Abstract
Purpose
To investigate the prognostic value of an integrative approach combining clinical variables and the Qanadli CT obstruction index (CTOI) in patients with nonmassive acute pulmonary embolism (PE).
Materials and Methods
This retrospective study included 705 consecutive patients (mean age, 63 years; range, 18–95 years) with proven PE. Clot burden was quantified using the CTOI, which reflects the ratio of fully or partially obstructed pulmonary arteries to normal arteries. Patients were subdivided into two groups according to the presence (group A) or absence (group B) of preexisting cardiopulmonary disease. Thirty-day and 3-month mortality was evaluated. CTOI thresholds of 20% and 40% were used to stratify patients regarding outcome (low, intermediate, and high risk). The predictive value of CTOI was assessed through logistic regression analysis.
Results
Analysis included 690 patients (mean age, 63.3 years ± 18 [standard deviation]) with complete follow-up data: 247 (36%) in group A and 443 (64%) in group B. The mean CTOI was 23% ± 19, 30-day mortality was 9.7%, and 3-month mortality was 11.6%. Three-month mortality was higher in group A than in group B (17.8% and 8.1%, respectively; P = .001). Within group B, CTOI predicted outcome and allowed stratification: significantly higher mortality with CTOI greater than 40% (P < .001) and lower mortality with CTOI less than 20% (P = .05). CTOI did not predict outcome in group A. Age was an independent mortality risk factor (P ≤ .04).
Conclusion
CTOI predicted outcome in this cohort of patients with PE and no cardiopulmonary disease, and it may provide a simple single-examination–based approach for risk stratification in this subset of patients.
© RSNA, 2020
See also the commentary by Kay and Abbara in this issue.
Summary
In patients with pulmonary embolism but without concomitant cardiopulmonary disease, the CT obstruction index can identify low-risk patients who may benefit from outpatient treatment as well as high-risk patients who may need more aggressive therapy.
Key Points
■ When combined with clinical factors, the CT obstruction index can predict outcomes in the setting of acute pulmonary embolism.
■ A CT obstruction index less than 20% predicts favorable outcome in patients with pulmonary embolism but without cardiopulmonary comorbidities or pulmonary malignancy.
■ Patients with pulmonary embolism as well as cardiopulmonary comorbidities or pulmonary malignancy are at increased risk for fatal outcome regardless of the CT obstruction index.
Introduction
CT pulmonary angiography is the study of choice for accurate and reproducible diagnosis of acute pulmonary embolism (PE) (1). Despite remarkable advances in diagnostic work-up, survival rates after acute PE still vary widely, with short-term survival ranging from 71% to 95% and long-term survival from 61% to 75% (2,3). Thus, it has become a clinical priority to develop a means of rapid and confident risk stratification in patients with acute PE, to improve both short- and long-term survival (4).
Prognostic approaches derived from CT pulmonary angiography are particularly attractive owing to their simplicity and availability. One such approach is the CT obstruction index (CTOI) described by Qanadli et al (5). The CTOI is a clot burden score for acute PE that assigns a heavier weight when the vessel is fully occluded than when it is only partially obstructed, providing information regarding blood flow in the parenchyma distal to the clot. However, controversial results have been reported regarding the ability of the CTOI to predict mortality in patients with acute PE (6–8). The discrepancies may be related to the limited numbers of patients in most prior studies, and the failure to account for the presence or absence of concomitant comorbidities. Other studies emphasize the importance of providing individualized patient care depending on PE severity (4,9), both to ensure adequate treatment and monitoring of patients with severe disease and to identify low-risk patients who will benefit from outpatient treatment.
In this study, our intent was to develop a mortality risk stratification model for patients presenting with nonmassive acute PE. Massive PE was defined as systemic hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes). The model accounted for CTOI, patient age, and the presence or absence of cardiopulmonary disease (CPD), under the assumption that CPD influences the predictive value of CTOI.
Materials and Methods
The institutional ethics committee approved this single-center retrospective study and waived the requirement for informed consent.
Study Population
This study included data from consecutive inpatients or outpatients who visited the emergency department with CT pulmonary angiography–proven nonmassive acute PE, as defined by the American Heart Association (10), between January 2013 and December 2015. To identify these patients, the institutional electronic health database was queried by a radiologist with 5 years of experience in cardiovascular imaging (D.C.R.). Patients were excluded if they were younger than 18 years or underwent thrombolysis. A total of 705 consecutive patients were identified, including 376 women and 329 men (mean age, 63 years; range, 18–95 years).
Image Acquisition, Analysis, and Obstruction Index
All patients underwent CT pulmonary angiography with a 64-row multidetector system (LightSpeed VCT; GE Healthcare, Milwaukee, Wis), following a standard protocol. The acquisition and reconstruction parameters were as follows: tube potential, 120 kVp; tube current, 100–300 mA; beam collimation, 64 mm × 0.625 mm; beam pitch, 0.984; gantry rotation, 0.6 second; acquisition direction, caudocranial; reconstruction kernel, standard; slice thickness, 1.25 mm; and section overlap, 1 mm. Bolus tracking was used after the injection of 80 mL iohexol (concentration: 300 mg I/mL) at a flow rate of 4 mL/sec, followed by a flush with 40 mL physiologic saline, during a single breath hold.
Images were reviewed with an Advantage Workstation (GE Healthcare, Buc, France), using standard mediastinal windows with real-time ability to change the window width and level settings for optimal vessel visualization. Image review was performed by two radiologists (S.D.Q. and D.C.R.) with 18 and 5 years, respectively, of experience in reading cardiothoracic CT examinations, who were blinded to the patient outcome. Images were reviewed in consensus rather than independently, since the interrater reliability for CTOI has been evaluated previously (11). The CT pulmonary angiography criteria for PE diagnosis included direct visualization of nonocclusive endoluminal embolus (central filling defect completely or partially outlined by contrast agent), or complete occlusion by thrombus in normal-sized or enlarged vessels. Clot burden was quantified following the method described by Qanadli et al (5), in which each lung is considered to have 10 segmental arteries (three to the upper lobes, two to the middle lobe or lingula, and five to the lower lobes). In the presence of a proximal or lobar clot, the segmental and subsegmental arteries were not interpreted. Clot burden was calculated using the following formula:
where n is the number of segmental vessels arising distally (minimum, 1, indicates occlusive disease in one segment; maximum, 20, indicates occlusive disease in both the right and left pulmonary arteries), and d is the degree of obstruction (0, patent vessel; 1, partial occlusion; 2, completely occluded vessel).
Outcome Predictors and Risk Stratification Model
The study population was subdivided into two groups according to the presence (group A) or absence (group B) of preexisting CPD, using a structured approach based on image analysis and retrospective case file review. Group A included patients with CPD presence within the 3 months preceding patient enrollment. A patient was considered to have cardiac disease (congestive heart failure, cardiomyopathy, ischemic heart disease, or valvular heart disease) if noted on electronic health records, and atrial fibrillation was considered CPD after a second episode. Pulmonary diseases included chronic obstructive pulmonary disease, which is defined by the World Health Organization as a history of chronic bronchitis or pulmonary emphysema as a thin-section CT imaging finding (12). We also included lung cancer and pulmonary metastases because they are significant risk factors for patients with PE (13).
We calculated 30-day and 3-month mortality rates based on death certificates retrieved from clinical files. Most PE-related deaths occur within 30 days, and the 3-month time point provided additional prognostic information about surviving patients (14).
The probability of death was computed using logistic regression analysis and plotted as a function of CTOI for groups A and B, and for the overall study population (mean of group A and B). We also plotted the proportion of patients who died within 3 months as a baseline risk reference, as well as two previously reported discrete CTOI severity thresholds (20% and 40%) (15,16).
In a second step, we assessed whether the 20% and 40% CTOI thresholds could be used to stratify patients into low-risk (1%–17.5%), intermediate-risk (20%–37.5%), or high-risk (≥40%) groups. To this end, we converted the CTOI score (continuous variable) into an ordinal scale and performed univariate logistic regression. The effects of sex and age were also assessed using univariate logistic regression. The probability of death as a function of age among group B patients was derived by logistic regression and plotted according to CTOI (low-, intermediate-, or high-risk category). Finally, outcome predictors (age, CPD, and CTOI) were tested in multivariate analysis.
Statistical Analysis
Statistical analysis was performed using Anaconda 2.7, a free distribution of the Python programming language (version 2.7; Python Software Foundation, Wilmington, Del), and the Python module rpy2 (available at https://pypi.python.org/pypi/rpy2) to link Python with the R 3.1.3 software package (R Core Team 2015, Vienna, Austria). The probability of death as a function of CTOI was computed using the following logistic regression equation:
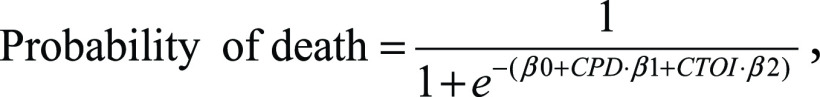 |
where βi are the regression coefficients, CPD equals 1 in group A, CPD equals 0 in group B, and CTOI is the numeric value of the CTOI. The regression coefficients are estimates describing the correlation between the predictor variable (CTOI) and the outcome variable (mortality), obtained directly from the logistic regression function. Bivariate statistical analysis was conducted using the χ2 (Fisher exact test for small samples) or Wilcoxon Mann-Whitney test, as appropriate. Both univariate and multivariate logistic regression were performed to assess the predictive values of age, CPD, and CTOI. Results are expressed as the number of patients and percentage, or as mean ± standard deviation, odds ratio (OR) with 95% confidence interval, and P value. A P value of less than or equal to .05 indicated a significant difference.
Results
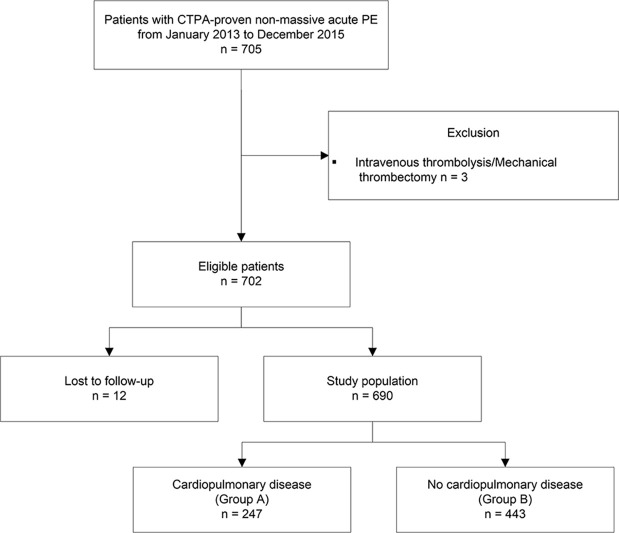
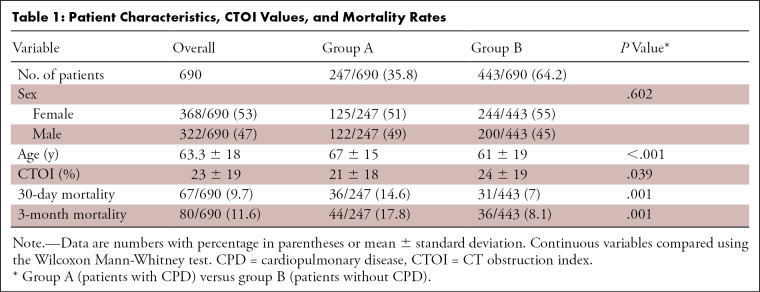
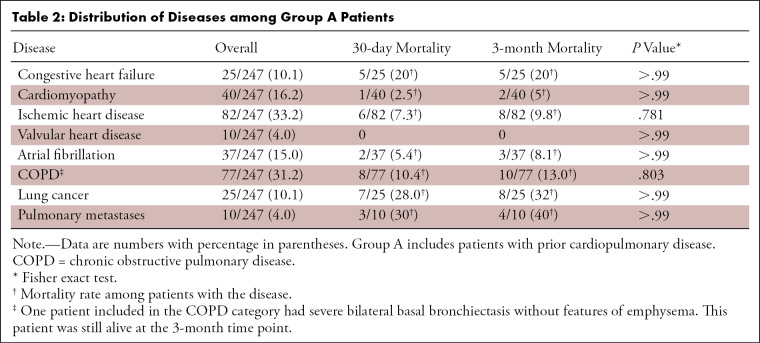
Of the eligible patients, 12 of 702 (1.7%) were excluded from analysis (Fig 1). Thus, our final analysis included 690 patients with 30-day and 3-month follow-up data (Table 1). Of these patients, 247 of 690 (35.8%) had preexisting CPD (group A), including 126 of 690 (18.3%) with preexisting cardiac disease, 84 of 690 (12.2%) with underlying pulmonary disease, and 37 of 690 (5.4%) with both cardiac and pulmonary disease (Table 2). The most common CPDs overall were ischemic heart disease (82 of 247 [33.2%]) and chronic obstructive pulmonary disease (77 of 247 [31.2%]). The highest mortality rate was found among patients with congestive heart failure (five of 25 [20%]) and malignancy (12 of 35 [34%]); however, no significant difference in CTOI was found in surviving patients versus those who have died (P = .40). The 30-day and 3-month mortality rates were not significantly different. The remaining 443 of 690 (64.2%) patients had no preexisting CPD (group B). Patients in group A were older than those in group B (P < .001). Sex distribution was similar between groups (Table 1).
Figure 1:
Inclusion and exclusion criteria, study population, and subgroups with cardiopulmonary disease (group A) and without cardiopulmonary disease (group B). CTPA = CT pulmonary angiography, PE = pulmonary embolism.
Table 1:
Patient Characteristics, CTOI Values, and Mortality Rates
Table 2:
Distribution of Diseases among Group A Patients
In the total study population, the mean CTOI was 23% ± 19 (range, 2.5%–82.5%), and 165 of 690 patients (23.9%) had a CTOI above 40%. Patients in group A had a significantly lower mean CTOI (21%) than those in group B (24%) (Table 1).
Among all included patients, the 30-day all-cause mortality rate was 9.7% (67 of 690 patients) and the 3-month mortality rate was 11.6% (80 of 690 patients). Despite having a significantly lower CTOI, patients in group A had significantly higher mortality rates at both time points. In group A, 36 of 247 patients (14.6%) died within 30 days and 44 of 247 patients (17.8%) died within 3 months of follow-up. In group B, 31 of 443 patients (7%) died within 30 days and 36 of 443 patients (8.1%) died within 3 months of follow-up (Table 1).
Figure 2 shows the estimated mortality rate as a function of CTOI within group A, group B, and the overall study population. Notably, the CTOI threshold of 20% corresponded to the intersection between baseline risk and probability of death as a function of CTOI in the overall population, which clinically represents the CTOI threshold from which the probability of fatal outcome in the overall population exceeds the baseline risk. The CTOI threshold of 40% corresponded to the intersection between baseline risk and probability of death as a function of CTOI in group B, which clinically represents the CTOI threshold from which the probability of fatal outcome among patients without preexisting CPD exceeds the baseline risk.
Figure 2:
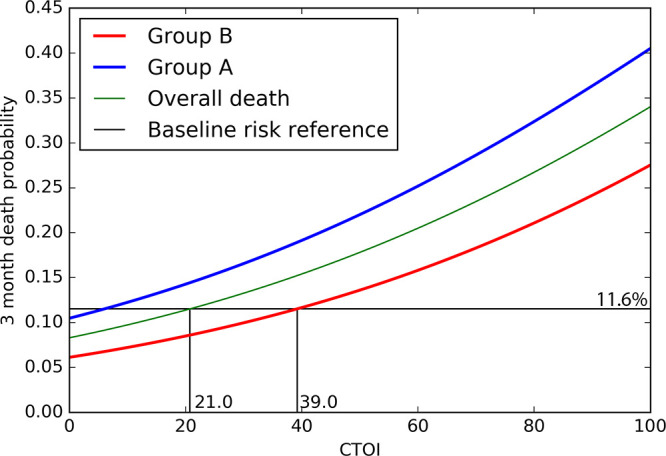
Estimated probability of 3-month mortality as a function of the CT obstruction index (CTOI), obtained from the logistic regression model for the overall population (green), group A (blue), and group B (red). The horizontal black line (baseline risk reference, 11.6%) represents the ratio of patients who died within 3 months in the overall population, indicating the overall probability of dying following the diagnosis of acute pulmonary embolism without taking into account any other variable. Group A includes patients with prior cardiopulmonary disease (CPD); group B includes patients without CPD.
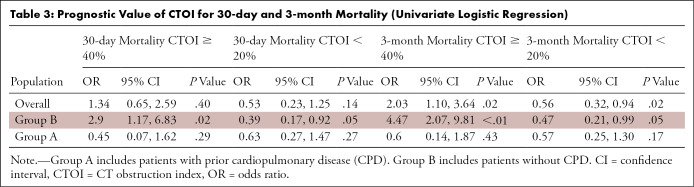
Table 3 shows the predictive value of CTOI. A CTOI of 40% or more was not associated with increased 30-day mortality within the overall study population or group A, but it was significantly associated with a threefold higher risk of 30-day mortality in group B. A CTOI of 40% or more was also significantly associated with a twofold increased risk of 3-month mortality within the overall population, and with a fourfold increased risk of 3-month mortality within group B, but not within group A. A CTOI of less than 20% was not associated with decreased 30-day mortality within the overall population or in group A, but it was significantly associated with a twofold lower risk of 30-day mortality in group B. A CTOI of less than 20% was significantly associated with a nearly twofold decreased risk of 3-month mortality within the overall population, and with a twofold decreased risk of 3-month mortality in group B, but not within group A. Compared with low-risk patients (CTOI < 20%), high-risk patients (CTOI ≥ 40%) showed a 2.7-fold increase of 30-day mortality (P = .036) and a 3.8-fold increase of 3-month mortality (P = .001) in group A.
Table 3:
Prognostic Value of CTOI for 30-day and 3-month Mortality (Univariate Logistic Regression)
In the overall population, sex was not associated with either 30-day mortality (P = .749) or 3-month mortality (P = .672). In contrast, each year of age was significantly associated with increased 30-day mortality (OR, 1.02; P = .04) and 3-month mortality (OR, 1.02; P = .02) in the overall population. Figure 3 shows the probability of death within 3 months in group B as a function of age, within the low-, intermediate-, and high-risk CTOI categories. This graph illustrates the greater probability of fatal outcome in elderly patients, with a 1.4% increase per decade from 30 to 80 years old in group B. In group A, the risk of mortality increased by 2.3% per decade.
Figure 3:
![Probability of 3-month mortality in group B (patients without cardiopulmonary disease [CPD]) as a function of age (in years), obtained from the logistic regression model for low- (blue), intermediate- (green), and high-risk (red) CT obstruction index (CTOI) scores. Group A patients are not included in this model since CTOI had no predictive value in group A (patients with prior CPD).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/0367/7978016/5708522b4dc3/ryct.2020190188.fig3.jpg)
Probability of 3-month mortality in group B (patients without cardiopulmonary disease [CPD]) as a function of age (in years), obtained from the logistic regression model for low- (blue), intermediate- (green), and high-risk (red) CT obstruction index (CTOI) scores. Group A patients are not included in this model since CTOI had no predictive value in group A (patients with prior CPD).
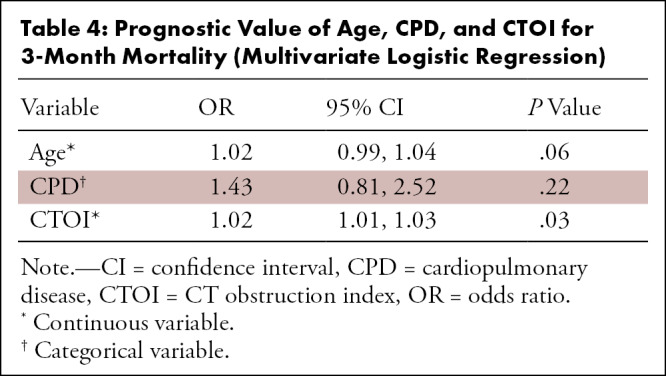
Finally, we performed multivariate logistic regression analysis with age, CPD presence or absence, and CTOI (continuous variable) as predictor variables, and 3-month mortality as the outcome variable (Table 4). CTOI was the only significant predictor of mortality (P = .03).
Table 4:
Prognostic Value of Age, CPD, and CTOI for 3-Month Mortality (Multivariate Logistic Regression)
Discussion
The results of this study indicated that a combination of CTOI, presence of cardiopulmonary comorbidities (CPD), and age could be used to predict outcomes in a large, representative cohort of patients with acute PE. We found that a lower CTOI (< 20%) could identify low-risk patients eligible for hospital discharge among those without associated cardiopulmonary comorbidities. Additionally, our results demonstrated that CTOI was only useful for identifying high-risk patients within the population who did not exhibit preexisting CPD. Patients with prior CPD are at increased risk of adverse outcome regardless of CTOI, and their mortality in our cohort may have been due to CPD. Congestive heart failure, lung cancer, and pulmonary metastases were the CPDs most often found among patients who died.
In this study, we focused on defining a scenario in which CT pulmonary angiography findings combined with clinical data could be used to predict the outcome of patients with acute PE, by stratifying patients based on short-term risk of death. The rationale behind developing this single-examination–based approach included three key components. First, the clinical consequences of PE result from pulmonary arterial obstruction and subsequent vasoconstriction (9,17), supporting the likelihood that pathophysiology is correlated with outcome. Second, while a high degree of obstruction may be correlated with high mortality risk, patients with a low degree of obstruction may still have adverse outcomes if they have comorbidities (18); thus, the integration of comorbidities is required to support therapeutic decision making. Third, defining a subgroup carrying a high risk of death is essential for patient stratification, since such patients may benefit from aggressive treatment (9). Conversely, it is also necessary to identify low-risk patients who will likely have a favorable outcome and could benefit from outpatient PE management (19,20).
Several prior reports have highlighted the CTOI as a useful and straightforward indicator of PE severity (6,15,21–27). However, its prognostic value remains controversial. Several authors found that CTOI predicted 1-month mortality (15,22), 45-day mortality (23), 3-month mortality (26), and 6-month mortality (21), with mortality rates similar to those found in our study (range, 8%–15%) and mortality CTOI cutoff values of 40% (26), 45% (15), and 60% (22). None reported a CTOI cutoff value for low mortality risk. In contrast, Vedovati et al (28) analyzed 579 patients and Furlan et al (6) analyzed 635 patients, and both studies found no significant correlation between CTOI and 30-day mortality. A recent meta-analysis, which includes 3884 patients from 16 studies, also failed to confirm a relationship between CTOI and 30-day mortality (8). There are multiple likely reasons for these conflicting results. The primary reason is likely patient selection bias, especially regarding patients with previous comorbidities that might affect patient outcome. In our study population reported in this article, 36% (247 of 690) of patients had previous CPD, and more than one-third had known ischemic heart disease. Almost 15% (36 of 247) of those patients had combined prior cardiac and pulmonary diseases. Distinguishing between patients with and without previous CPD enabled us to discriminate a subgroup in which the CTOI was an independent indicator of short-term outcome.
Interestingly, the importance of prior CPD has been previously demonstrated in another context. Studies based on transcatheter angiography have reported a good correlation between the degree of arterial obstruction and the mean pulmonary arterial pressure (23), revealing that clinical and physiologic manifestations of PE are directly related to embolic burden in patients without prior CPD. In contrast, patients with prior CPD typically manifest a greater severity of cardiovascular failure with a lesser degree of arterial obstruction (29). Miller et al (30) reported that there is no consistent relationship between the severity of cardiovascular and right ventricle (RV) functional impairment and the degree of arterial obstruction in patients without prior CPD, which is consistent with our results reported herein.
It is important to be able to identify not only the patients with high mortality risk, but also those with low mortality risk. For this purpose, we used previously reported CTOI thresholds of 20% and 40%. The 40% threshold is consistent with those most commonly used to predict high mortality risk (21,26,31) and was correlated with RV dilatation in a report by Qanadli et al (5). Our results indicated that patients without prior CPD and with CTOI greater than 40% were at high risk, while patients without prior CPD and with CTOI less than 20% were at low risk of a fatal outcome. CTOI could not be used for risk stratification among patients with prior CPD, and other parameters likely should be considered in this subgroup. The most commonly reported parameter for predicting high mortality risk is the RV-to–left ventricle ratio. However, patients with severe PE and previously dilated left ventricle might have a false-negative ratio, while patients with subsegmental PE and previously dilated RV for any other reason might have a false-positive ratio. Accordingly, a study showed that an increased right-to-left ventricle diameter ratio (> 0.9) was not associated with worse outcome among low-risk patients (simplified Pulmonary Embolism Severity Index = 0) (32). More evaluations are needed to better understand the consequences of PE in selected patient groups, such as patients with chronic obstructive pulmonary disease and patients with ischemic heart disease who have limited cardiorespiratory reserve, in whom RV and left ventricle function may carry more prognostic information.
Further indicators of hemodynamic compromise that might play a significant role in risk stratification are reduced left atrial size, indicating underfilling by the pulmonary veins (33) and measurement of pulmonary perfusion. However, these indicators have been evaluated to identify high-risk patients rather than to establish a real stratification identifying both high- and low-risk patients. Current CT systems are increasingly equipped with wide-area detectors and dual-energy technology, offering further options to depict compromised blood flow in the lung parenchyma. Specifically, wide-area detectors facilitate dynamic CT perfusion imaging of the lung, a technique that yields a quantitative assessment of pulmonary blood flow (34), whereas dual-energy CT pulmonary angiography can map iodine distribution in the lung at a single time point, providing a semiquantitative assessment of pulmonary perfusion (35). Both of these techniques may help determine PE severity and, ultimately, the risk for fatal outcome, but scarce data have been published to date. Using dynamic CT perfusion technique in 26 patients, Mirsadraee et al (34) found that 70% of patients had persistent perfusion abnormalities 3–6 months following acute PE, but none had RV dilation. Sakamoto et al (35) performed dual-energy CT pulmonary angiography and iodine-based material decomposition in 72 patients with acute PE to estimate perfused blood volume, an indirect descriptor of lung perfusion. They found reduced perfused blood volume owing to acute PE to be correlated to RV dilation and therefore concluded that perfused blood volume can be used to determine PE severity.
As expected, we found age to be a significant indicator of fatal outcome. Group A patients were significantly older than group B patients, which may partly explain why the CTOI was poorly correlated with mortality among unselected elderly patients, as previously published (36). Since mortality increased by 1.4% per decade in group A versus 2.3% in group B, and patients from group B were, on average, 6 years older than those in group A, the significantly higher mortality rate in group A was mainly driven by other parameters, of which CPDs likely play a determining role. We observed no effect of sex, which is in agreement with the findings of an extensive study that includes 2096 patients (37).
This study had several limitations. First, it was a retrospective uncontrolled study that lacked a propensity-matched cohort without PE. Second, we did not include the analysis of cardiac cavities on CT pulmonary angiography, which also carries an important prognostic value. The right-to-left ventricular ratio, as well as left atrium assessment, might help to identify high-risk patients; however, the incremental value of these variables in risk stratification as defined in our current study is questionable, since no data regarding their usefulness to identify low-risk patients exists. Third, we did not evaluate any biomarkers (eg, troponin), since they were not routinely measured at our institution during the study period. Elevated serum troponin levels are associated with adverse outcome in patients with acute PE (38), especially when associated with RV dilatation (39). Consequently, troponin measurements are recommended to support the decision to pursue aggressive treatments such as US-assisted catheter-directed thrombolysis for intermediate-risk patients (40). However, troponin levels have no established role to identify low-risk patients as defined in the present study. Fourth, we did not assess either the effect of the type or duration of medical therapy, or the presence of known deep vein thrombosis. Fifth, we could not provide a reliable method to account for CPD severity or CTOI predictions per CPD category, which could help to better understand the prognostic value of the CTOI in group A. Finally, the statistical analyses considered 30-day and 3-month mortality rates from all causes. We did not have a valid method for distinguishing PE-related deaths from other causes, although some interesting approaches were published by Méan et al (36) in 2017. In that prospective multicenter study, a committee consisting of three blinded clinical experts determined the cause of death in consensus, based on sudden, unexpected death following a clinically severe PE, or autopsy.
To conclude, we were able to demonstrate that among patients without CPD, the CTOI had prognostic value for identifying patients at both high risk and low risk of fatal outcome. It is difficult to determine the relative contributions of cardiopulmonary comorbidities, pulmonary malignancy, and embolism magnitude to the risk of adverse outcome, which is why the CTOI was not a prognostic factor among patients with CPD. However, our simple and clinically relevant approach could be applied in about two-thirds of the consecutive patients with PE in our cohort.
D.C.R. supported by the Leenaards Foundation.
Disclosures of Conflicts of Interest: D.C.R. disclosed no relevant relationships. J.F.K. disclosed no relevant relationships. A.M.J. disclosed no relevant relationships. A.G. disclosed no relevant relationships. S.D.Q. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution receives payment from BD Bard for consulting. Other relationships: disclosed no relevant relationships.
Abbreviations:
- CPD
- cardiopulmonary disease
- CTOI
- CT obstruction index
- OR
- odds ratio
- PE
- pulmonary embolism
- RV
- right ventricle
References
- 1.Tamjeedi B, Correa J, Semionov A, Mesurolle B. Interobserver Agreement between On-Call Radiology Resident and General Radiologist Interpretations of CT Pulmonary Angiograms and CT Venograms. PLoS One 2015;10(5):e0126116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based, cohort study. Arch Intern Med 1999;159(5):445–453. [DOI] [PubMed] [Google Scholar]
- 3.Klok FA, Zondag W, van Kralingen KW, et al. Patient outcomes after acute pulmonary embolism: A pooled survival analysis of different adverse events. Am J Respir Crit Care Med 2010;181(5):501–506. [DOI] [PubMed] [Google Scholar]
- 4.Becattini C, Agnelli G, Lankeit M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J 2016;48(3):780–786. [DOI] [PubMed] [Google Scholar]
- 5.Qanadli SD, El Hajjam M, Vieillard-Baron A, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol 2001;176(6):1415–1420. [DOI] [PubMed] [Google Scholar]
- 6.Furlan A, Aghayev A, Chang C-CH, et al. Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology 2012;265(1):283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen HS, Abakay Ö, Cetincakmak MG, et al. A single imaging modality in the diagnosis, severity, and prognosis of pulmonary embolism. BioMed Res Int 2014;2014:470295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vedovati MC, Germini F, Agnelli G, Becattini C. Prognostic role of embolic burden assessed at computed tomography angiography in patients with acute pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost 2013;11(12):2092–2102. [DOI] [PubMed] [Google Scholar]
- 9.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35(43):3033–3069, 3069a–3069k. [Published corrections appear in Eur Heart J 2015;36(39):2666 and Eur Heart J 2015;36(39):2642.]. [DOI] [PubMed] [Google Scholar]
- 10.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123(16):1788–1830 [Published corrections appear in Circulation 2012;126(7):e104 and Circulation 2012;125(11):e495.]. [DOI] [PubMed] [Google Scholar]
- 11.Rotzinger DC, Breault S, Knebel JF, Beigelman-Aubry C, Jouannic AM, Qanadli SD. Can a Trained Radiology Technician Do Arterial Obstruction Quantification in Patients With Acute Pulmonary Embolism? Front Cardiovasc Med 2019;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. COPD: Definition. World Health Organization website. https://www.who.int/respiratory/copd/definition/en/. Accessed September 20, 2019. [Google Scholar]
- 13.Ma L, Wen Z. Risk factors and prognosis of pulmonary embolism in patients with lung cancer. Medicine (Baltimore) 2017;96(16):e6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacMahon B. The National Death Index. Am J Public Health 1983;73(11):1247–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu AS, Pezzullo JA, Cronan JJ, Hou DD, Mayo-Smith WW. CT pulmonary angiography: quantification of pulmonary embolus as a predictor of patient outcome--initial experience. Radiology 2004;230(3):831–835. [DOI] [PubMed] [Google Scholar]
- 16.Guo ZJ, Liu HT, Bai ZM, et al. A new method of CT for the cardiac measurement: correlation of computed tomography measured cardiac parameters and pulmonary obstruction index to assess cardiac morphological changes in acute pulmonary embolism patients. J Thromb Thrombolysis 2018;45(3):410–416. [DOI] [PubMed] [Google Scholar]
- 17.Smulders YM. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res 2000;48(1):23–33. [DOI] [PubMed] [Google Scholar]
- 18.den Exter PL, van Es J, Klok FA, et al. Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood 2013;122(7):1144–1149; quiz 1329. [DOI] [PubMed] [Google Scholar]
- 19.Klil-Drori AJ, Coulombe J, Suissa S, Hirsch A, Tagalakis V. Temporal trends in outpatient management of incident pulmonary embolism and associated mortality. Thromb Res 2018;161:111–116. [DOI] [PubMed] [Google Scholar]
- 20.Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost 2017;15(4):685–694. [DOI] [PubMed] [Google Scholar]
- 21.Attinà D, Valentino M, Galiè N, et al. Application of a new pulmonary artery obstruction score in the prognostic evaluation of acute pulmonary embolism: comparison with clinical and haemodynamic parameters. Radiol Med (Torino) 2011;116(2):230–245. [DOI] [PubMed] [Google Scholar]
- 22.Praveen Kumar BS, Rajasekhar D, Vanajakshamma V. Study of clinical, radiological and echocardiographic features and correlation of Qanadli CT index with RV dysfunction and outcomes in pulmonary embolism. Indian Heart J 2014;66(6):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu T, Yuan M, Zhang Q, Shi H, Wang D. Evaluation of computed tomography obstruction index in guiding therapeutic decisions and monitoring percutanous catheter fragmentation in massive pulmonary embolism. J Biomed Res 2011;25(6):431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dogan H, Kroft LJ, Huisman MV, et al. Assessment of right ventricular function in acute pulmonary embolism using ECG-synchronized MDCT. AJR Am J Roentgenol 2010;195(4):909–915. [DOI] [PubMed] [Google Scholar]
- 25.Venkatesh SK, Wang SC. Central clot score at computed tomography as a predictor of 30-day mortality after acute pulmonary embolism. Ann Acad Med Singapore 2010;39(6):442–447. [PubMed] [Google Scholar]
- 26.van der Meer RW, Pattynama PMT, van Strijen MJL, et al. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology 2005;235(3):798–803. [DOI] [PubMed] [Google Scholar]
- 27.Collomb D, Paramelle PJ, Calaque O, et al. Severity assessment of acute pulmonary embolism: evaluation using helical CT. Eur Radiol 2003;13(7):1508–1514. [DOI] [PubMed] [Google Scholar]
- 28.Vedovati MC, Becattini C, Agnelli G, et al. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest 2012;142(6):1417–1424. [DOI] [PubMed] [Google Scholar]
- 29.McIntyre KM, Sasahara AA. Determinants of right ventricular function and hemodynamics after pulmonary embolism. Chest 1974;65(5):534–543. [DOI] [PubMed] [Google Scholar]
- 30.Miller RL, Das S, Anandarangam T, et al. Association between right ventricular function and perfusion abnormalities in hemodynamically stable patients with acute pulmonary embolism. Chest 1998;113(3):665–670. [DOI] [PubMed] [Google Scholar]
- 31.Inönü H, Acu B, Pazarlı AC, Doruk S, Erkorkmaz Ü, Altunkaş A. The value of the computed tomographic obstruction index in the identification of massive pulmonary thromboembolism. Diagn Interv Radiol 2012;18(3):255–260. [DOI] [PubMed] [Google Scholar]
- 32.Côté B, Jiménez D, Planquette B, et al. Prognostic value of right ventricular dilatation in patients with low-risk pulmonary embolism. Eur Respir J 2017;50(6):1701611. [DOI] [PubMed] [Google Scholar]
- 33.Zuin M, Rigatelli G, Turchetta S, Zonzin P, Zuliani G, Roncon L. Left atrial size measured on CT pulmonary angiography: another parameter of pulmonary embolism severity? A systematic review. J Thromb Thrombolysis 2019 Nov 21 [Epub ahead of print]. [DOI] [PubMed]
- 34.Mirsadraee S, Reid JH, Connell M, et al. Dynamic (4D) CT perfusion offers simultaneous functional and anatomical insights into pulmonary embolism resolution. Eur J Radiol 2016;85(10):1883–1890. [DOI] [PubMed] [Google Scholar]
- 35.Sakamoto A, Sakamoto I, Nagayama H, Koike H, Sueyoshi E, Uetani M. Quantification of lung perfusion blood volume with dual-energy CT: assessment of the severity of acute pulmonary thromboembolism. AJR Am J Roentgenol 2014;203(2):287–291. [DOI] [PubMed] [Google Scholar]
- 36.Méan M, Tritschler T, Limacher A, et al. Association between computed tomography obstruction index and mortality in elderly patients with acute pulmonary embolism: A prospective validation study. PLoS One 2017;12(6):e0179224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrios D, Morillo R, Guerassimova I, et al. Sex differences in the characteristics and short-term prognosis of patients presenting with acute symptomatic pulmonary embolism. PLoS One 2017;12(11):e0187648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajaj A, Saleeb M, Rathor P, Sehgal V, Kabak B, Hosur S. Prognostic value of troponins in acute nonmassive pulmonary embolism: A meta-analysis. Heart Lung 2015;44(4):327–334. [DOI] [PubMed] [Google Scholar]
- 39.Carroll BJ, Heidinger BH, Dabreo DC, et al. Multimodality Assessment of Right Ventricular Strain in Patients With Acute Pulmonary Embolism. Am J Cardiol 2018;122(1):175–181. [DOI] [PubMed] [Google Scholar]
- 40.Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129(4):479–486. [DOI] [PubMed] [Google Scholar]