Abstract
Artificial intelligence (AI) describes the use of computational techniques to perform tasks that normally require human cognition. Machine learning and deep learning are subfields of AI that are increasingly being applied to cardiovascular imaging for risk stratification. Deep learning algorithms can accurately quantify prognostic biomarkers from image data. Additionally, conventional or AI-based imaging parameters can be combined with clinical data using machine learning models for individualized risk prediction. The aim of this review is to provide a comprehensive review of state-of-the-art AI applications across various noninvasive imaging modalities (coronary artery calcium scoring CT, coronary CT angiography, and nuclear myocardial perfusion imaging) for the quantification of cardiovascular risk in coronary artery disease.
© RSNA, 2021
Summary
This review covers state-of-the-art artificial intelligence applications across various noninvasive imaging modalities for the quantification of cardiovascular risk in coronary artery disease.
Essentials
■ Artificial intelligence (AI) is increasingly being applied to noninvasive cardiovascular imaging modalities for risk stratification in coronary artery disease.
■ Deep learning algorithms can perform automated measurements of prognostic biomarkers directly from image data.
■ Conventional or AI-based imaging parameters can be integrated with clinical data using machine learning models for per-patient risk prediction.
■ The objective ranking of variables in individualized machine learning prediction models increases the explainability of AI findings.
Introduction
Artificial intelligence (AI) is being increasingly applied in cardiovascular medicine for identifying new disease genotypes and phenotypes, enabling cost-effectiveness, and importantly, risk stratification (1). Noninvasive imaging, with a well-established role in assessing the anatomic and functional significance of coronary artery disease (CAD) and guiding subsequent management, is well-primed for scalable AI applications (2,3). This review covers state-of-the-art AI techniques in three-dimensional cardiovascular imaging for the quantification of cardiovascular risk.
Terminology and Techniques
AI is a field of computer science that aims to mimic human cognition in performing tasks such as object or pattern recognition, planning, and problem-solving. The term big data refers to large and heterogeneous data sets that require computational techniques such as AI for their analysis and interpretation (4). In health care, this includes data from the omics fields (eg, genomics, metabolomics, proteomics, lipidomics), tabular data from electronic health records, and imaging data. Within big data exist individual measurable characteristics or datapoints known as features. In imaging, this could be represented by voxel intensity, the spatial distribution of voxels, a vector of motion, or conventional metrics used in clinical practice (2). The quality, accuracy, and richness of features in data are key determinants of AI model performance.
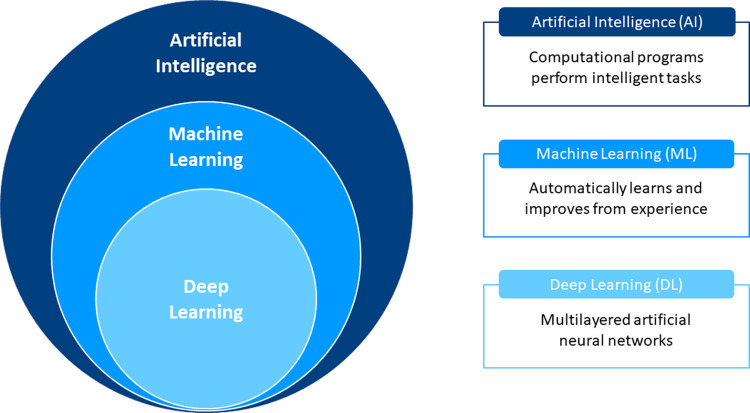
Machine learning (ML), a subfield of AI, enables computer algorithms to automatically learn and improve from experience through exposure to vast amounts of data (Fig 1). While many different algorithmic approaches—or models—exist within ML, they all aim to achieve one of two tasks. The first is supervised learning, in which a labeled data set is used to predict a known outcome. This involves the iterative selection and weighting of individual features to learn the underlying patterns within the data that best fit the outcome (5). Examples of supervised learning algorithms include linear regression, support vector machines, and random forest. The second task is unsupervised learning, whereby unlabeled data are used to predict unknown associations. These models attempt to capture relationships inherent to the structure of the features themselves; examples include clustering and principal component analysis (5).
Figure 1:
Basics of artificial intelligence (AI), machine learning (ML), and deep learning. Artificial intelligence describes the use of computational techniques to perform tasks characteristic of human intelligence. ML is a subfield of AI that enables computers to automatically learn by being exposed to large amounts of data. Deep learning is a specific form of ML that uses multilayered artificial neural networks to make predictions directly from input data.
Deep learning (DL) is a specific form of ML that uses multilayered artificial neural networks to make predictions directly from input data. The most commonly used DL networks for image analysis are convolutional neural networks (CNNs). These contain many layers stacked on top of each other, including one or more convolutional layers that create a feature map summarizing the presence of detected features in the input (6). In health care, some of the greatest successes of DL have been in the field of computer vision, which handles tasks such as object classification, detection, and segmentation from digital images or videos (6). Raw image data are typically transformed to features prior to input into the CNNs. Another emerging application of DL has been for processing of tabular data contained in electronic health records for per-patient predictions. This includes structured data such as laboratory results, diagnostic codes, and demographics, as well as unstructured data that requires standardization and sequencing (6).
Radiomics is the process of extracting thousands of computational quantitative features (most of which are invisible to the human eye) from medical images. These features capture the complex spatial relationship between voxels by describing textural patterns or geometric properties within a given imaging region of interest. ML techniques can then be applied to these data sets to identify imaging biomarkers of significant clinical value (7).
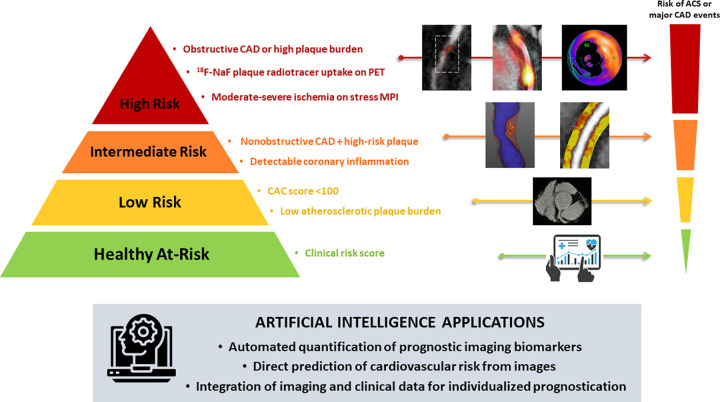
In cardiovascular imaging, AI algorithms can be used to both identify new imaging biomarkers and integrate data from many different sources to provide patient-tailored risk prediction. Figure 2 describes how clinical risk stratification can be enhanced by AI applications in anatomic and functional imaging assessment of CAD. The Table summarizes key recent studies using AI algorithms for risk stratification in noncontrast CT, coronary CT angiography (CCTA), and nuclear cardiology.
Figure 2:
Artificial intelligence (AI) in cardiovascular imaging for risk stratification. Clinical risk stratification based on anatomic and functional imaging assessment of coronary artery disease (CAD). The image case examples (from left to right) correspond to the descriptions (from top to bottom) adjacent to the risk pyramid. AI algorithms can perform automated measurements of prognostic biomarkers from image data. Additionally, conventional or AI-based imaging parameters can be combined with clinical data using machine learning models for individualized risk prediction. ACS = acute coronary syndrome, CAC = coronary artery calcium, 18F-NaF = fluorine 18 sodium fluoride, MPI = myocardial perfusion imaging.
Overview of AI Applications for Risk Stratification in Cardiovascular Imaging Studies
Noncontrast Cardiac CT
Coronary Artery Calcium Scoring
Coronary artery calcium (CAC), a specific marker of coronary atherosclerosis, is a powerful predictor of angiographically significant obstructive CAD (26) and incident cardiovascular events (27). Incremental prognostic value over standard clinical risk scores has also been consistently reported (28,29). In current clinical practice, CAC scoring from noncontrast electrocardiographically gated cardiac CT requires manual interaction by a human operator. Using commercially available software, the reader identifies and labels all voxels above a threshold of 130 HU to quantify the Agatston score (30) or volume score (31). This approach can be labor-intensive and time-consuming; thus, a more automated workflow that reduces the need for human interaction is highly desirable.
ML approaches for the rapid and automated quantification of CAC from dedicated noncontrast cardiac CT have shown promising results. Wolterink et al (32) trained a random forest classifier comprising numerous individual decision trees to distinguish between true calcifications and other candidate calcifications based on size, shape, position, and intensity features. The resultant per-patient Agatston and volume scores demonstrated a strong correlation (r = 0.94) with expert manual measurements. In an extension of this work, the authors introduced a multiclass classification of candidate coronary calcifications to detect CAC per artery, along with an ambiguity detection system that flagged difficult cases for expert review (8). Intraclass correlation coefficients between automatic and fully manual CAC volume scores improved with expert review of difficult cases that had been flagged by the algorithm: left anterior descending artery (0.98 vs 1.00), left circumflex artery (0.69 vs 0.95), and right coronary artery (0.95 vs 0.99). No ambiguous candidates were identified in close to half of all CT scans. Automatic scoring combined with expert review led to a faster processing time of 45 seconds, compared with 128 seconds per scan for fully manual scoring.
CAC scores can also be derived from chest CT performed for other indications, such as lung cancer screening, thus paving the way for application of AI algorithms in large sets of screening data. Fully automated ML- and DL-based quantification of CAC has been evaluated in large data sets of low-dose, ungated CT scans from national lung cancer screening trials in Europe and the United States, demonstrating feasibility and high reliability compared with manual measurements (33,34). Similar results were observed for a CAC scoring DL algorithm trained using radiation therapy–planning CT scans of patients with breast cancer acquired during free breathing (35).
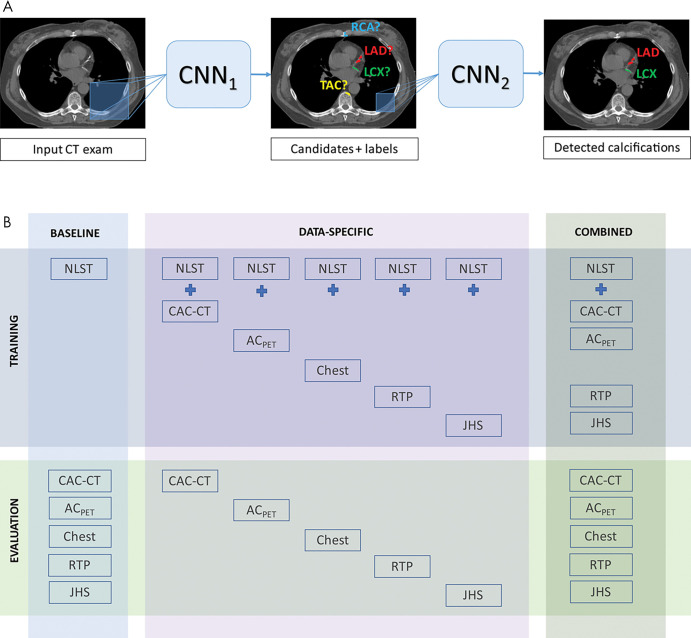
Recently, van Velzen et al (9) sought to validate a DL method for automated CAC scoring using multiple cardiac and chest CT protocols. In 7240 patients who underwent various types of noncontrast chest CT scans, the investigators used two consecutive CNNs to quantify the Agatston score. The first CNN detected candidate calcifications on each image and assigned them to a coronary artery, and the second CNN classified these calcifications as true positive or false positive (Fig 3, A). The DL algorithm was trained and tested on three separate data sets stratified by scan type (Fig 3, B). Compared with manual CAC scoring, the DL algorithm produced reliable measurements, with intraclass correlation coefficients of 0.79–0.97 across the range of scan types. In cardiovascular risk categorization according to the Agatston score, DL had excellent agreement with manual CAC scoring (κ = 0.90 for all test scans). These findings lend support to the use of such a DL method to aid clinicians in CAC scoring across a wide range of noncontrast chest CT images.
Figure 3:
Automatic coronary artery calcium scoring. A, The deep learning algorithm consists of two convolutional neural networks (CNNs). The first CNN (CNN1) detects candidate calcifications (voxels) with attenuation of greater than 130 HU and assigns them to a coronary artery, and the second CNN (CNN2) detects true calcified voxels among candidates detected by the first CNN. B, The baseline algorithm was trained with National Lung Screening Trial (NLST) scans, and its performance was evaluated in each CT protocol type. Five data-specific algorithms were trained, according to CT protocol type, and evaluated in the respective CT type. The combined algorithm was trained and evaluated using all available CT protocol types. CT types used for training were NLST CT scans, coronary artery calcium scoring CT (CAC-CT), PET attenuation correction CT (ACPET), diagnostic chest (Chest) CT, radiation therapy treatment planning (RTP) CT, and Jackson Heart Study (JHS) CT scans. exam = examination, LAD = left anterior descending artery, LCX = left circumflex artery, RCA = right coronary artery, TAC = thoracic aorta calcification. (Reprinted, with permission, from reference 9.)
While AI enables direct computation of CAC measures from CT images, the Agatston score quantified by conventional methods can also be used as an input into AI models for risk prediction. Al’Aref et al (10) trained an ML algorithm with extreme gradient boosting (XGBoost; https://xgboost.ai/) using clinical data from 35 281 patients either suspected of having CAD or with established CAD. An ML model incorporating clinical variables (age, sex, risk factors, and cholesterol levels) yielded an area under the receiver operating characteristic curve (AUC) of 0.77 for predicting the presence of obstructive CAD at CCTA. The addition of CAC score to the ML model resulted in a significantly higher AUC (0.88), which also outperformed a statistical model containing the CAC score and CAD consortium clinical score (AUC, 0.86) and the CAD consortium clinical score alone (AUC, 0.73; P < .05 for all comparisons). Hence, integration of the CAC score and clinical data with ML could potentially be adopted in clinical practice to enhance risk stratification and inform referral for downstream testing such as CCTA.
In 66 636 asymptomatic individuals from the CAC Consortium, Nakanishi et al (11) developed a LogitBoost-based (58) ML model incorporating 20 CAC measures (total and per-vessel Agatston, volume, and density scores), 11 non-CAC metrics (including thoracic and aortic calcium scores), and 46 clinical variables for the prediction of CAD-related death at 10-year follow-up. The ML model (AUC, 0.86) provided superior risk prediction compared with either the atherosclerotic cardiovascular disease (ASCVD) risk score (AUC, 0.83) or the CAC score (AUC, 0.82; P < .001 for both). This comprehensive ML approach also outperformed ML with clinical data alone or CT data alone. Hence, an ML model that can use all available information has the potential to become a routine risk assessment tool, especially as electronic health records are widely adopted in clinical practice and their platforms enable increasingly seamless integration of clinical and imaging data.
Epicardial Adipose Tissue Quantification
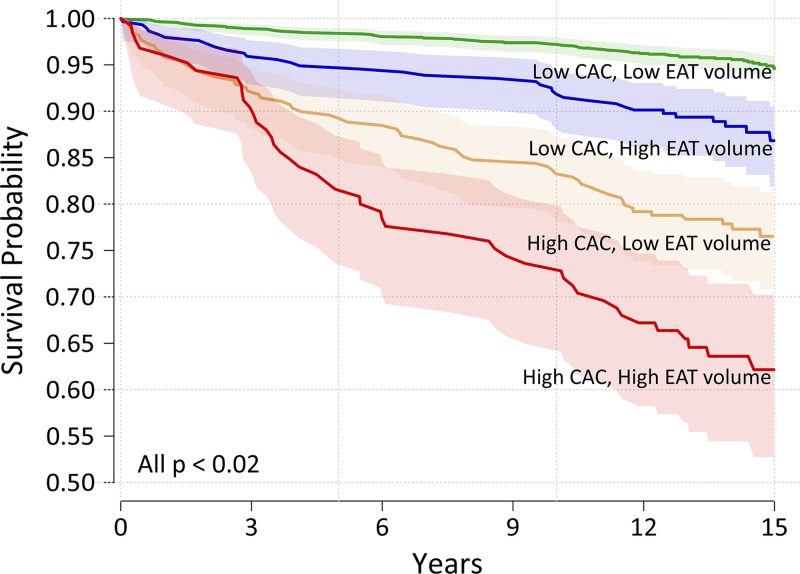
Epicardial adipose tissue is a metabolically active fat depot located between the myocardium and visceral pericardium that modulates coronary arterial function (36). Epicardial adipose tissue volume, which traditionally has been quantified from routine CAC scoring CT using manual or semiautomated techniques (37,38), has been shown to associate with coronary atherosclerosis and incident cardiac events (36). Recently, Commandeur et al (12) developed a fully automated DL-based method for epicardial adipose tissue quantification (Fig 4), which was tested in a large multicenter study and showed a strong correlation with expert manual measurements (r = 0.97; P < .001). DL computation was rapid, with a time of approximately 6 seconds per case, compared with a time of 15 minutes per case for experts. Epicardial adipose tissue measures derived using this DL algorithm were subsequently used for prognostication in 2068 asymptomatic participants from the Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research (EISNER) trial (39). In that study, DL-based epicardial adipose tissue volume was independently associated with increased risk of major adverse cardiovascular events (MACEs) (hazard ratio, 1.35 [95% CI: 1.07, 1.68] per doubling; P = .009), following adjustment for ASCVD risk score and CAC score (Fig 5). Epicardial adipose tissue attenuation by this method was inversely associated with MACE risk (hazard ratio, 0.83 [95% CI: 0.72, 0.96] per 5 HU increase; P = .01). Such rapid, automated measurements of epicardial adipose tissue have the potential for integration into routine reporting of calcium scoring CT, providing real-time, complementary information on cardiovascular risk.
Figure 4:
Deep learning–based quantification of epicardial adipose tissue. A, Three-dimensional rendering of epicardial adipose tissue (pink overlay) derived from coronary artery calcium scoring CT, B, as manually measured by an expert, and C, as automatically quantified by a deep learning algorithm embedded in research software (QFAT [version 2.0; Cedars-Sinai Medical Center]). (Reprinted, with permission, from reference 12.)
Figure 5:
Risk stratification by coronary artery calcium (CAC) score and deep learning–based epicardial adipose tissue (EAT) volume. Kaplan-Meier curves of MACE-free survival in asymptomatic individuals from the Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research trial stratified by low (< 100) versus high (≥ 100) CAC score and low (< 113 cm3) versus high (≥ 113 cm3) EAT volume. Risk was highest in individuals with a high CAC score and high EAT volume. (Reprinted, with permission, from reference 39.)
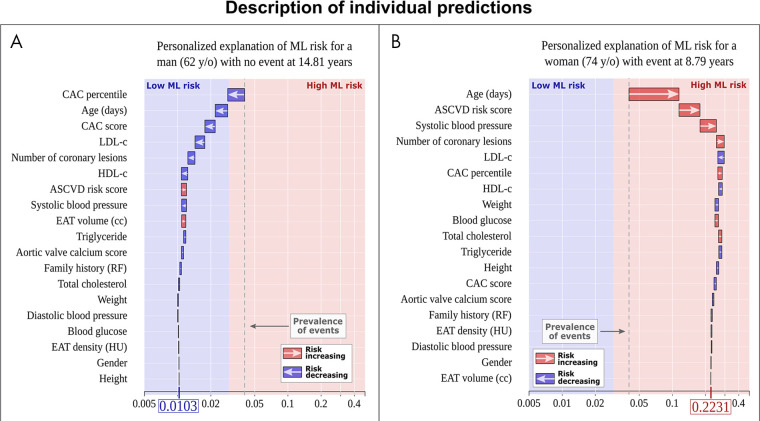
In addition, Commandeur et al (13) combined DL-based epicardial adipose tissue metrics with clinical variables and the CAC score using an XGBoost algorithm for risk stratification in a separate EISNER substudy. The ML risk score (AUC, 0.82) outperformed the ASCVD risk score or CAC score alone (AUC, 0.77 for both; P < .05) for long-term prediction of MACE. A major advantage of this ML approach is its ability to explicitly describe the influence of each variable for individualized prediction and provide so-called explainable AI (Fig 6). This is particularly important given that many ML algorithms are considered “black boxes” with no clear justification for their decision-making. Further, such stratification of significant clinical and imaging parameters could potentially guide therapy targeted at the specific factors affecting cardiovascular outcomes.
Figure 6:
Explainable artificial intelligence through individualized machine learning (ML) risk prediction. ML model for the prediction of myocardial infarction and cardiac death with patient-specific variable importance for A, a 62-year-old man with no event and B, a 74-year-old woman with myocardial infarction. The x-axis corresponds to the ML risk score. The arrows represent the influence of each variable on the overall prediction—either decreasing (blue) or increasing (red) the risk of future events. The final ML risk score is determined by the weighting of each variable and variable-variable interactions within the model. The patient in A has a low ML risk score (0.0103), while the patient in B has a high ML risk score (0.2231). The blue and red colors provide the separation between low and high ML risk as determined by the Youden index. The gray dashed lines represent the overall prevalence of events in the population (approximately 4%). ASCVD = atherosclerotic cardiovascular disease, CAC = coronary artery calcium, EAT = epicardial adipose tissue, HDL = high-density lipoprotein, LDL = low-density lipoprotein, RF = risk factor. (Reprinted, with permission, from reference 13.)
Coronary CT Angiography
Coronary Artery Stenosis Detection and Grading
The prognostic value of anatomic assessment of CAD with CCTA is well established (40), with coronary stenosis detection and quantification being the cornerstone of routine clinical reporting. Currently, these tasks rely on visual assessment and are therefore subject to substantial interobserver variability (41). A number of AI approaches have been able to automatically determine the degree of coronary stenosis directly from image data. Kang et al (14) used a support vector machine incorporating geometric and plaque features to detect coronary artery lesions with at least 25% stenosis in a data set of 42 patients, yielding high sensitivity (93%) and specificity (95%) compared with expert visual assessment. Kelm et al (42) trained a random forest ML algorithm to automatically estimate luminal cross-sectional area and detect obstructive (> 50%) stenosis, achieving a sensitivity of 95% and specificity of 67% compared with experts, at an average speed of 1.8 seconds per case. Hong et al (15) used a CNN to automatically quantify the percentage diameter stenosis and minimal luminal area, with DL measures correlating strongly with expert manual annotations (r = 0.957 for stenosis, r = 0.984 for minimal luminal area; P < .001 for both).
CCTA-derived measures of coronary stenosis have also been integrated into ML models for outcome prediction. In 10 030 patients, Motwani et al (16) trained a LogitBoost algorithm using 25 clinical variables and 44 visually determined CCTA parameters of CAD extent and severity for the prediction of 5-year all-cause mortality. The resultant ML score (AUC, 0.79) outperformed traditional CCTA-based segment scores (AUC, 0.64 for both segment stenosis score and segment involvement score) and the Framingham risk score (AUC, 0.61; P < .001 for all) (Fig 7). Among the highest ranked variables in the ML model were the number of noncalcified segments and number of vessels with diameter stenosis less than 50%.
Figure 7:
![Machine learning (ML) model for the prediction of all-cause mortality. Area under the receiver operating characteristic curves (AUCs) for prediction of 5-year all-cause mortality: an ML risk score integrating clinical and coronary CT angiography data had a higher AUC than the Framingham risk score (FRS) and CT angiography severity scores (segment stenosis score [SSS], segment involvement score [SIS], modified Duke index [DI]; P < .001†). AUCs for the SSS and SIS were greater than FRS (P < .005*). (Reprinted, with permission, from reference 16.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c09a/7978004/4d296cec4956/ryct.2021200512.fig7.jpg)
Machine learning (ML) model for the prediction of all-cause mortality. Area under the receiver operating characteristic curves (AUCs) for prediction of 5-year all-cause mortality: an ML risk score integrating clinical and coronary CT angiography data had a higher AUC than the Framingham risk score (FRS) and CT angiography severity scores (segment stenosis score [SSS], segment involvement score [SIS], modified Duke index [DI]; P < .001†). AUCs for the SSS and SIS were greater than FRS (P < .005*). (Reprinted, with permission, from reference 16.)
Coronary Plaque Characterization and Quantification
CCTA also enables assessment of plaque morphology, which previously could only be evaluated using invasive intracoronary imaging. Qualitative “high-risk plaque” features (positive remodeling, low attenuation plaque, spotty calcification, and napkin-ring sign) derived from CCTA have predictive value for acute coronary syndrome (43,44). Of these features, identification of the napkin-ring sign is most prone to interreader variability as it relies exclusively on visual assessment (45).
Kolossváry et al (46) sought to use CCTA-based radiomics to improve the detection of napkin-ring sign. Of 4400 radiomic features analyzed in this study, 440 (9.9%) exhibited high diagnostic accuracy (AUC > 0.80). Most of these features were textural or geometric, describing the complex spatial distribution of voxels. In another study, radiomic analysis was shown to outperform qualitative features at CCTA for the identification of vulnerable plaques determined by using intracoronary imaging (47). Finally, Kolossváry et al (48) combined CCTA-based radiomic analysis with ML for the identification of histologically determined advanced atherosclerotic lesions. After using 1919 radiomic features to train eight independent ML models, a test model based on least angle regression and fitted with the 13 most predictive parameters was able to outperform visual CCTA assessment (AUC, 0.73 vs 0.65; P = .04).
In a study by Motoyama et al (49), the presence of qualitative high-risk plaque at CCTA (defined by positive remodeling and/or low attenuation plaque) was an independent predictor of future acute coronary syndrome. Importantly, however, the cumulative number of events was similar among patients with and without high-risk plaques; this was primarily attributed to the diffuse nature of coronary atherosclerosis. In more recent studies, CCTA-based quantification of plaque volume and burden in the entire coronary tree using software applications has demonstrated independent prognostic value (50,51). In a landmark post hoc analysis of the multicenter Scottish COmputed Tomography of the HEART (SCOT-HEART) trial with 5-year follow-up, Williams et al (51) showed that patients with a high >4%) low-attenuation noncalcified plaque burden were five times more likely to develop myocardial infarction than patients with a low (≤4%) plaque burden, independent of risk factors, stenosis severity, or CAC score.
CCTA-derived quantitative plaque measures have been incorporated into ML models to enhance outcome prediction. In a lesion-level analysis of a matched cohort of patients with and without acute coronary syndrome, Al’Aref et al (17) input qualitative and quantitative plaque features measured by conventional methods into an XGBoost algorithm for the prediction of culprit lesions associated with acute coronary syndrome. The ML model provided superior performance (AUC, 0.77) compared with quantitative plaque features or qualitative high-risk plaque features alone.
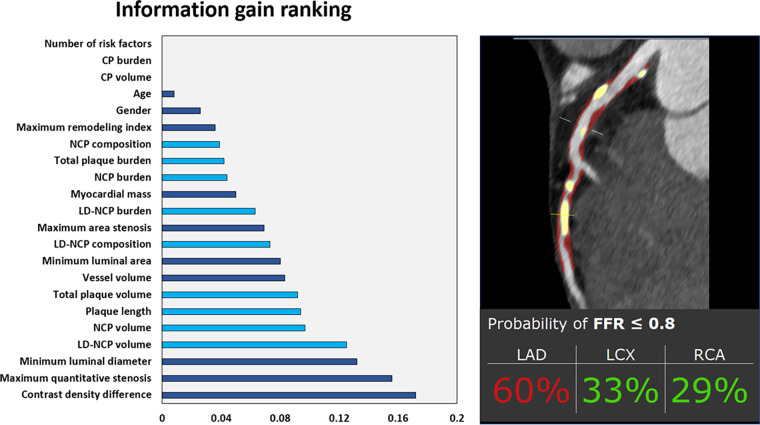
Lesion-specific ischemia by invasive fractional flow reserve, which has a well-established association with adverse cardiac events (52), has been used as the outcome for training AI algorithms. Dey et al (18) combined conventional quantitative plaque and vessel-based metrics in an ML model that provided superior prediction for lesion-specific ischemia (AUC, 0.84) compared with stenosis severity (AUC, 0.76) or pretest probability of CAD (AUC, 0.63). The burdens of noncalcified plaque and low-attenuation noncalcified plaque provided the highest ML information gain (Fig 8). Noninvasive fractional flow reserve measurement derived from CCTA can also be performed with the assistance of DL techniques that identify lumen boundaries and thus enable assessment of computational fluid dynamics. In a real-world study, this method (AUC, 0.94) outperformed conventional CCTA stenosis assessment (AUC, 0.83) and PET (AUC, 0.87; P < .01 for both) for the detection of per-vessel ischemia when compared with invasive angiography as the reference standard (53).
Figure 8:
Machine learning (ML) prediction of lesion-specific ischemia. The left panel shows information gain ranking of variables in a coronary CT angiography (CCTA)–based ML model used to predict lesion-specific ischemia by invasive fractional flow reserve. Quantitative plaque measures are shown in light blue, and other CCTA and clinical variables are shown in dark blue. The right panel is a case example of the ML prediction applied to a symptomatic patient undergoing CCTA, with noncalcified plaque (NCP) and calcified plaque (CP) shown in red and yellow overlay, respectively. Invasive fractional flow reserve (FFR) of the left anterior descending artery (LAD) was positive (0.73). LCX = left circumflex artery, LD-NCP = low-density noncalcified plaque, RCA = right coronary artery. (Reprinted, with permission, from reference 2.)
An ML framework has also demonstrated promising results in predicting the risk of rapid plaque progression. In patients undergoing serial CCTA over a median interval of 3.3 years, Han et al (54) showed a LogitBoost-based model incorporating baseline clinical variables as well as qualitative and quantitative plaque parameters to have greater discriminatory value in identifying patients with rapid plaque progression compared with ML models with clinical variables or qualitative plaque features alone, and also compared with traditional risk scores. Quantitative plaque parameters provided the highest information gain for ML, followed by qualitative plaque parameters and clinical variables.
Detecting Coronary Inflammation
Beyond coronary assessment, CCTA imaging of pericoronary adipose tissue (PCAT) enables detection of coronary inflammation (55), and several AI techniques have recently been applied to PCAT quantification. Oikonomou et al (19) input radiomic features extracted from PCAT into a random forest ML model to develop a patient-level “fat radiomic profile.” When tested in 1575 participants from the SCOT-HEART trial, this metric had incremental value for MACE prediction beyond traditional CCTA-based risk stratification (AUC, 0.88 vs 0.75; P < .001). In a prospective case-control study, Lin et al (56) examined 1103 radiomic features of PCAT in patients with and without acute myocardial infarction. Using XGBoost, an ML model integrating clinical data, the average CT attenuation of PCAT, and PCAT radiomic features (AUC, 0.87) outperformed a model with clinical data and PCAT attenuation (AUC, 0.77; P = .001) and clinical data alone (AUC, 0.87 vs 0.76; P < .001) in identifying patients with myocardial infarction (Fig 9, A). Textural features of PCAT provided the greatest variable importance in the radiomics-based ML model (Fig 9, B). Together, these studies show how combining advanced CCTA-based quantification of PCAT with ML can identify new imaging biomarkers of the so-called vulnerable patient.
Figure 9:
Coronary CT angiography–based radiomics and machine learning (ML) for identifying patients with myocardial infarction. A, Performance of ML models for patients with myocardial infarction. A model integrating clinical data, pericoronary adipose tissue (PCAT) attenuation, and PCAT radiomic features (area under the receiver operating characteristic curve [AUC], 0.87) outperformed a model with clinical data and PCAT attenuation (AUC, 0.77; P = .001) and clinical data alone (AUC, 0.87 vs 0.76; P < .001). B, Textural features of PCAT at coronary CT angiography were highest ranked in the final radiomics-based model. HDL-C = high-density lipoprotein cholesterol, hs-CRP = high-sensitivity C-reactive protein, LDL-C = low-density lipoprotein cholesterol. (Reprinted, with permission, from reference 56.)
Nuclear Cardiology
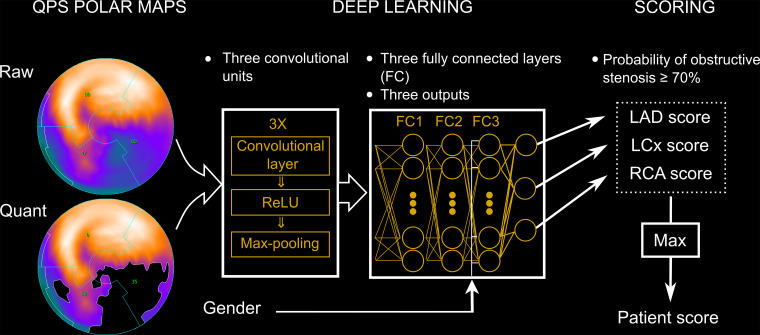
Early studies of AI in nuclear cardiology used quantitative and functional parameters from SPECT myocardial perfusion imaging (MPI) for the prediction of obstructive CAD at invasive angiography. Arsanjani et al (20) combined imaging measures (including stress total perfusion deficit and inducible ischemic and ejection fraction changes) with clinical data and stress electrocardiogram changes using LogitBoost. The integrated ML model provided superior accuracy for detecting obstructive CAD compared with not only total perfusion deficit alone or visual analysis, but also with an ML model without clinical data. Recently, Betancur et al (21) used 1638 MPI scans from REgistry of Fast Myocardial Perfusion Imaging with NExt generation SPECT (REFINE SPECT) for training of a DL algorithm to directly analyze polar map images and detect obstructive CAD (Fig 10). This model outperformed standard total perfusion deficit assessment (AUC, 0.80 vs 0.78; P < .01), with a computation time of less than 0.5 second per patient.
Figure 10:
Deep learning prediction of obstructive coronary artery disease (CAD) from myocardial perfusion imaging. SPECT polar map images are directly connected to the convolutional neural network, and patterns of perfusion defects are identified by feature extraction (left). These image features then pass through three fully connected layers (FC) in a deep learning process (center), which predicts the probability of obstructive CAD in each vascular territory (right). LAD = left anterior descending artery, LCx = left circumflex artery, Max = maximum probability of obstructive CAD, QPS = quantitative perfusion SPECT, ReLU = rectified linear unit (linear function mapping input to output values), RCA = right coronary artery. (Reprinted, with permission, from reference 21.)
MPI data have also been input into ML models for outcome prediction. In another REFINE SPECT substudy, Hu et al (22) used LogitBoost to combine measures of regional perfusion deficits with stress test and clinical variables to predict early revascularization. The per-vessel and per-patient prediction by ML was higher than that of standard quantitative analysis using total perfusion deficit or expert visual analysis. In a subsequent report, Hu et al (23) used clinical and stress perfusion parameters from all 20 414 patients from the REFINE SPECT registry to develop an ML model for automatic rest scan cancellation based on prognostic safety. An XGBoost algorithm was trained on the data to predict MACE, and the resultant ML score was used to select patients for simulated cancellation of their rest scans. This resulted in lower annualized MACE rates compared with rest scan cancellation by conventional clinical criteria. For example, the algorithm could potentially cancel 60% of the rest scans, and in these patients the annual MACE risk would be 1.4%; the same cancellation rate based on visual scoring would result in an annual 2.1% MACE rate. These findings lend support to the clinical adoption of such an ML approach, which could reduce radiation exposure and health care costs while ensuring prognostic safety.
Betancur et al (24) applied a LogitBoost algorithm for the prediction of 3-year MACE risk in 2619 patients undergoing clinically indicated exercise or pharmacologic MPI. They showed a comprehensive ML model integrating imaging, stress test, and clinical variables to outperform expert visual interpretation, automated measures of total perfusion deficit, or ML with only imaging variables (AUC, 0.81 vs 0.73, 0.71, and 0.65, respectively; P < .01 for all). Further, the combined ML model provided a 26% (P < .001) risk reclassification for MACE compared with visual analysis. Different ML algorithms using MPI and clinical data have been tested in parallel against traditional statistical methods for the prediction of cardiac death. In an analysis by Haro Alonso et al (25), a support vector machine model with 49 features had the highest accuracy compared with a baseline logistic regression model with 122 features (AUC, 0.83 vs 0.76; P < .0001). A least absolute shrinkage and selection operator approach minimized the number of predictive features to six, while providing slightly better accuracy than logistic regression (AUC, 0.77; P = .045). These findings highlight the role of ML in selecting the most important predictive variables, enabling the relevance of risk factors to be readily appreciated within the time constraints of clinical practice.
Automated cardiovascular risk assessment can also be performed using a hybrid imaging approach. In patients undergoing 82Rb PET/CT, Išgum et al (57) applied an ML method of automated CAC scoring (8) to low-dose CT attenuation correction images, which are routinely acquired after the PET studies. There was excellent agreement between automated CT attenuation correction scoring and the reference standard of dedicated CAC scoring CT with manual quantification (κ = 0.79–0.82). Hence, this AI approach is not only highly feasible but may enable clinical cardiovascular risk assessment using the CT attenuation correction component of PET/CT MPI without the need for any additional protocols or radiation exposure.
Challenges to AI Implementation
Although the described AI applications hold great promise for cardiovascular risk stratification, there are several barriers to their clinical implementation. First, the accuracy of ML models will always be limited by the availability and quality of data used for training. Hence, ML algorithms may not perform as well in areas of cardiovascular imaging that do not generate large data sets, such as with rare diseases, uncommon presentations of common diseases, or historic archived images. Further, standardization of image acquisition protocols and formats is required before data from different institutions can be input into a standard AI model. Second, running AI algorithms to handle vast data sets can be computationally expensive, thereby limiting their widespread availability. However, advances in increasingly affordable hardware (eg, graphics processing units) and software (eg, cloud-based computing services) solutions will facilitate the integration and storage of imaging big data. Finally, AI algorithms trained and tested at a single institution may not be generalizable to different cohorts. Thus, all models will require external validation across multiple centers and imaging vendors before their widespread clinical adoption. Moreover, as patient characteristics, disease patterns, and image acquisition and reconstruction techniques change over time, AI models may benefit from continuous updating using large and dynamic data sets.
Summary
Noninvasive cardiovascular imaging is data-rich and primed for AI-powered solutions. As shown in this review, AI can be used to quantify cardiovascular risk by two main methods: (a) through the direct application of DL algorithms to image data for automated quantification of prognostic biomarkers; or (b) through integration of conventional or AI-based imaging metrics with tabular data in ML models for individualized outcome prediction. For all the potential applications, however, high-quality data and model validation on unseen data sets are key to success. Ongoing advancements in AI algorithms and an increasing standardization of imaging protocols and parameters will likely improve the accuracy and explainability of AI-based risk quantification. In the near future, one can envisage AI working in the background of standard cardiac image analysis software and clinical reporting, automatically collecting data and computing risk scores to provide real-time prognostication and guide personalized therapy.
Supported in part by the National Heart, Lung, and Blood Institute (grants 1R01HL133616 and 1R01HL148787-01A1).
Disclosures of Conflicts of Interest: A.L. disclosed no relevant relationships. M.K. disclosed no relevant relationships. M.M. disclosed no relevant relationships. I.I. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institutional research grants from Pie Medical Imaging BV (Dutch Technology Foundation with participation of Pie Medical Imaging and Philips Healthcare), The Netherlands Organisation for Health Research and Development with participation of Pie Medical Imaging; Dutch Cancer Foundation; author has patent issued with Pie Medical Imaging; cofounder, scientific lead, and shareholder of Quantib-U. Other relationships: disclosed no relevant relationships. P.M.H. disclosed no relevant relationships. P.J.S. Activities related to the present article: institution has grant from NIH. Activities not related to the present article: institution has grant from Siemens; author receives fund from patent of CT analysis software; author receives software royalties from Cedars-Sinai Medical Center. Other relationships: patent pending with Cedars-Sinai for explainable AI models for myocardial perfusion images. D.D. Activities related to the present article: institution received grant from National Heart, Lung, and Blood Institute (1R01HL133616 and 1R01HL148787-01A1). Activities not related to the present article: author receives software royalties from Cedars-Sinai Medical Center and holds patent. Other relationships: disclosed no relevant relationships.
Abbreviations:
- AI
- artificial intelligence
- ASCVD
- atherosclerotic cardiovascular disease
- AUC
- area under the receiver operating characteristic curve
- CAC
- coronary artery calcium
- CAD
- coronary artery disease
- CCTA
- coronary CT angiography
- CNN
- convolutional neural network
- DL
- deep learning
- EISNER
- Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research
- MACE
- major adverse cardiovascular events
- ML
- machine learning
- MPI
- myocardial perfusion imaging
- PCAT
- pericoronary adipose tissue
- REFINE SPECT
- Registry of Fast Myocardial Perfusion Imaging with NExt generation SPECT
- SCOT-HEART
- Scottish COmputed Tomography of the HEART
References
- 1.Krittanawong C, Zhang H, Wang Z, Aydar M, Kitai T. Artificial Intelligence in Precision Cardiovascular Medicine. J Am Coll Cardiol 2017;69(21):2657–2664. [DOI] [PubMed] [Google Scholar]
- 2.Dey D, Slomka PJ, Leeson P, et al. Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;73(11):1317–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin A, Kolossváry M, Išgum I, Maurovich-Horvat P, Slomka PJ, Dey D. Artificial intelligence: improving the efficiency of cardiovascular imaging. Expert Rev Med Devices 2020;17(6):565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Mauro A, Greco M, Grimaldi M. A formal definition of Big Data based on its essential features. Library Rev 2016;65(3):122–135. [Google Scholar]
- 5.Deo RC. Machine Learning in Medicine. Circulation 2015;132(20):1920–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteva A, Robicquet A, Ramsundar B, et al. A guide to deep learning in healthcare. Nat Med 2019;25(1):24–29. [DOI] [PubMed] [Google Scholar]
- 7.Kolossváry M, Kellermayer M, Merkely B, Maurovich-Horvat P. Cardiac Computed Tomography Radiomics: A Comprehensive Review on Radiomic Techniques. J Thorac Imaging 2018;33(1):26–34. [DOI] [PubMed] [Google Scholar]
- 8.Wolterink JM, Leiner T, Takx RAP, Viergever MA, Isgum I. Automatic Coronary Calcium Scoring in Non-Contrast-Enhanced ECG-Triggered Cardiac CT With Ambiguity Detection. IEEE Trans Med Imaging 2015;34(9):1867–1878. [DOI] [PubMed] [Google Scholar]
- 9.van Velzen SGM, Lessmann N, Velthuis BK, et al. Deep Learning for Automatic Calcium Scoring in CT: Validation Using Multiple Cardiac CT and Chest CT Protocols. Radiology 2020;295(1):66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al’Aref SJ, Maliakal G, Singh G, et al. Machine learning of clinical variables and coronary artery calcium scoring for the prediction of obstructive coronary artery disease on coronary computed tomography angiography: analysis from the CONFIRM registry. Eur Heart J 2020;41(3):359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakanishi R, Slomka P, Rios R, et al. Machine Learning Adds to Clinical and CAC Assessments in Predicting 10-Year CHD and CVD Deaths. JACC Cardiovasc Imaging doi:10.1016/j.jcmg.2020.08.024. Published online October 28, 2020. Accessed November 21, 2020. [DOI] [PMC free article] [PubMed]
- 12.Commandeur F, Goeller M, Razipour A, et al. Fully Automated CT Quantification of Epicardial Adipose Tissue by Deep Learning: A Multicenter Study. Radiol Artif Intell 2019;1(6):e190045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Commandeur F, Slomka PJ, Goeller M, et al. Machine learning to predict the long-term risk of myocardial infarction and cardiac death based on clinical risk, coronary calcium, and epicardial adipose tissue: a prospective study. Cardiovasc Res 2020;116(14):2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang D, Dey D, Slomka PJ, et al. Structured learning algorithm for detection of nonobstructive and obstructive coronary plaque lesions from computed tomography angiography. J Med Imaging (Bellingham) 2015;2(1):014003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong Y, Commandeur F, Cadet S, et al. Deep learning-based stenosis quantification from coronary CT Angiography. Proc SPIE Int Soc Opt Eng 2019;10949:109492I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motwani M, Dey D, Berman DS, et al. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur Heart J 2017;38(7):500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al’Aref SJ, Singh G, Choi JW, et al. A Boosted Ensemble Algorithm for Determination of Plaque Stability in High-Risk Patients on Coronary CTA. JACC Cardiovasc Imaging 2020;13(10):2162–2173. [DOI] [PubMed] [Google Scholar]
- 18.Dey D, Gaur S, Ovrehus KA, et al. Integrated prediction of lesion-specific ischaemia from quantitative coronary CT angiography using machine learning: a multicentre study. Eur Radiol 2018;28(6):2655–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oikonomou EK, Williams MC, Kotanidis CP, et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur Heart J 2019;40(43):3529–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arsanjani R, Xu Y, Dey D, et al. Improved accuracy of myocardial perfusion SPECT for detection of coronary artery disease by machine learning in a large population. J Nucl Cardiol 2013;20(4):553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betancur J, Commandeur F, Motlagh M, et al. Deep Learning for Prediction of Obstructive Disease From Fast Myocardial Perfusion SPECT: A Multicenter Study. JACC Cardiovasc Imaging 2018;11(11):1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu LH, Betancur J, Sharir T, et al. Machine learning predicts per-vessel early coronary revascularization after fast myocardial perfusion SPECT: results from multicentre REFINE SPECT registry. Eur Heart J Cardiovasc Imaging 2020;21(5):549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu LH, Miller RJH, Sharir T, et al. Prognostically safe stress-only single-photon emission computed tomography myocardial perfusion imaging guided by machine learning: report from REFINE SPECT. Eur Heart J Cardiovasc Imaging doi:10.1093/ehjci/jeaa134. Published online June 12, 2020. Accessed November 21, 2020 . [DOI] [PubMed]
- 24.Betancur J, Otaki Y, Motwani M, et al. Prognostic Value of Combined Clinical and Myocardial Perfusion Imaging Data Using Machine Learning. JACC Cardiovasc Imaging 2018;11(7):1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haro Alonso D, Wernick MN, Yang Y, Germano G, Berman DS, Slomka P. Prediction of cardiac death after adenosine myocardial perfusion SPECT based on machine learning. J Nucl Cardiol 2019;26(5):1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budoff MJ, Diamond GA, Raggi P, et al. Continuous probabilistic prediction of angiographically significant coronary artery disease using electron beam tomography. Circulation 2002;105(15):1791–1796. [DOI] [PubMed] [Google Scholar]
- 27.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49(18):1860–1870. [DOI] [PubMed] [Google Scholar]
- 28.Erbel R, Möhlenkamp S, Moebus S, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010;56(17):1397–1406. [DOI] [PubMed] [Google Scholar]
- 29.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012;308(8):788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15(4):827–832. [DOI] [PubMed] [Google Scholar]
- 31.Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 1998;208(3):807–814. [DOI] [PubMed] [Google Scholar]
- 32.Wolterink JM, Leiner T, Takx RAP, Viergever MA, Išgum I. An automatic machine learning system for coronary calcium scoring in clinical non-contrast enhanced, ECG-triggered cardiac CT. In: Aylward S, Hadjiiski LM, eds. Proceedings of SPIE: medical imaging 2014—computer-aided diagnosis. Vol 9035. Bellingham, Wash: International Society for Optics and Photonics, 2014; 90350E. [Google Scholar]
- 33.Takx RAP, de Jong PA, Leiner T, et al. Automated coronary artery calcification scoring in non-gated chest CT: agreement and reliability. PLoS One 2014;9(3):e91239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lessmann N, van Ginneken B, Zreik M, et al. Automatic Calcium Scoring in Low-Dose Chest CT Using Deep Neural Networks With Dilated Convolutions. IEEE Trans Med Imaging 2018;37(2):615–625. [DOI] [PubMed] [Google Scholar]
- 35.Gernaat SAM, van Velzen SGM, Koh V, et al. Automatic quantification of calcifications in the coronary arteries and thoracic aorta on radiotherapy planning CT scans of Western and Asian breast cancer patients. Radiother Oncol 2018;127(3):487–492. [DOI] [PubMed] [Google Scholar]
- 36.Lin A, Dey D, Wong DTL, Nerlekar N. Perivascular Adipose Tissue and Coronary Atherosclerosis: from Biology to Imaging Phenotyping. Curr Atheroscler Rep 2019;21(12):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorter PM, de Vos AM, van der Graaf Y, et al. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol 2008;102(4):380–385. [DOI] [PubMed] [Google Scholar]
- 38.Dey D, Wong ND, Tamarappoo B, et al. Computer-aided non-contrast CT-based quantification of pericardial and thoracic fat and their associations with coronary calcium and Metabolic Syndrome. Atherosclerosis 2010;209(1):136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eisenberg E, McElhinney PA, Commandeur F, et al. Deep Learning-Based Quantification of Epicardial Adipose Tissue Volume and Attenuation Predicts Major Adverse Cardiovascular Events in Asymptomatic Subjects. Circ Cardiovasc Imaging 2020;13(2):e009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Min JK, Dunning A, Lin FY, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings: results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58(8):849–860. [DOI] [PubMed] [Google Scholar]
- 41.Arbab-Zadeh A, Hoe J. Quantification of coronary arterial stenoses by multidetector CT angiography in comparison with conventional angiography methods, caveats, and implications. JACC Cardiovasc Imaging 2011;4(2):191–202. [DOI] [PubMed] [Google Scholar]
- 42.Kelm BM, Mittal S, Zheng Y, et al. Detection, grading and classification of coronary stenoses in computed tomography angiography. Med Image Comput Comput Assist Interv 2011;14(Pt 3):25–32. [DOI] [PubMed] [Google Scholar]
- 43.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54(1):49–57. [DOI] [PubMed] [Google Scholar]
- 44.Otsuka K, Fukuda S, Tanaka A, et al. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc Imaging 2013;6(4):448–457. [DOI] [PubMed] [Google Scholar]
- 45.Maurovich-Horvat P, Hoffmann U, Vorpahl M, Nakano M, Virmani R, Alkadhi H. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc Imaging 2010;3(4):440–444. [DOI] [PubMed] [Google Scholar]
- 46.Kolossváry M, Karády J, Szilveszter B, et al. Radiomic Features Are Superior to Conventional Quantitative Computed Tomographic Metrics to Identify Coronary Plaques With Napkin-Ring Sign. Circ Cardiovasc Imaging 2017;10(12):e006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolossváry M, Park J, Bang JI, et al. Identification of invasive and radionuclide imaging markers of coronary plaque vulnerability using radiomic analysis of coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2019;20(11):1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolossváry M, Karády J, Kikuchi Y, et al. Radiomics versus Visual and Histogram-based Assessment to Identify Atheromatous Lesions at Coronary CT Angiography: An ex Vivo Study. Radiology 2019;293(1):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Motoyama S, Ito H, Sarai M, et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J Am Coll Cardiol 2015;66(4):337–346. [DOI] [PubMed] [Google Scholar]
- 50.Chang HJ, Lin FY, Lee SE, et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J Am Coll Cardiol 2018;71(22):2511–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams MC, Kwiecinski J, Doris M, et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results From the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020;141(18):1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson NP, Tóth GG, Lai D, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol 2014;64(16):1641–1654. [DOI] [PubMed] [Google Scholar]
- 53.Driessen RS, Danad I, Stuijfzand WJ, et al. Comparison of Coronary Computed Tomography Angiography, Fractional Flow Reserve, and Perfusion Imaging for Ischemia Diagnosis. J Am Coll Cardiol 2019;73(2):161–173. [DOI] [PubMed] [Google Scholar]
- 54.Han D, Kolli KK, Al’Aref SJ, et al. Machine Learning Framework to Identify Individuals at Risk of Rapid Progression of Coronary Atherosclerosis: From the PARADIGM Registry. J Am Heart Assoc 2020;9(5):e013958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Antonopoulos AS, Sanna F, Sabharwal N, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 2017;9(398):eaal2658. [DOI] [PubMed] [Google Scholar]
- 56.Lin A, Kolossváry M, Yuvaraj J, et al. Myocardial Infarction Associates With a Distinct Pericoronary Adipose Tissue Radiomic Phenotype: A Prospective Case-Control Study. JACC Cardiovasc Imaging 2020;13(11):2371–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Išgum I, de Vos BD, Wolterink JM, et al. Automatic determination of cardiovascular risk by CT attenuation correction maps in Rb-82 PET/CT. J Nucl Cardiol 2018;25(6):2133–2142 [Published correction appears in J Nucl Cardiol 2018;25(6):2143.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedman J, Hastie T, Tibshirani R. Additive logistic regression: a statistical view of boosting (with discussion and a rejoinder by the authors). Ann Stat 2000;28:337–407. [Google Scholar]




![Deep learning–based quantification of epicardial adipose tissue. A, Three-dimensional rendering of epicardial adipose tissue (pink overlay) derived from coronary artery calcium scoring CT, B, as manually measured by an expert, and C, as automatically quantified by a deep learning algorithm embedded in research software (QFAT [version 2.0; Cedars-Sinai Medical Center]). (Reprinted, with permission, from reference 12.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c09a/7978004/7172bbb7ac51/ryct.2021200512.fig4.jpg)
![Coronary CT angiography–based radiomics and machine learning (ML) for identifying patients with myocardial infarction. A, Performance of ML models for patients with myocardial infarction. A model integrating clinical data, pericoronary adipose tissue (PCAT) attenuation, and PCAT radiomic features (area under the receiver operating characteristic curve [AUC], 0.87) outperformed a model with clinical data and PCAT attenuation (AUC, 0.77; P = .001) and clinical data alone (AUC, 0.87 vs 0.76; P < .001). B, Textural features of PCAT at coronary CT angiography were highest ranked in the final radiomics-based model. HDL-C = high-density lipoprotein cholesterol, hs-CRP = high-sensitivity C-reactive protein, LDL-C = low-density lipoprotein cholesterol. (Reprinted, with permission, from reference 56.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c09a/7978004/8ef3b0ca174f/ryct.2021200512.fig9.jpg)