
Abbreviations:
- AAE
- acute adverse event
- ACR
- American College of Radiology
- CI
- confidence interval
- EMA
- European Medicines Agency
- ESCR
- European Society of Cardiovascular Radiology
- GBCA
- gadolinium-based contrast agent
- OR
- odds ratio
References
- 1.Pfeiffer MP, Biederman RWW. Cardiac MRI: A General Overview with Emphasis on Current Use and Indications. Med Clin North Am 2015;99(4):849–861. [DOI] [PubMed] [Google Scholar]
- 2.Bruder O, Wagner A, Lombardi M, et al. European Cardiovascular Magnetic Resonance (EuroCMR) registry--multi national results from 57 centers in 15 countries. J Cardiovasc Magn Reson 2013;15(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paiman EHM, Lamb HJ. When should we use contrast material in cardiac MRI? J Magn Reson Imaging 2017;46(6):1551–1572. [DOI] [PubMed] [Google Scholar]
- 4.Uhlig J, Lücke C, Vliegenthart R, et al. Acute adverse events in cardiac MR imaging with gadolinium-based contrast agents: results from the European Society of Cardiovascular Radiology (ESCR) MRCT Registry in 72,839 patients. Eur Radiol 2019;29(7):3686–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumura T, Hayakawa M, Shimada F, et al. Safety of gadopentetate dimeglumine after 120 million administrations over 25 years of clinical use. Magn Reson Med Sci 2013;12(4):297–304. [DOI] [PubMed] [Google Scholar]
- 6.Bleicher AG, Kanal E. Assessment of adverse reaction rates to a newly approved MRI contrast agent: review of 23,553 administrations of gadobenate dimeglumine. AJR Am J Roentgenol 2008;191(6):W307–W311. [DOI] [PubMed] [Google Scholar]
- 7.Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol 2011;196(2):W138–W143. [DOI] [PubMed] [Google Scholar]
- 8.Abujudeh HH, Kosaraju VK, Kaewlai R. Acute adverse reactions to gadopentetate dimeglumine and gadobenate dimeglumine: experience with 32,659 injections. AJR Am J Roentgenol 2010;194(2):430–434. [DOI] [PubMed] [Google Scholar]
- 9.Bruder O, Schneider S, Pilz G, et al. 2015 Update on Acute Adverse Reactions to Gadolinium based Contrast Agents in Cardiovascular MR. Large Multi-National and Multi-Ethnical Population Experience With 37788 Patients From the EuroCMR Registry. J Cardiovasc Magn Reson 2015;17(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt CH, Hartman RP, Hesley GK. Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. AJR Am J Roentgenol 2009;193(4):1124–1127. [DOI] [PubMed] [Google Scholar]
- 11.Aran S, Shaqdan KW, Abujudeh HH. Adverse allergic reactions to linear ionic gadolinium-based contrast agents: experience with 194, 400 injections. Clin Radiol 2015;70(5):466–475. [DOI] [PubMed] [Google Scholar]
- 12.Bellin MF, Van Der Molen AJ. Extracellular gadolinium-based contrast media: an overview. Eur J Radiol 2008;66(2):160–167. [DOI] [PubMed] [Google Scholar]
- 13.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270(3):834–841. [DOI] [PubMed] [Google Scholar]
- 14.Radbruch A. Are some agents less likely to deposit gadolinium in the brain? Magn Reson Imaging 2016;34(10):1351–1354. [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency . EMA’s final opinion confirms restrictions on use of linear gadolinium agents in body scans. London, England: European Medicines Agency, 2017. [Google Scholar]
- 16.Idée JM, Port M, Robic C, Medina C, Sabatou M, Corot C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging 2009;30(6):1249–1258. [DOI] [PubMed] [Google Scholar]
- 17.American College of Radiology . Manual on Contrast Media Version 10.3. Reston, VA: American College of Radiology, 2018. [Google Scholar]
- 18.R Development Core Team. R : A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2008. [Google Scholar]
- 19.RStudio Team . RStudio: Integrated Development for R. Boston, Mass: RStudio Team, 2015. [Google Scholar]
- 20.Nava S, Larovere MT, Fanfulla F, Navalesi P, Delmastro M, Mortara A. Orthopnea and inspiratory effort in chronic heart failure patients. Respir Med 2003;97(6):647–653. [DOI] [PubMed] [Google Scholar]
- 21.Bruder O, Schneider S, Nothnagel D, et al. Acute adverse reactions to gadolinium-based contrast agents in CMR: multicenter experience with 17,767 patients from the EuroCMR Registry. JACC Cardiovasc Imaging 2011;4(11):1171–1176. [DOI] [PubMed] [Google Scholar]
- 22.Ananthasubramaniam K, Weiss R, McNutt B, Klauke B, Feaheny K, Bukofzer S. A randomized, double-blind, placebo-controlled study of the safety and tolerance of regadenoson in subjects with stage 3 or 4 chronic kidney disease. J Nucl Cardiol 2012;19(2):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karamitsos TD, Ntusi NAB, Francis JM, Holloway CJ, Myerson SG, Neubauer S. Feasibility and safety of high-dose adenosine perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pape M, Zacho HD, Aarøe J, Eggert Jensen S, Petersen LJ. Safety and tolerability of regadenoson for myocardial perfusion imaging - first Danish experience. Scand Cardiovasc J 2016;50(3):180–186. [DOI] [PubMed] [Google Scholar]
- 25.Brink HL, Dickerson JA, Stephens JA, Pickworth KK. Comparison of the Safety of Adenosine and Regadenoson in Patients Undergoing Outpatient Cardiac Stress Testing. Pharmacotherapy 2015;35(12):1117–1123. [DOI] [PubMed] [Google Scholar]
- 26.Sherry AD, Caravan P, Lenkinski RE. Primer on gadolinium chemistry. J Magn Reson Imaging 2009;30(6):1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behzadi AH, Zhao Y, Farooq Z, Prince MR. Immediate Allergic Reactions to Gadolinium-based Contrast Agents: A Systematic Review and Meta-Analysis. Radiology 2018;286(2):731. [DOI] [PubMed] [Google Scholar]
- 28.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006;17(9):2359–2362. [DOI] [PubMed] [Google Scholar]
- 29.Uhlig J, Lücke C, Bremerich J, Gutberlet M. Reply to Letter to the Editor: How to document adverse reactions induced by gadolinium-based contrast agents? A plea for type A and type B reactions. Eur Radiol 2020;30(3):1757–1758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.