Abstract
Purpose
To compare the diagnostic performance of the original Lake Louise criteria (LLC) and the 2018 LLC for the diagnosis of acute myocarditis and simultaneously validate previously reported cutoff values for parametric mapping techniques.
Materials and Methods
A total of 40 patients with acute myocarditis and 26 control participants underwent cardiac MRI. Cardiac MRI protocol allowed for assessment of T2 signal intensity ratio, early gadolinium enhancement ratio, late gadolinium enhancement, T1 relaxation times, extracellular volume fraction, and T2 relaxation times. The original and the 2018 LLC were assessed, and differences between sensitivities and specificities were calculated with the McNemar test.
Results
The 2018 LLC yielded a sensitivity of 87.5% (95% confidence interval [CI]: 73.9%, 94.5%) and a specificity of 96.2% (95% CI: 81.1%, 99.3%). The original LLC had a sensitivity of 72.5% (95% CI: 57.2%, 83.9%) and a specificity of 96.2% (95% CI: 81.1%, 99.3%). Sensitivity of the 2018 LLC was significantly higher compared with the sensitivity of original LLC (P = .031). No differences in specificity were observed between both scores (P = .999)
Conclusion
Multiparametric cardiac MRI has a high diagnostic value for the diagnosis of patients clinically suspected of having acute myocarditis. The 2018 LLC further improve the diagnostic performance of cardiac MRI by increasing its sensitivity. An implementation of the new score into routine diagnostic protocols should be considered.
© RSNA, 2019
See also the commentary by Gutberlet and Lücke in this issue.
Summary
The 2018 Lake Louise criteria provide a high diagnostic accuracy for the diagnosis of acute myocarditis and significantly increase the sensitivity compared with the original score.
Key Points
■ Multiparametric cardiac MRI has a high diagnostic value for the diagnosis of patients with acute myocarditis.
■ The 2018 Lake Louise criteria improve the diagnostic performance of cardiac MRI by significantly increasing its sensitivity compared with the original criteria.
Introduction
Myocardial inflammation in acute myocarditis can be provoked from a wide variety of infectious, immune-mediated, and toxic causes (1). The clinical presentation of myocarditis is also heterogeneous; patients can present with different severities of chest pain and palpitations, which can be accompanied by several rhythm disorders ranging from transient electrocardiographic changes to life-threatening cardiogenic shock and ventricular arrhythmias (1–3). Furthermore, myocarditis is an important cause of sudden cardiac death and as a late sequela, dilated cardiomyopathy may develop in patients with ongoing inflammation due to an inadequate immune response (2,3). Cardiac MRI is often used for diagnostic work-up, which allows noninvasive characterization of myocardial tissue (4,5). Since 2009, cardiac MRI–based diagnosis of myocarditis has been based on the Lake Louise criteria (LLC), which target three aspects of myocardial inflammation: edema, hyperemia, and necrosis and/or fibrosis (6). Image interpretation of the original LLC depend on a qualitative or semiquantitative analysis of signal intensities on T2-weighted, early gadolinium enhancement, and late gadolinium enhancement (LGE) images. However, when no significant signal intensity differences between diseased and normal myocardium exist, cases of diffuse or subtle myocardial inflammation can be missed by the original LLC, thereby limiting its diagnostic accuracy (7–10). Myocardial T1 and T2 mapping are robust cardiac MRI techniques and allow for a pixelwise quantitative characterization of myocardial tissue. Several studies have investigated the diagnostic benefits of mapping techniques and could show distinct advantages over the original LLC for the evaluation and characterization of myocardial inflammation (7–10). Consequently, the original LLC were revised in 2018 with the implementation of mapping techniques (11). According to the 2018 LLC, cardiac MRI–based diagnosis of myocarditis is based on at least one T1-based criterion (increased myocardial T1 relaxation times, extracellular volume fraction, or LGE) with at least one T2-based criterion (increased myocardial T2 relaxation times, visible myocardial edema, or increased T2 signal intensity ratio) (11). For analysis of T1 and T2 relaxation times, the application of published or local cutoff values is recommended (11). However, many published cutoff values have not been validated in a validation cohort of patients with myocarditis, and the direct implication of such cutoff values remain undetermined.
The aim of this study was to compare the diagnostic performance of the original LLC and the 2018 LLC for the diagnosis of acute myocarditis. The secondary aim was to validate previously reported cutoff values for myocardial T1 relaxation times, extracellular volume fraction, and myocardial T2 relaxation times (7).
Materials and Methods
The institutional review committee approved this prospective study, and all participants gave written informed consent prior to cardiac MRI. Patients with clinically defined acute myocarditis and healthy control participants were included in this study.
All patients with acute myocarditis have not been included in a previous study. Acute myocarditis was diagnosed based on diagnostic criteria for clinically suspected myocarditis as recommended by the European Society of Cardiology Working Group on myocardial and pericardial diseases (1). Patients with clinically acute myocarditis had the following: acute chest pain, signs of acute myocardial injury (electrocardiographic changes and/or elevated troponin level), and increased laboratory markers of inflammation (eg, C-reactive protein level). Coronary artery disease was excluded prior to cardiac MRI. Patients with preexisting cardiovascular disease were excluded. Cardiac MRI results were not part of the diagnostic algorithm and had no influence on clinical diagnosis. The study used a clinical validation approach for the diagnosis of acute myocarditis as previously reported (7–10), and myocarditis in all patients was diagnosed by using clinical criteria. This clinical evidence was the reference standard against which the diagnostic performance of cardiac MRI parameters was tested. Acute myocarditis was the final diagnosis of all included patients with myocarditis at hospital discharge. The control group consisted of healthy volunteers and outpatients referred for nonspecific cardiac symptoms or thoracic pain. All control participants had an unremarkable past medical history of cardiovascular disease. Electrocardiographic results were unremarkable and no cardiac risk factors were present. All control participants had normal cardiac MRI results without structural abnormalities or signs of previous myocarditis.
Cardiac MRI
All imaging was performed with a clinical whole-body cardiac MRI system (Ingenia 1.5T; Philips Healthcare, Best, the Netherlands). A 32-channel torso coil with digital interface was used for signal reception. For functional analysis, electrocardiographic-gated steady-state free precession cine images were obtained in short-axis, four-chamber, and two-chamber views. Edema-sensitive black-blood T2-weighted short-tau inversion-recovery, or STIR, sequences were performed in short-axis, two-chamber, and transversal views. To correct for torso coil–related signal inhomogeneities, an inherent signal intensity correction algorithm (constant level appearance; Philips Medical Systems) was used. Early gadolinium enhancement was assessed by using transverse free-breathing precontrast and postcontrast fast spin-echo T1-weighted images. For LGE imaging, segmented inversion-recovery gradient-echo sequences in short-axis, two-chamber, and four-chamber views were performed. Optimal inversion time was determined by using the Look-Locker technique (12). T1 and T2 mapping were performed in end diastole in short-axis views. Basal, midventricular, and apical sections were acquired. For myocardial T1 mapping, a standard 3(3)3(3)5 modified Look-Locker inversion recovery, or MOLLI (13), acquisition scheme was used. Native and postcontrast T1 maps were acquired in the same orientations. Postcontrast T1 maps were performed 10 minutes after contrast agent administration. For myocardial T2 mapping, a six-echo gradient spin-echo sequence was used as previously described (14). Blood hematocrit levels were determined on the day of examination. For contrast enhancement, a bolus of 0.2 mmol/kg of body weight of gadobutrol (Gadovist; Bayer Healthcare, Leverkusen, Germany) was administered. Detailed sequence parameters are given in Table E1 (supplement).
Image Analysis
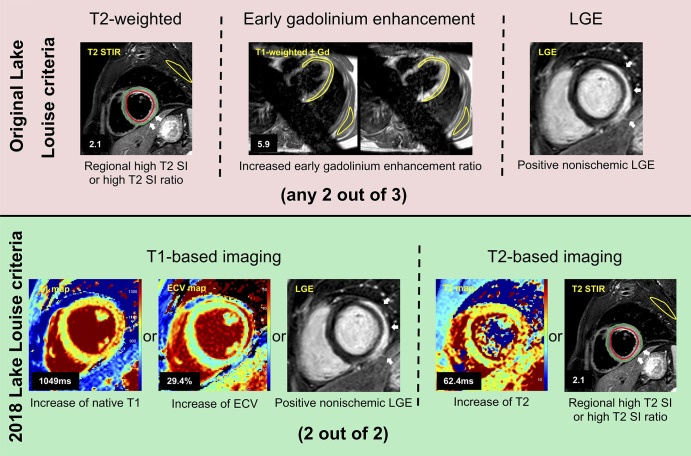
Image analysis was performed by two radiologists experienced in cardiac MRI (J.A.L. and D.T.). Readers were blinded to the clinical information. Cardiac MRI analysis was performed offline by using dedicated software (IntelliSpace Portal, version 10.1; Philips Medical Systems). The presence of focal regional high signal intensities on T2 STIR and on LGE images in a nonischemic distribution pattern was assessed visually by consensus agreement of the two readers. Semiquantitative T2 signal intensity ratio as a marker of global myocardial edema and early gadolinium enhancement ratio as a marker of hyperemia and capillary leak were calculated as recommended for the assessment of the LLC (6,11,15,16). Myocardial T1 and T2 relaxation maps were reconstructed offline by using a software-implemented motion-correction algorithm (fast elastic image registration). Myocardial contours were drawn throughout the T1 and T2 maps to investigate the entire myocardium, and global T1 and T2 relaxation times were calculated. Hematocrit-corrected global extracellular volume fraction values were calculated separately from pre- and postcontrast T1 values as previously described (8,17). The original and the 2018 LLC were applied as recommended (6,11) (see Fig 1). Cutoff values for T2 signal intensity ratio, early gadolinium enhancement ratio, T1 relaxation times, extracellular volume fraction, and T2 relaxation times were derived from a previously reported initial cohort (7).
Figure 1:
Illustration shows original and 2018 Lake Louise criteria (LLC) in a 24-year-old man with acute myocarditis. Original LLC consisted of three main criteria: regional high T2 signal intensities on T2-weighted images (white arrows) or increased global T2 signal intensity ratio, increased early gadolinium enhancement ratio on T1-weighted images, and areas with high signal intensities in nonischemic distribution pattern on late gadolinium enhancement (LGE) images (white arrows). 2018 LLC consist of two main criteria (T1-based criterion and T2-based criterion). T1-based criterion is considered to be positive if increase of native T1 relaxation times, increase of extracellular volume (ECV), or positive LGE (white arrows) exist. T2-based criterion is positive in cases of increased T2 relaxation times or in cases with regional high T2 signal intensities on T2-weighted images (white arrows) or increased global T2 signal intensity ratio. Gd = gadolinium, SI = signal intensity, STIR = short tau inversion recovery.
Statistical Analysis
Prism (version 7.0d; GraphPad Software, La Jolla, Calif), SPSS Statistics (version 23; IBM, Armonk, NY), and MedCalc (version 18.11.3; MedCalc Software, Ostend, Belgium) were used for statistical analysis. Patient characteristics are presented as mean ± standard deviation or as absolute frequency. Continuous variables between two groups were compared by using Student t test. Dichotomous variables were compared by using the χ2 test (with a cell count greater than five) or Fisher exact test (with a cell count less than or equal to five). Correlation analysis was performed by using Pearson correlation coefficient. A receiver operating characteristic analysis was performed to calculate areas under the curve. Cutoff values for continuous variables were chosen as previously determined (7) and sensitivities, specificities, accuracies, positive predictive values, and negative predictive values were calculated. Differences between sensitivities and specificities were tested with McNemar test. The level of statistical significance was set to P < .05.
Results
General Characteristics
A total of 66 participants were included in this study (40 patients with acute myocarditis and 26 control participants). The mean age of patients with myocarditis was 41.1 years ± 18.4 (range, 18–86 years). The mean age of healthy control participants was 41.2 years ± 16.6 (range, 18–71 years). Age (P = .995), sex (P = .959), height (P = .189), and weight (P = .467) did not differ significantly between both groups. Cardiac MRI was performed 3.9 days ± 3.7 after hospital admission. Clinical characteristics of patients with myocarditis and healthy control participants are given in Table 1.
Table 1:
Clinical Characteristics of Patients with Myocarditis and Healthy Control Participants
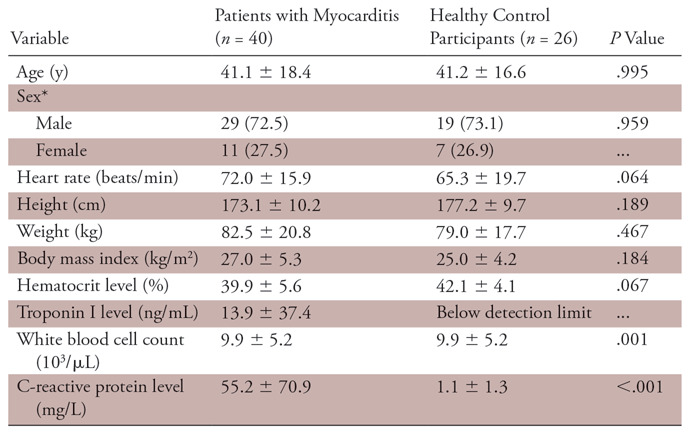
Note.—Unless otherwise specified, data are means ± standard deviations.
*Data are absolute frequencies, with percentages in parentheses.
Cardiac MRI Results
Patients with acute myocarditis had elevated parameters of myocardial inflammation at cardiac MRI when compared with the control group, as follows: T2 signal intensity ratio (1.7 ± 0.3 vs 1.5 ± 0.3; P = .006), early gadolinium enhancement ratio (3.6 ± 3.5 vs 2.1 ± 1.4; P = .036), native T1 relaxation times (1047.0 msec ± 53.8 vs 965.8 msec ± 25.1; P < .001), extracellular volume fraction (28.6% ± 5.3 vs 26.1% ± 4.2; P = .038), and T2 relaxation times (61.8 msec ± 8.2 vs 52.8 msec ± 2.4; P < .001). Focal-regional high signal intensities on T2 STIR images indicating focal myocardial edema were present in 25 of 40 (63%) patients (P < .001 vs control participants). LGE in a nonischemic distribution was positive in 31 of 40 (78%) patients (P < .001 vs control participants). Patients without visible myocardial edema had significantly lower T2 relaxation times compared with patients with visible myocardial edema (57.3 msec ± 5.6 vs 64.7 msec ± 8.3; P = .005). In patients who were LGE negative, T1 relaxation times were markedly reduced compared with patients with visible LGE (1009.6 msec ± 41.3 vs 1057.9 msec ± 52.5; P = .016). Cardiac MRI characteristics of patients with myocarditis and healthy control participants are given in Table 2.
Table 2:
Cardiac MRI Characteristics of Patients with Myocarditis and Healthy Control Participants

Note.—Unless otherwise specified, data are means ± standard deviations.
*Data are absolute frequencies, with percentages in parentheses.
Patients with positive 2018 LLC for acute myocarditis had higher T1 and T2 relaxation times compared with patients with negative 2018 LLC (T1 relaxation times, 1057.5 msec ± 48.9 vs 974.1 msec ± 17.4; P < .001; T2 relaxation times, 63.2 msec ± 7.7 vs 51.9 msec ± 2.2; P = .002). Twenty-three of 40 (58%) patients had a preserved left ventricular ejection fraction (ejection fraction ≥55%). Compared with patients with reduced ejection fraction, patients with preserved left ventricular ejection fraction had a lower proportion of positive LGE (eight of 23 [35%] vs 16 of 17 [94%]; P = .033) and a lower frequency of visible myocardial edema (11 of 23 [48%] vs 14 of 17 [82%]; P = .027). T2 relaxation times were lower in patients with preserved ejection fraction (58.5 msec ± 5.4 vs 66.6 msec ± 9.2; P = .004). Early gadolinium enhancement ratio (r = −0.46; P < .001), T1 relaxation times (r = −0.41; P = .001), and T2 relaxation times (r = −0.52; P < .001) were correlated with left ventricular ejection fraction.
Diagnostic Performance of Original and 2018 LLC
The original LLC yielded a sensitivity of 72.5% (95% confidence interval [CI]: 57.2%, 83.9%) and a specificity of 96.2% (95% CI: 81.1%, 99.3%). The sensitivity of the 2018 LLC was 87.5% (95% CI: 73.9%, 94.5%) and the specificity was 96.2% (95% CI: 81.1%, 99.3%). Sensitivity of the 2018 LLC was significantly higher compared with the sensitivity of original LLC (P = .031). However, no differences in specificity were present between the two scores (P = .999). Compared with the original LLC, the 2018 LLC allowed the diagnosis of acute myocarditis in six additional patients. In three of six (50%) patients with negative LGE, diagnosis was made possibly due to increased myocardial T1 and T2 relaxations times. In three of six (50%) patients with positive LGE, the additional detection of myocardial edema on myocardial T2 mapping was responsible for the added diagnostic value. No patient who was diagnosed by the original criteria was missed by the 2018 version.
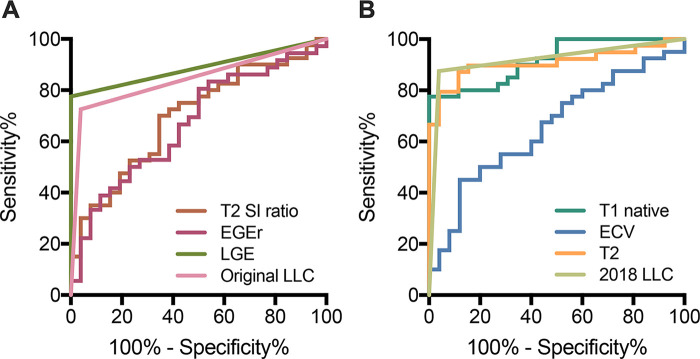
Using only T1-based criteria, an area under the curve of 0.85 was achieved (sensitivity: 90.0% [95% CI: 76.9%, 96.0%], specificity: 76.9% [95% CI: 57.9%, 89.0%], accuracy: 84.8% [95% CI: 74.3%, 91.6%]). Using only T2-based criteria, an area under the curve of 0.87 was yielded (sensitivity: 84.6% [95% CI: 70.3%, 92.8%], specificity: 88.5% [95% CI: 71.0%, 96.0%], accuracy: 86.2% [95% CI: 75.7%, 92.5%]). Sensitivity and specificity of both criteria did not differ significantly from the “two out of two” approach of the 2018 LLC (P > .05, respectively). Table 3 summarizes all sensitivities, specificities, accuracies, positive predictive values, and negative predictive values for all evaluated parameters for previously defined cutoff values. Figure 2 visualizes the area under the curve values for single variables and the LLC. The diagnostic accuracies for single variables and the LLC are illustrated in Figure 3.
Table 3:
Diagnostic Performance of Different Cardiac MRI Parameters for Diagnosis of Acute Myocarditis

Note.—Data in parentheses are 95% confidence intervals. Cutoff values were adopted from an initial study cohort (7). NPV = negative predictive value, PPV = positive predictive value.
Figure 2:
Graphs show receiver operating characteristic curves for, A, T2 signal intensity (SI) ratio (area under curve [AUC], 0.70), early gadolinium enhancement ratio (EGEr) (AUC, 0.67), late gadolinium enhancement (LGE) (AUC, 0.89), and Lake Louise criteria (LLC) (AUC, 0.84) and for, B, native T1 relaxation times (AUC, 0.92), extracellular volume (ECV) (AUC, 0.66), T2 relaxation times (AUC, 0.91), and 2018 LLC (AUC, 0.92).
Figure 3:
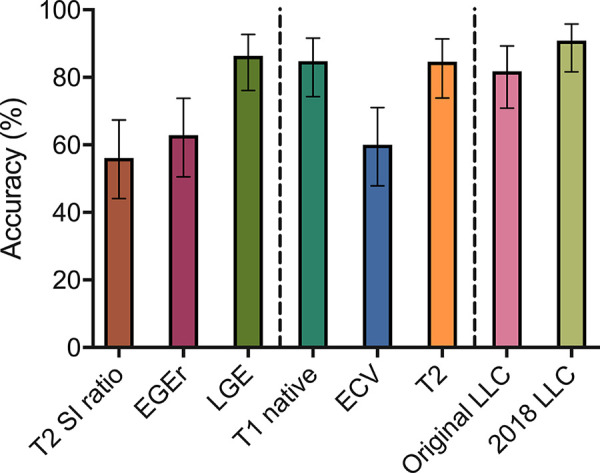
Column graph shows overall diagnostic accuracies for single parameters and for Lake Louise criteria (LLC). Error bars indicate 95% confidence intervals. ECV = extracellular volume fraction, EGEr = early gadolinium enhancement ratio, LGE = late gadolinium enhancement, SI = signal intensity.
Discussion
In this prospective study, we evaluated different diagnostic cardiac MRI criteria for acute myocarditis in a validation cohort by using previously defined cutoff values. The main findings of this study are that the diagnostic performance of the 2018 LLC was significantly higher compared with the original LLC, and previously derived local cutoff values for single parameters (especially for myocardial T1 and T2 mapping) seem to be sufficient for diagnosis when exactly the same imaging techniques are applied.
Since the introduction of the original MOLLI T1 mapping sequence (13), several studies investigated the diagnostic performance of various myocardial T1 mapping schemes in acute myocarditis (7,8,18,19). According to a recent meta-analysis of 17 studies, myocardial T1 mapping in acute myocarditis yielded a pooled sensitivity of 85% and a pooled specificity of 86% (10). Another meta-analysis of 22 studies showed a pooled sensitivity of 89%, a specificity of 90%, and an area under the curve of 0.95 for diagnosing acute myocarditis (9). In our study, T1 mapping achieved similar diagnostic values with an area under the curve of 0.92 (sensitivity, 78%; specificity, 96%). These results show that myocardial T1 is a valuable tool in detecting diseased myocardium in acute myocarditis. The increase of myocardial T1 in acute myocarditis is mainly driven by an intracellular and extracellular edema (20), but also vasodilatation, hyperemia, and the expansion of the extracellular space can increase myocardial T1 relaxation times (7,11,20). Notably, myocardial T1 is also increased in more chronic forms of myocarditis (5,18,21,22) and can be also increased in other entities of chronic myocardial disease, which are accompanied by myocardial fibrosis (23). Therefore, myocardial T1 relaxation times are not specific for acute myocarditis, but they can provide a high diagnostic performance when interpreted in an appropriate clinical setting. Consequently, myocardial T1 relaxation times were implemented in the 2018 LLC as a new T1-based criterion (11). However, no statement was made about specific cutoff values and it was only referred to already existing local or published cutoff values (11). Because cardiac mapping is a rapidly evolving field, and standardized protocols and techniques are still in the process of being established, the lack of more specific guidelines and standardized imaging protocols could hamper the use of myocardial mapping techniques in many nonspecialty imaging departments. Consequently, published cutoff values for myocardial T1 mapping in acute myocarditis range from 852 msec to 1140 msec (7,8,10). This heterogeneity of cutoff values demonstrates the challenges it may pose to integrate myocardial mapping techniques into wide clinical routine. Myocardial T1 values depend on multiple factors, including field strength, T1 mapping sequence, manufacturer, software, image quality, and standardized methods of analysis (24–26). In acute myocarditis, several forms of MOLLI and shortened MOLLI sequences have been investigated (7,9,10), which influence myocardial T1 values. Also, the time interval between admission or onset of symptoms to cardiac MRI was different in many studies and ranged from 2 days (21) to a median of 2 weeks (18), another factor that can bias disease activity and cutoff values between two groups. Furthermore, it is important to note that cutoff values are always a trade-off between sensitivity and specificity and can be adjusted accordingly. Many previous studies used the Youden index as a criterion for selecting optimum cutoff points for myocardial T1 mapping (18,22,27), which is an appropriate method when investigating the diagnostic performance of a single variable. However, in a multiparametric score such as the LLC, more customized cutoff values for single variables might increase the diagnostic performance of the final score. Finally, it is important to validate cutoff values by using an independent validation cohort. In our study, we provide a validation cohort of patients with acute myocarditis, which were evaluated by using previously defined cutoff values (7). Myocardial T1 mapping yielded a diagnostic accuracy of 85%, which is comparable to the previously reported accuracy of 86%–92% (7). Our results therefore indicate that previously defined local cutoff values for myocardial T1 mapping are sufficient to diagnose acute myocarditis if the sequence is acquired, processed, and analyzed exactly the same way. In the current clinical recommendations for cardiac MRI mapping from the Society for Cardiovascular Magnetic Resonance, it is suggested that local reference values should be primarily used for myocardial mapping techniques and local results should be benchmarked against published reported ranges (28). Therefore, the presented cutoff values in this study are not intended to be used in wide praxis.
Myocardial T2 mapping is also a sensitive parameter for myocardial edema in the clinical setting of acute myocarditis (5,14). In contrast to T1 relaxation times, myocardial T2 relaxation times can discriminate between acute and healed stages of myocarditis, likely because they are insensitive to the detection of areas of scarring and fibrosis (27). In patients with chronic symptoms and biopsy-proven myocarditis, myocardial T2 mapping can more accurately diagnose inflammatory changes compared with myocardial T1 mapping (22). Because of its specificity for inflammatory alterations, myocardial T2 relaxation times were also implemented in the 2018 LLC as a T2-based criterion (11). However, some of the drawbacks of myocardial T1 mapping also exist for myocardial T2 mapping. For myocardial T2 mapping, the most widely applied imaging approaches used T2-prepared steady-state free precession (27) and gradient (7) or fast spin-echo multiecho readouts (22). Although myocardial T2 mapping is less dependent on field strength compared with myocardial T1 mapping, reported cutoff values for the assessment of acute myocarditis range from 52.3 msec to 61.0 msec (10,18,27). In our study, the cutoff value of 55.8 msec yielded a diagnostic accuracy of 85%, which is comparable to the 87% of the initial study cohort (7). The diagnostic performance of myocardial T2 mapping was high, which is illustrated by an area under the curve of 0.91. Together with myocardial T1 mapping, myocardial T2 mapping is one of the two best-performing single parameters for diagnosing acute myocarditis (9). By using the same techniques and cutoff values as previously reported, a similar discrimination capability can be expected, indicating a robust reproducibility of the techniques.
The increased diagnostic performance of the 2018 LLC compared with the original LLC in our study was mainly driven due to high sensitivity and overall diagnostic performance of myocardial T1 and T2 mapping. The original LLC yielded an area under the curve of 0.84, which is comparable to the results of previous studies (9). The original LLC might be further used in clinical practice; the classic elements of the score allow for a fast, well-studied, and reliable investigation of patients with acute myocarditis, which can be adequately performed with every cardiac MRI system. However, the implementation of the new 2018 LLC will enhance the diagnostic performance of multiparametric cardiac MRI in acute myocarditis.
Because endomyocardial biopsy is not clinical practice at our institution and has been reported to be prone to a sampling error that can lead to false-negative results, especially in a patient with focal myocardial inflammation (29), no systematic endomyocardial biopsy as a reference standard was performed in our study. Instead, the presence of acute myocarditis was defined by combining typical clinical features, exclusion of coronary artery disease, and elevated biomarkers as reported previously in multiple cardiac MRI validation studies (18,19). However, the selected study approach does not represent the patient composition in a real-world setting and the reported parameters of diagnostic performance (especially negative and positive predictive value) have to be regarded as study specific. In our study, the period between hospital admission and cardiac MRI was very short. In this regard, it is important to mention that diagnostic performance of cardiac MRI can be reduced when patients with acute myocarditis are examined later in the course of the disease (5). Also, most of the included patients presented with an acute infarctlike myocarditis, and therefore the proposed cutoff values are primarily valid for the described subgroup of patients with acute myocarditis. Other cutoff values might be used when transferring the results to patients with more chronic clinical symptoms and presentations. Because of the small sample size and the single-center design, our results have to be considered as preliminary and further prospective studies are necessary to substantiate the results of this study.
In conclusion, multiparametric cardiac MRI has a high diagnostic value for the diagnosis of patients with acute myocarditis. The 2018 LLC significantly enhance the diagnostic performance of cardiac MRI compared with the original LLC and an implementation of the new parametric imaging techniques into routine diagnostic protocols is highly recommended.
SUPPLEMENTAL TABLES
Disclosures of Conflicts of Interest: J.A.L. disclosed no relevant relationships. A.F. disclosed no relevant relationships. A.I. disclosed no relevant relationships. D.D. disclosed no relevant relationships. D.K. disclosed no relevant relationships. A.F. disclosed no relevant relationships. F.C.S. disclosed no relevant relationships. A.M.S. disclosed no relevant relationships. D.T. disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- LGE
- late gadolinium enhancement
- LLC
- Lake Louise criteria
References
- 1.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34(33):2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 2.Drory Y, Turetz Y, Hiss Y, et al. Sudden unexpected death in persons less than 40 years of age. Am J Cardiol 1991;68(13):1388–1392. [DOI] [PubMed] [Google Scholar]
- 3.Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis—diagnosis, treatment options, and current controversies. Nat Rev Cardiol 2015;12(11):670–680. [DOI] [PubMed] [Google Scholar]
- 4.Luetkens JA, Schlesinger-Irsch U, Kuetting DL, et al. Feature-tracking myocardial strain analysis in acute myocarditis: diagnostic value and association with myocardial oedema. Eur Radiol 2017;27(11):4661–4671. [DOI] [PubMed] [Google Scholar]
- 5.Luetkens JA, Homsi R, Dabir D, et al. Comprehensive cardiac magnetic resonance for short-term follow-up in acute myocarditis. J Am Heart Assoc 2016;5(7):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 2009;53(17):1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luetkens JA, Homsi R, Sprinkart AM, et al. Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging 2016;17(2):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luetkens JA, Doerner J, Thomas DK, et al. Acute myocarditis: multiparametric cardiac MR imaging. Radiology 2014;273(2):383–392. [DOI] [PubMed] [Google Scholar]
- 9.Kotanidis CP, Bazmpani MA, Haidich AB, Karvounis C, Antoniades C, Karamitsos TD. Diagnostic accuracy of cardiovascular magnetic resonance in acute myocarditis: a systematic review and meta-analysis. JACC Cardiovasc Imaging 2018;11(11):1583–1590. [DOI] [PubMed] [Google Scholar]
- 10.Pan JA, Lee YJ, Salerno M. Diagnostic performance of extracellular volume, native T1, and T2 mapping versus Lake Louise Criteria by cardiac magnetic resonance for detection of acute myocarditis: a meta-analysis. Circ Cardiovasc Imaging 2018;11(7):e007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72(24):3158–3176. [DOI] [PubMed] [Google Scholar]
- 12.Look DC, Locker DR. Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum 1970;41(2):250–251. [Google Scholar]
- 13.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52(1):141–146. [DOI] [PubMed] [Google Scholar]
- 14.Sprinkart AM, Luetkens JA, Träber F, et al. Gradient Spin Echo (GraSE) imaging for fast myocardial T2 mapping. J Cardiovasc Magn Reson 2015;17(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Aty H, Boyé P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 2005;45(11):1815–1822. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 1998;97(18):1802–1809. [DOI] [PubMed] [Google Scholar]
- 17.Luetkens JA, Klein S, Träber F, et al. Quantification of liver fibrosis at T1 and T2 mapping with extracellular volume fraction MRI: preclinical results. Radiology 2018;288(3):748–754. [DOI] [PubMed] [Google Scholar]
- 18.Radunski UK, Lund GK, Stehning C, et al. CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging 2014;7(7):667–675. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira VM, Piechnik SK, Dall’Armellina E, et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging 2013;6(10):1048–1058. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira VM, Piechnik SK, Dall’Armellina E, et al. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinojar R, Foote L, Arroyo Ucar E, et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging 2015;8(1):37–46. [DOI] [PubMed] [Google Scholar]
- 22.Lurz P, Luecke C, Eitel I, et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer-Trial. J Am Coll Cardiol 2016;67(15):1800–1811. [DOI] [PubMed] [Google Scholar]
- 23.Ambale-Venkatesh B, Lima JA. Cardiac MRI: a central prognostic tool in myocardial fibrosis. Nat Rev Cardiol 2015;12(1):18–29. [DOI] [PubMed] [Google Scholar]
- 24.Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 mapping: basic techniques and clinical applications. JACC Cardiovasc Imaging 2016;9(1):67–81. [DOI] [PubMed] [Google Scholar]
- 25.Dabir D, Child N, Kalra A, et al. Reference values for healthy human myocardium using a T1 mapping methodology: results from the International T1 Multicenter cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2014;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers T, Dabir D, Mahmoud I, et al. Standardization of T1 measurements with MOLLI in differentiation between health and disease—the ConSept study. J Cardiovasc Magn Reson 2013;15(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Knobelsdorff-Brenkenhoff F, Schüler J, Dogangüzel S, et al. Detection and monitoring of acute myocarditis applying quantitative cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2017;10(2):10. [DOI] [PubMed] [Google Scholar]
- 28.Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19(1):75 [Published correction appears in J Cardiovasc Magn Reson 2018;20(1):9.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc 1989;64(10):1235–1245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.