Abstract
Purpose
To explore the safety and clinical utility of MRI in participants with non–MRI-conditional cardiac implantable electronic devices, by establishing the Patient Registry of Magnetic Resonance Imaging in Non-Approved DEvices (PROMeNADe).
Materials and Methods
From September 2015 to June 2019, 532 participants (211 women) with a mean age of 69 years ± 14 (standard deviation) were enrolled prospectively in the PROMeNADe registry (ClinicalTrials.gov identifier: NCT03081364) and underwent a total of 608 MRI examinations (61 cardiac MRI examinations). All participants had device interrogations performed before and after each MRI. Pacemaker-dependent patients received asynchronous pacing. Patients with an implantable cardioverter defibrillator (ICD) had tachycardia therapies disabled during the MRI. An electrophysiology nurse monitored participants for any hemodynamic or rhythm abnormalities. Referring physicians were surveyed regarding the clinical utility of the MRI. Standard descriptive analyses included summary statistics with percentages and means.
Results
Cardiac devices included pacemakers (46%), ICDs (30%), cardiac resynchronization therapy (CRT) pacemakers (4%), and CRT defibrillators (17%), as well as abandoned leads (2%). Pacemaker-dependent patients comprised 27% of all MRI examinations. There were no patient- or device-related complications. Clinical utility surveys of MRI examinations were completed by 150 physicians. According to the survey responses, these MRI examinations changed the suspected diagnosis 25% of the time and changed suspected prognosis in 26% of participants, with planned medical or surgical treatment being changed 42% of the time.
Conclusion
This registry demonstrates that MRI examinations, including thoracic MRI examinations, can be performed safely in patients who have non–MRI-conditional devices, in pacemaker-dependent patients with ICDs, and in patients with abandoned leads. These MRI examinations can have a substantial impact on patient care, justifying the extensive resources used to perform them.
Supplemental material is available for this article.
© RSNA, 2020
See also the commentary by Peshock in this issue.
Summary
This registry demonstrates that MRI examinations, including thoracic MRI examinations, can be performed safely in patients who have non–MRI-conditional devices, in pacemaker-dependent patients with implantable cardioverter defibrillators, and patients with abandoned leads.
Key Points
■ MRI examinations, including cardiac and thoracoabdominal MRI examinations, can be performed safely in patients who have non–MRI-conditional cardiac devices, including those who are pacemaker dependent or have abandoned leads.
■ These MRI examinations frequently influenced management plans and patient care, justifying the extensive resources utilized to perform them.
■ This study adds to the growing literature demonstrating that MRI examinations can be performed safely in patients who have non–MRI-conditional devices; this finding may prompt more institutions to start offering MRI examinations to these patients.
Introduction
Over 2 million people within the United States have cardiovascular implantable electronic devices (CIEDs), including pacemakers and implantable cardioverter defibrillators (ICDs); and half of these patients are predicted to eventually require an MRI examination (1). Although there are an increasing number of patients with MRI-conditional CIEDs, a large number of patients implanted with non–MRI-conditional CIEDs were unable to undergo an MRI until March 2011, when a change to Centers for Medicare and Medicaid Services National Coverage Determination was granted to allow coverage for MRI examinations in prospective registries designed to assess the risk of MRI examinations in this population (2). Several reported studies over the past 2 decades have demonstrated few important adverse events among patients with legacy CIEDs undergoing MRI examinations (1,3–19). These studies have reported the experience of several institutions that created registries based upon similar safety protocols (1,3). However, most of the published studies excluded imaging of the thorax, including cardiac MRI examinations, excluded pacemaker-dependent patients, and excluded those with fragmented or abandoned leads. These three populations of patients (ie, thoracic MRI examinations, pacemaker-dependent ICDs, and abandoned leads) that were not included in many previous studies represent an important segment of patients who often require MRI examinations clinically. The objective of this registry was to determine the safety and clinical utility of performing MRI examinations, including thoracic examinations, in participants who have non–MRI-conditional CIEDs and by including participants who were pacemaker dependent and those with abandoned leads.
Materials and Methods
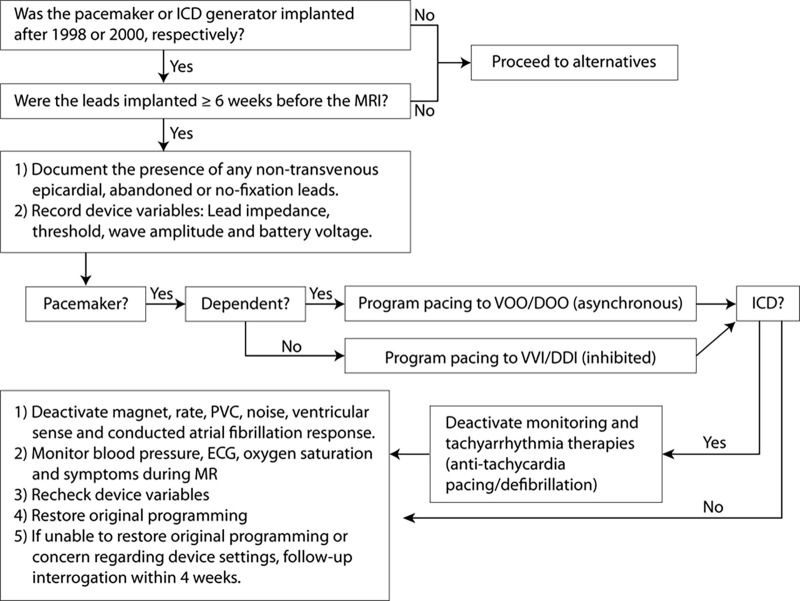
At our institution, we created an institutional review board–approved registry (Patient Registry of Magnetic Resonance Imaging in Non-Approved DEvices [PROMeNADe]), with a protocol (Fig 1) similar to those previously published (1,3), but also including thoracic MRI examinations, pacemaker-dependent patients with ICDs, and abandoned leads. The Food and Drug Administration considers an MRI-conditional device to be a device and leads that have been demonstrated to pose no known hazards in a specified MRI environment with specified conditions of use. A non–MRI-conditional device may include older devices and leads that were not specifically studied or include abandoned, fractured, or epicardial leads. The study was registered at ClinicalTrials.gov (identifier: NCT 03081364). This registry is supported by a grant from the Frank and Evangeline Thompson Foundation. The full protocol is available upon request by contacting the investigators. Participants were enrolled when a clinically indicated MRI was ordered and if other imaging modalities lacked the necessary spatial resolution and tissue characterization to answer the patients’ clinical question. Between September 7, 2015, and June 14, 2019, participants with non–MRI-conditional CIEDs were prospectively enrolled in the PROMeNADe registry. The inclusion criteria were that the participant had a non–MRI-conditional CIED and was planned to undergo a clinically indicated MRI examination. The exclusion criteria included participants with pacemakers implanted before 1998, ICDs implanted before 2000, participants with recent appropriate ICD therapies, and unresponsive participants without durable power of attorney, from whom informed consent could not be obtained. In addition, all devices implanted with new or revised leads within 6 weeks of the MRI request date were excluded to prevent microdislodgement of lead tips that could theoretically impair proper endothelialization. There were four patients excluded due to unresponsive state and four patients excluded due to recent ICD therapies. Informed consent was obtained, and baseline device interrogation was performed immediately before each MRI to assess if battery voltage was adequate, the leads were functional, there were no recent appropriate therapies for participants with ICDs, and to determine if participants were pacemaker dependent. Pacemaker dependency was defined as the absence of a native ventricular rate above 40 beats per minute. Pacemaker-dependent patients were paced asynchronously at 60 beats per minute during the study. For patients with ICDs, all tachycardia therapies were disabled during MRI examinations. Participants with abandoned or epicardial leads or patches were imaged according to the same protocol with no deviations from the protocol listed in Figure 1. All MRI examinations were performed with a 1.5-T scanner (Optima MR450 W; GE Healthcare, Waukesha, Wis) according to standard MRI protocols. All examinations were performed with hemodynamic monitoring in the presence of an Advanced Cardiovascular Life Support–certified electrophysiology nurse. Two nurses, each with more than 20 years of clinical experience, also assessed the patient for any symptoms of chest pain or burning, near syncope, or palpitations during the examination, or for changes in vital signs (eg, heart rate, blood pressure, and oxygen saturation). A physician was immediately available in the event of an emergency but was not physically present in the MRI suite. After each study, a complete device interrogation was performed, followed by reprogramming back to original device settings. All participants had follow-up in a device clinic within 3–4 months after the examination, either via remote download or in-clinic check. Adverse device events were defined as a marked change in battery voltage, a reset of programmed parameters, lead dislodgement, or a persistent change in lead sensing, impedance, or pacing threshold that occurred within 6 months of the MRI examination. An adverse patient event was defined as a sensation of burning or pain at the device site or in the chest, bradyarrhythmia, tachyarrhythmia, syncope or near syncope, cardiac arrest, or death that occurred during the examination or within 24 hours of examination completion. After the examination was completed, all physicians who referred a patient to the PROMeNADe registry were asked to complete a survey to assess the clinical utility of the MRI (Fig 2) within 2 months of examination completion. Survey completion was voluntary and at least two attempts were made to contact the referring physician to complete the survey. Any patient without a completed survey was excluded from the analysis of clinical utility.
Figure 1:
Flowchart demonstrates algorithm of patient enrollment and protocol for device evaluation and programming. BP = blood pressure, ECG = electrocardiogram, ICD = implantable cardioverter defibrillator, PVC = premature ventricular contraction, VOO/DOO = asynchronous pacing, VVI/DDI = inhibited pacing.
Figure 2:
The Patient Registry of Magnetic Resonance Imaging in Non-Approved DEvices survey form sent to referring physicians. The referring physician could select more than one option when answering the fourth question in the survey.
Statistical Analysis
In a previously published registry of MRI examinations in patients with non–MRI-conditional devices, the rate of generator failure requiring replacement and observed atrial arrhythmias occurred at 0.2% (1). Assuming a more conservative event rate of 1% in our population, a sample size of 600 MRI examinations would provide 95% probability of obtaining a 95% confidence interval half-width of ± 1%. Given that 76 patients underwent two MRI examinations performed at the same time (most commonly MRI of head and neck or MRI of abdomen and pelvis), there was a potential impact of ± 1% on estimated margin of error due to clustering of samples. For the clinical utility surveys, if 568 MRI examinations were performed in the registry, to achieve representative results with a 95% confidence interval, a total of 146 completed surveys were necessary. A P value of .05 was used to establish significance. Standard descriptive analyses included summary statistics with percentages and means. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient Populations
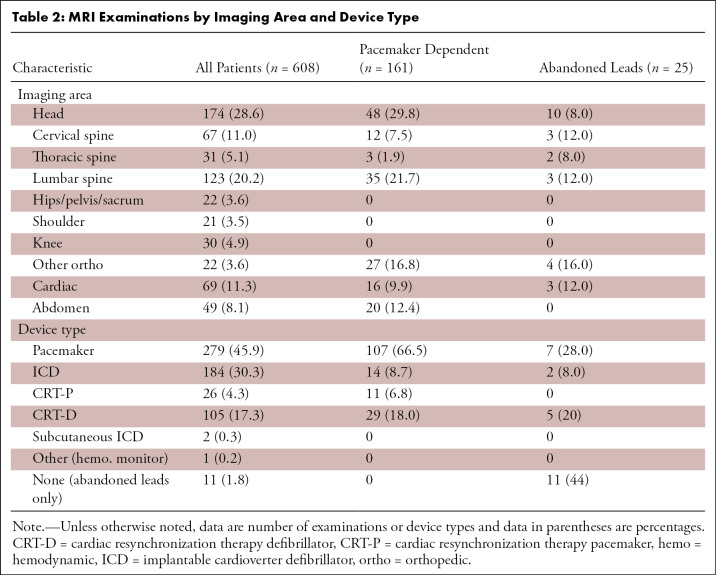
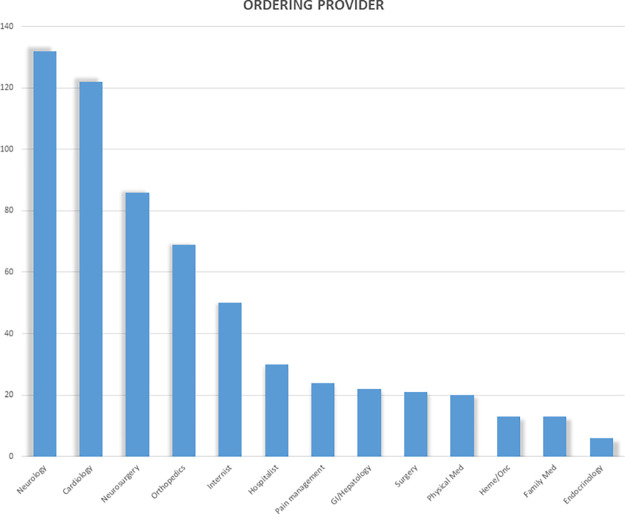
A total of 608 MRI examinations were performed in 532 patients (211 women) with non–MRI-conditional CIEDs. Patient demographics (mean age, 69 years ± 14; 35% women) and device manufacturer are listed in Table 1. The device types and the imaging areas are listed in Table 2. The referring physician was a neurologic specialist in 36% of ordered studies and a cardiologist in 20% (Fig 3). Among the 608 MRI examinations, 161 (26%) examinations were in 121 pacemaker-dependent patients, of which 43 examinations were in defibrillator patients, including 14 with dual-chamber ICDs and 29 with cardiac resynchronization therapy defibrillators (CRT-D). The remaining pacemaker-dependent patients had pacemakers and CRT pacemakers (CRT-Ps), as shown in Table 2. The manufacturer of pacemaker-dependent devices was Medtronic (Mannsfield, Mass) for 112 (70%) examinations, Abbott/St Jude Medical (Abbott Park, Ill) for 38 (24%) examinations, and Boston Scientific (Natick, Mass) for 11 (7%) examinations. The imaging areas for examinations in pacemaker-dependent patients are shown in Table 2. Of note, 86 (53%) of the examinations were in the thoracoabdominal region, of which 16 (10%) were cardiac MRI examinations.
Table 1:
Patient Demographics, Device Type, and Device Manufacturer
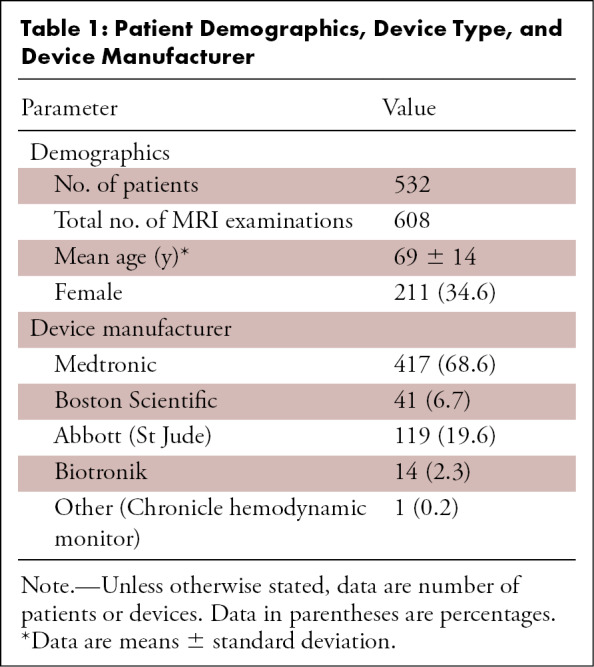
Table 2:
MRI Examinations by Imaging Area and Device Type
Figure 3:
MRI examinations in patients with non–MRI-conditional cardiac implantable electronic devices according to the referring physician by specialty.
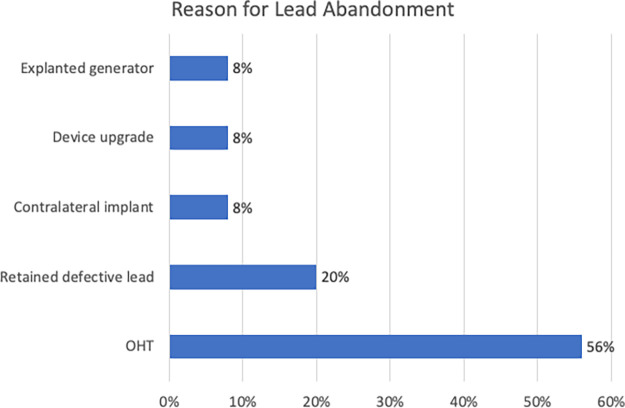
A total of 25 MRI examinations were performed in 15 participants with abandoned leads, of which three examinations were cardiac MRI examinations (Table 2 and Table E1 [supplement]). There was an average of 1.28 abandoned leads per patient with a range from 0.5 lead to three leads. In 11 examinations (44%), there was no implanted device, only abandoned leads (Table 2 and Table E1[supplement]; Fig 4). The remainder of the participants had concomitant pacemakers (28%), ICDs (8%), and CRT-Ds (20%). The most common reason for lead abandonment in our patient population was orthotopic heart transplant (56%), usually with an abandoned right ventricular lead and superior vena cava coil (Fig 5). Other reasons for lead abandonment were defective leads that could not be extracted (20%), device upgrades (8%, typically from a pacemaker to defibrillator), generator explantation (8%), and contralateral device implant when device upgrade was not possible from the ipsilateral side (8%). Some examples of these types of participants are shown in Figures 4, 6–8. One patient had three abandoned leads: one epicardial patch (from a previous abdominal ICD), a coronary sinus pacing lead, and a right ventricular defibrillator lead (Fig 8). Two participants had subcutaneous ICDs that were included prior to these devices obtaining MRI-conditional status.
Figure 4:
Chest radiograph, posteroanterior view, in a 64-year-old woman with history of heart transplantation, demonstrates retained portion of right ventricular pacing lead and defibrillator lead with superior vena cava coil. This patient underwent five MRI examinations as part of this registry.
Figure 5:
MRI examinations in patients with non–MRI-conditional cardiac implantable electronic devices and abandoned leads according to reason for lead abandonment. OHT = orthotopic heart transplant.
Figure 6:
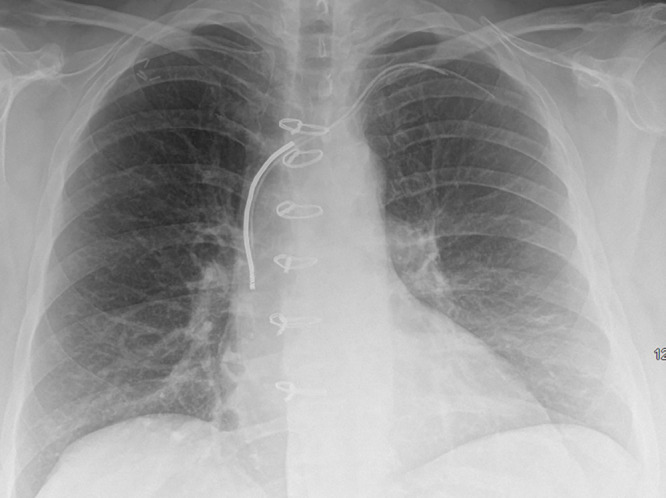
Chest radiograph, posteroanterior view, in an 85-year-old woman with history of previous pacemaker that was explanted and retained right ventricular pacing lead. There is evidence of vertebroplasties at multiple levels.
Figure 8:
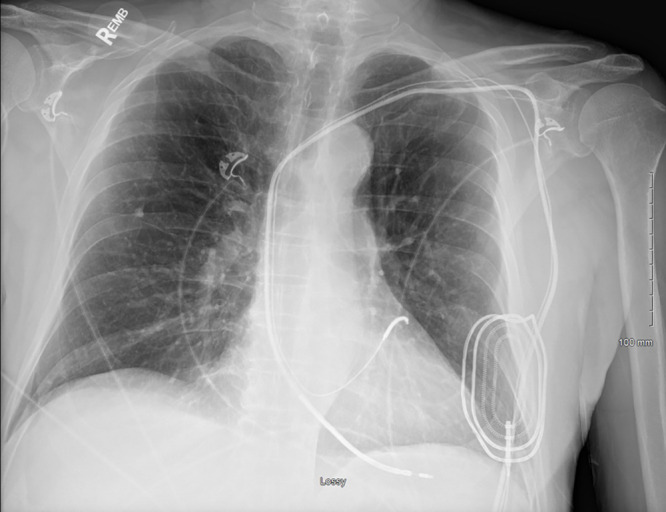
Chest radiograph, anteroposterior view, in a 76-year-old man with history of previous abdominal cardiac resynchronization therapy defibrillator that was explanted, with retained right ventricular defibrillator lead, coronary sinus lead, and an epicardial patch.
Figure 7:
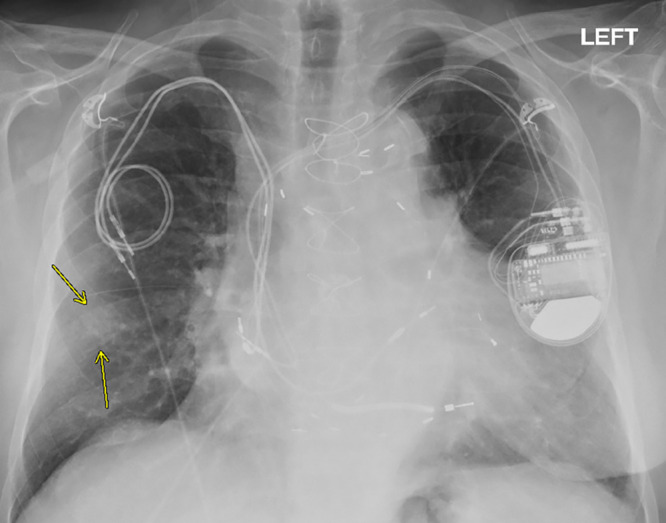
Chest radiograph, posteroanterior view, in a 79-year-old man with history of previous pacemaker, with abandoned right atrial and right ventricular pacing leads on the right side at time of new cardiac resynchronization therapy defibrillator implant on the left side. Arrows indicate a nodular opacity in the right midlung concerning for mass.
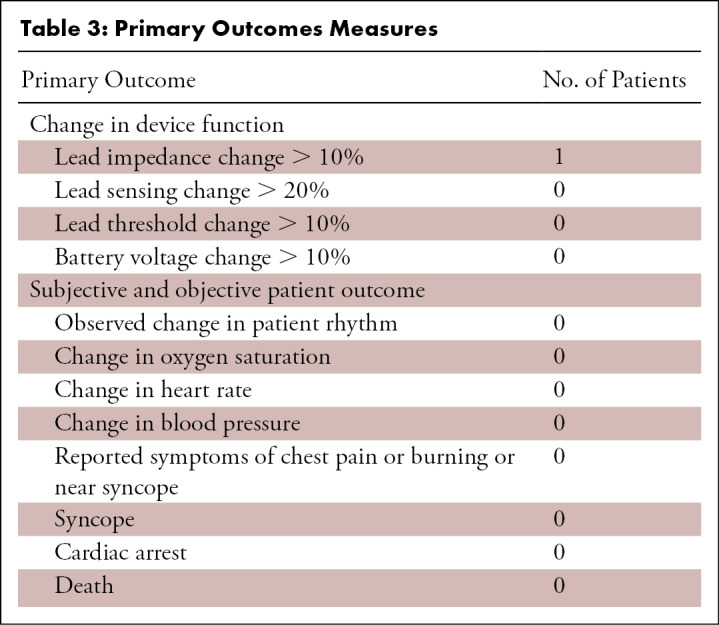
Follow-up data, including device interrogation, were available on all participants at 6 months after MRI. There were no substantial adverse device events or adverse patient events noted (Table 3). Heating of the device and components was determined by assessing the participants for chest discomfort or a sensation of warmth, and this was not reported by any participants in this registry. Three participants were unable to complete the MRI examination, two due to claustrophobia and one due to hip pain (the MRI was of the hip). In 607 of 608 examinations (99.8%), no changes in device programming parameters and function were observed after the MRI examination. In one patient, we found a transient change in coronary sinus impedance in a CRT-P device (510 ohms before the MRI and 1075 ohms afterward). A clinic follow-up was scheduled within 1 week of the MRI and repeat device interrogation showed a return of impedance to baseline (490 ohms), and no intervention was required. There were 76 participants (14%) who underwent multiple MRI studies and no transient changes in device programming parameters were observed in this subset of participants. A physician was summoned to the MRI suite in two cases out of concern for the development of unstable arrhythmia in medically complex participants; however, there were no adverse events in these two participants.
Table 3:
Primary Outcomes Measures
Physician Follow-up
Surveys were requested of consecutive referring physicians until a total of 150 surveys were completed (a total of 348 surveys sent with a response rate of 43%). Of these, 25% of completed surveys were in participants undergoing cardiac MRI. In participants undergoing cardiac MRI, the diagnosis was altered in 35% and confirmed in 54%, while prognosis was altered in 35% and confirmed in 51%. In noncardiac MRI examinations, diagnosis was altered in 25% and confirmed in 69%; prognosis was altered in 26% and confirmed in 66% of these cases.
Impact on treatment plan, including changes in medical management, changes in surgical management, and assistance in planning surgery was assessed (Fig 9b). In 31% of participants, the MRI results changed medical management, based upon the survey results of the referring physician. For surgical participants, MRI assisted in surgical management in 28% of participants and changed surgical management in 11% of participants. In 27% of participants, the MRI obviated further testing, and in 17% of participants, it led to other testing. Based on survey responses, images were of insufficient quality due to imaging artifacts from the device or patient factors in 5% of examinations performed for diagnostic utility, 8% of examinations for prognostic utility, and 3% of examinations performed to guide medical or surgical treatment.
Figure 9b:
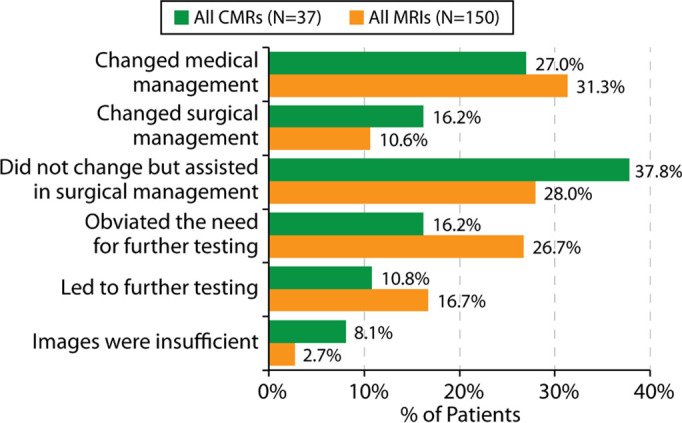
(a-b) Description of survey responses regarding diagnostic utility and prognostic utility (a) as well as impact on treatment plans (b) for patients undergoing cardiac and noncardiac MRI examinations in the presence of nonconditional cardiac implanted electronic devices. CMR = cardiac MRI.
Figure 9a:
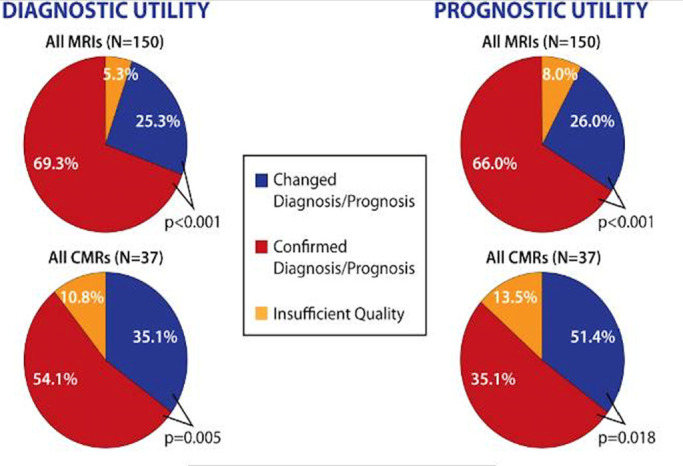
(a-b) Description of survey responses regarding diagnostic utility and prognostic utility (a) as well as impact on treatment plans (b) for patients undergoing cardiac and noncardiac MRI examinations in the presence of nonconditional cardiac implanted electronic devices. CMR = cardiac MRI.
An example of the clinical utility of these examinations includes a patient with significant ventricular arrhythmias who was determined to have cardiac sarcoidosis on late gadolinium imaging (Figure E1 [supplement]). Another example is a patient with severe aortic stenosis and the inability to exclude a left ventricular thrombus on contrast material–enhanced echocardiography prior to transcatheter valve replacement (Figure E2 [supplement]).
Discussion
This analysis of the PROMeNADe registry of patients with non–MRI-conditional implanted cardiac devices who underwent clinically indicated MRI examinations according to a prespecified protocol indicates that these examinations can be performed safely. These data support a growing body of literature attesting to the safety of MRI examinations in patients with CIEDs. Potential risks of thermal lead injury, device displacement, or lethal arrhythmias were not observed in prior studies that carefully adhered to site-specific protocols (1,3–23,24). Initially, it was thought that MRI examinations performed in the thoracic region, including cardiac MRI examinations, would have a higher risk of complications (1,10). Previous studies did not show a significant difference in complication rates between thoracic and nonthoracic MRI examinations (21). The 83 MRI examinations performed in the thoracoabdominal region, without an adverse event, reported in this study add to this growing body of evidence on the safety of thoracic MRI examinations.
Performing MRI examinations in patients with non–MRI-conditional CIEDs in accordance with this protocol requires a substantial investment of time and personnel. Despite this substantial investment of time and resources, the finding of MRI examinations having positive clinical impact in patients with nonapproved devices from the perspective of the referring physicians has not been well established in the literature. Previously published findings indicated that MRI examinations in patients with nonconditional devices were interpretable in 98% of patients and changed treatment in 75% of patients. However, this study only assessed clinical utility by an independent chart review by a physician not involved in the care of the patient (23). Another similar study assessed the clinical utility of MRI examinations in patients with non–MRI-conditional cardiac devices by surveying the MRI physicians and technologists who performed the examination (25). While our study justifies the clinical impact of MRI examinations in patients with non–MRI-conditional devices, it is important to screen the indications for these MRI examinations to ensure that they are absolutely necessary, to justify using additional resources to complete these examinations according to a safety protocol.
Our study differs from previous studies in several elements. First, unlike the MagnaSafe registry, we included thoracic MRI examinations and pacemaker-dependent patients with ICDs (1). In fact, to our knowledge, this is the largest published series of MRI examinations in pacemaker-dependent patients with ICDs. Second, unlike the pioneering work of both the MagnaSafe registry and the study by Nazarian et al, we included patients with abandoned leads. To our knowledge, this is the second largest published series on MRI examinations in patients with abandoned leads and adds incremental evidence to support the safety of performing examinations in this situation. Our study also differs from previous reports in terms of the number of device-related adverse events. In the study by Nazarian et al, the investigators reported nine cases of power-on reset in 1509 patients who underwent 2100 MRI examinations (3). Power-on reset was also reported in other studies (1,11). In the MagnaSafe registry, which included 1500 patients, 1000 with pacemakers and 500 with ICDs, investigators reported one case of inability to interrogate the ICD following MRI, which deviated from the prespecified safety protocol, and the device was consequently replaced immediately. They also report six cases of arrhythmia and six cases of partial electrical reset (1). In contrast, we report a minimal number of device-related adverse events in this study. On the basis of the results of prior studies, we specifically avoided older devices that had been known to have the issue of power-on reset, which likely contributed to a lower rate of adverse events in our registry.
These findings should be interpreted in the context of the following potential limitations. First, MRI safety is not clear in those patients who were excluded from the study, including patients within 6 weeks of undergoing a device implant, hemodynamically unstable patients, or patients requiring recent defibrillator therapy, for whom the referring clinician felt it was unwise to turn off arrhythmia detection while in the MRI. In addition, the surveys sent to referring physicians were retrospective, so recall bias may have influenced physician responses. There is selection bias in that these MRI examinations were only ordered in patients where an MRI examination was deemed to be clinically relevant by the referring provider. Selection bias also exists in that these patients were referred for MRI examination at our institution due to the presence of the PROMeNADe registry, whereas similar patients would not have undergone an MRI at other institutions without such a protocol. Finally, an important portion of this study was continuous hemodynamic and clinical monitoring of the patient during the MRI. As a result, those patients who were intubated, unresponsive, or unable to answer questions regarding discomfort, and/or did not have a designated power of attorney were excluded from this registry.
In conclusion, we found no serious adverse consequences of performing MRI examinations, including cardiac MRI examinations, in a large consecutive cohort of patients with non–MRI-conditional CIEDs. This experience not only adds to the growing body of evidence that MRI examinations can be performed safely in patients with nonapproved cardiac devices under careful protocols, but extends this to include patients with ICDs who are pacemaker-dependent, those with abandoned leads, and those undergoing thoracic MRI examinations. This study also offers support for the extensive nonphysician resources necessary to perform these MRI examinations safely by identifying a substantial impact on clinical care in a large proportion of surveyed cases.
SUPPLEMENTAL TABLE
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgments
We would like to acknowledge the contributions of Gail Kendall, RN, Marcia Price, RN, and Tina Baker, RN, for their contributions to the screening, monitoring, and follow-up of the participants in this study. We also acknowledge the generous support of the Frank and Evangeline Thompson Foundation for supporting this registry.
Disclosures of Conflicts of Interest: S.K.G. disclosed no relevant relationships. L.Y. disclosed no relevant relationships. A.P.W. disclosed no relevant relationships. S.F. disclosed no relevant relationships. I.M.S. disclosed no relevant relationships.
Current address: INOVA Heart and Vascular and Virginia Heart, Falls Church, Va
Supported by a grant from the Frank and Evangeline Thompson Foundation.
Abbreviations:
- CIED
- cardiac implantable electronic devices
- CRT
- cardiac resynchronization therapy
- ICD
- implantable cardioverter defibrillator
- PROMeNADe
- Patient Registry of Magnetic Resonance Imaging in Non-Approved DEvices
References
- 1.Russo RJ, Costa HS, Silva PD, et al. Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. N Engl J Med 2017;376(8):755–764. [DOI] [PubMed] [Google Scholar]
- 2.Faris OP, Shein MJ. Government viewpoint: U.S. Food & Drug Administration: Pacemakers, ICDs and MRI. Pacing Clin Electrophysiol 2005;28(4):268–269. [DOI] [PubMed] [Google Scholar]
- 3.Nazarian S, Hansford R, Rahsepar AA, et al. Safety of Magnetic Resonance Imaging in Patients with Cardiac Devices. N Engl J Med 2017;377(26):2555–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimbel JR, Johnson D, Levine PA, Wilkoff BL. Safe performance of magnetic resonance imaging on five patients with permanent cardiac pacemakers. Pacing Clin Electrophysiol 1996;19(6):913–919. [DOI] [PubMed] [Google Scholar]
- 5.Sommer T, Vahlhaus C, Lauck G, et al. MR imaging and cardiac pacemakers: in-vitro evaluation and in-vivo studies in 51 patients at 0.5 T. Radiology 2000;215(3):869–879. [DOI] [PubMed] [Google Scholar]
- 6.Gimbel JR, Kanal E, Schwartz KM, Wilkoff BL. Outcome of magnetic resonance imaging (MRI) in selected patients with implantable cardioverter defibrillators (ICDs). Pacing Clin Electrophysiol 2005;28(4):270–273. [DOI] [PubMed] [Google Scholar]
- 7.Naehle CP, Strach K, Thomas D, et al. Magnetic resonance imaging at 1.5-T in patients with implantable cardioverter-defibrillators. J Am Coll Cardiol 2009;54(6):549–555. [DOI] [PubMed] [Google Scholar]
- 8.Gimbel JR, Bailey SM, Tchou PJ, Ruggieri PM, Wilkoff BL. Strategies for the safe magnetic resonance imaging of pacemaker-dependent patients. Pacing Clin Electrophysiol 2005;28(10):1041–1046. [DOI] [PubMed] [Google Scholar]
- 9.Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med 2011;155(7):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sommer T, Naehle CP, Yang A, et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac pacemakers in non-pacemaker-dependent patients: a prospective study with 115 examinations. Circulation 2006;114(12):1285–1292. [DOI] [PubMed] [Google Scholar]
- 11.Martin ET, Coman JA, Shellock FG, Pulling CC, Fair R, Jenkins K. Magnetic resonance imaging and cardiac pacemaker safety at 1.5-Tesla. J Am Coll Cardiol 2004;43(7):1315–1324. [DOI] [PubMed] [Google Scholar]
- 12.Shirley ST, Yeung MC, Loshak H. Magnetic Resonance Imaging for Patients with Implantable Cardiac Devices: A Review of Safety and Guidelines. CADTH Rapid Response Report: Summary with Critical Appraisal. Ottawa, Canada: Canadian Agency for Drugs and Technologies in Health, 2019. [PubMed] [Google Scholar]
- 13.Higgins JV, Gard JJ, Sheldon SH, et al. Safety and outcomes of magnetic resonance imaging in patients with abandoned pacemaker and defibrillator leads. Pacing Clin Electrophysiol 2014;37(10):1284–1290. [DOI] [PubMed] [Google Scholar]
- 14.Friedman HL, Acker N, Dalzell C, et al. Magnetic resonance imaging in patients with recently implanted pacemakers. Pacing Clin Electrophysiol 2013;36(9):1090–1095. [DOI] [PubMed] [Google Scholar]
- 15.Sommer T, Bauer W, Fischbach K, et al. MR imaging in patients with cardiac pacemakers and implantable cardioverter defibrillators. Rofo 2017;189(3):204–217. [DOI] [PubMed] [Google Scholar]
- 16.Padmanabhan D, Kella DK, Mehta R, et al. Safety of magnetic resonance imaging in patients with legacy pacemakers and defibrillators and abandoned leads. Heart Rhythm 2018;15(2):228–233. [DOI] [PubMed] [Google Scholar]
- 17.Morris MF, Verma DR, Sheikh H, Su W, Pershad A. Outcomes after magnetic resonance imaging in patients with pacemakers and defibrillators and abandoned leads. Cardiovasc Revasc Med 2018;19(6):685–688. [DOI] [PubMed] [Google Scholar]
- 18.Rahsepar AA, Zimmerman SL, Hansford R, et al. The Relationship between MRI Radiofrequency Energy and Function of Nonconditional Implanted Cardiac Devices: A Prospective Evaluation. Radiology 2020;295(2):307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shellock FG. MRI and Patients with Non-MRI-conditional Cardiac Devices: Further Evidence of Safety. Radiology 2020;295(2):314–315. [DOI] [PubMed] [Google Scholar]
- 20.Nazarian S, Roguin A, Zviman MM, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation 2006;114(12):1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luechinger R, Zeijlemaker VA, Pedersen EM, et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur Heart J 2005;26(4):376–383; discussion 325–327. [DOI] [PubMed] [Google Scholar]
- 22.Langman DA, Goldberg IB, Judy J, Paul Finn J, Ennis DB. The dependence of radiofrequency induced pacemaker lead tip heating on the electrical conductivity of the medium at the lead tip. Magn Reson Med 2012;68(2):606–613. [DOI] [PubMed] [Google Scholar]
- 23.Strom JB, Whelan JB, Shen C, Zheng SQ, Mortele KJ, Kramer DB. Safety and utility of magnetic resonance imaging in patients with cardiac implantable electronic devices. Heart Rhythm 2017;14(8):1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Do DH, Eyvazian V, Bayoneta AJ, et al. Cardiac magnetic resonance imaging using wideband sequences in patients with nonconditional cardiac implanted electronic devices. Heart Rhythm 2018;15(2):218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samar H, Yamrozik JA, Williams RB, et al. Diagnostic Value of MRI in Patients With Implanted Pacemakers and Implantable Cardioverter-Defibrillators Across a Cross Population: Does the Benefit Justify the Risk? A Proof of Concept Study. JACC Clin Electrophysiol 2017;3(9):991–1002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.