Abstract
Background:
Globally, case fatality rate is more in males compared to females. Some studies have suggested. It is hypothesized that estrogen hormone may decrease susceptibility to severe acute respiratory syndrome coronavirus 2 (SARS CoV-2.)
Objective:
The objective of the study was to evaluate the gender differences in SARS CoV-2 outcomes and to analyze if there are any differences in outcomes in premenopausal females compared to postmenopausal females.
Materials and Methods:
Patients tested positive for SARS CoV-2 through real-time reverse transcription-polymerase chain reaction by Thermo Fischer Taqpath assay approved by the Indian Council of Medical Research were included in the study. The data obtained was analyzed for the epidemiological, clinical, and laboratory characteristics from their medical records.
Results:
The mortality rate in females was 12.6%, whereas mortality in males was 19.4%. In between-group analysis, 8.6% (16/185) of females died in premenopausal age group versus 12.8% (27/211) in postmenopausal group. The proportion of females who expired due to COVID significantly differ by age and postmenopausal status X2 (1, n = 293) = 7.2, the P value is 0.007. The difference is statistically significant at P < 0.05. Postmenopausal women were more likely to expire due to COVID-19 infection compared to premenopausal women.
Conclusion:
The mortality rate in postmenopausal age group was greater than mortality in premenopausal females emphasizing the protection provided by estrogens hormone in them. Postmenopausal women are also at higher risk of severe COVID-19 infection than premenopausal women. Mortality is greater in males compared to females, further strengthening the role of estrogens.
KEYWORDS: Coronavirus, estrogen, gender differences, perimenopause, postmenopause
INTRODUCTION
The novel coronavirus disease 2019 (nCoV19) pandemic is one of the most catastrophic events in the recent history of human beings. Severe acute respiratory syndrome coronavirus 2 (SARS COV-2) belongs to the same β-coronavirus subgroup, and it has genome similarity with SARS CoV and the Middle East Respiratory Syndrome (MERS)-CoV.[1] Men are more susceptible to viral infections than women, and globally case fatality ratio is more in males versus females. The objective of the study was to evaluate the gender differences in SARS CoV-2 outcomes and also if there are any differences in outcomes in premenopausal females compared to postmenopausal females.
MATERIALS AND METHODS
This study was conducted in a dedicated COVID-19 facility. Retrospective analysis of all SARCov2 positive patients admitted from April 2020 to July 2020 was done after taking institutional permission. All nCoV19 reverse transcription-polymerase chain reaction (RT-PCR)-positive patients were selected for the study. Nasal and oral swabs were collected from patients suspected of having the COVID-19 infection. Patients who had a clinical diagnosis of COVID-19 but RT-PCR negative were excluded from this study. Patients who were still admitted to the hospital were also omitted from the study. Informed consent was obtained from all patients. Ethical clearance was obtained from our institutional committee (SNMC/EC/2020-23) and it follows the ethical principles of the World Medical Association.
Declaration of Helsinki. Epidemiological, clinical, laboratory, chronic illnesses (comorbidities) and radiological characteristics and treatment and outcomes data were obtained from medical records and data were reviewed and analyzed. Laboratory and radiological parameters included complete blood count, erythrocyte sedimentation rate, liver function test, renal function test, blood sugar, C reactive protein (CRP), serum ferritin levels. Fever was defined as oral temperature >99°F. Disease onset was defined as the day when the symptoms were first noticed. Patients were discharged after two negative RTPCR reports 24 h apart as per the Indian Council of Medical Research protocol. Our hospital is dedicated for the treatment of Level 2 and Level 3 patients, and we had admitted only moderate and severe cases of nCoV19. Menopause was defined as complete cessation of menses for the past 1 year. Those women who met the criteria of menopause were labeled postmenopausal. We classified women into two groups, based on their menopause status as premenopausal and postmenopausal. The average age of natural menopause in India is 46 years.[2,3]
Statistical analysis
Chi-Square test was used to compare data among different cohorts of males and females. Student's t-test was used to compare different populations of males with females to analyze the differences in mortality among them. Data were analyzed using SPSS software (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp).
RESULTS
Total 720 SARS CoV-2 positive patients were admitted from April to July 2020, of which 293 patients were females and 427 were males. Out of 293 females, 185 females were premenopausal and 108 females were postmenopausal. The median age at admission was 58.30 (42–82 years).
Maximum women were housewives, 18 patients were working in different occupation and one was the paramedical staff working at our Covid hospital. Out of the 108 postmenopausal females, 71 (65.70%) presented with Severe acute respiratory infection (SARI), average oxygen saturation in patients who presented with SARI was 81.26% (45%–95%) on room air at admission. Respiratory failure/acute respiratory distress syndrome (ARDS) lead to mortality in 17/108 (15.78%) patients inspite of being given ventilatory support.
Symptoms
Fever was the most prevalent symptom 57.4% (62/108) in patients with nCoV19 followed by breathlessness 57.4% (62/108), cough 37% (40/108), myalgia 8.33% (9/108), sore throat 8.33% (9/108), and chest pain and diarrhea in 10.1% (11/108) equally [Table 1]. The other symptoms in these patients were abdominal pain in 2, epistaxis in 1, hemoptysis in 1, bleeding per rectum, and altered sensorium in 1 patient.
Table 1.
Clinical characteristics of subjects
| Clinical characteristics | Total | Premenopaus al females | Postmenopaus al females | Male ≤48 years | Male >48 years |
|---|---|---|---|---|---|
| n | 720 | 185 | 108 | 211 | 216 |
| Deceased | 120 | 16 | 21 | 27 | 56 |
| Test statistic, P | 7.2, 0.007 | 11.7, 0.001 | |||
| Mortality rate (%) | 16.66 | 8.6 | 19.4 | 12.8 | 25.9 |
| Mortality rate overall | 12.6 | 19.4 | |||
| Symptoms in alive patients | |||||
| Fever | 54 | 62 | 103 | 119 | |
| P | 0.00 | 0.19 | |||
| Breathlessness | 29 | 62 | 89 | 131 | |
| P | 0.00 | 0.00 | |||
| Cough | 39 | 40 | 80 | 92 | |
| P | 0.003 | 0.32 | |||
| Myalgia | 28 | 9 | 39 | 24 | |
| P | 0.14 | 0.03 | |||
| Sore throat | 13 | 9 | 20 | 6 | |
| P | 0.68 | 0.44 | |||
| Diarrhoea | 8 | 4 | 1 | 1 | |
| P | 0.79 | 0.98 | |||
| Chest pain | 5 | 7 | 10 | 7 | |
| P | 0.11 | 0.42 | |||
Urinary tract infection was present in 18%. Nine patients had ketones in their urine and out of which five were diabetic. About 38% of patients presented with increased random blood sugar levels at the time of admission, and this level of hyperglycemia was associated with poor outcome. When stratified by severity, those patients who had thrombocytopenia, increased CRP/ferritin, and decreased calcium/albumin had a poor outcome.
Laboratory and Chest X-ray findings
The chest X-ray findings in postmenopausal females who survived revealed that 50.5% (44/87) were normal, 21.8% (19/87) had unilateral consolidation, while 27.5% (24/87) had bilateral consolidation at the time of admission. The left lung (12/19) was involved more than the right lung (7/19). Among those patients who expired, unilateral consolidation was found in the chest X-ray of 33.33% (7/21), 57.1% (12/21) had bilateral consolidation, and 2/21 had normal X-ray. Increases CRP is most consistent pathology report present in 78% of subjects followed by raised serum Ferritin levels (63%). Pathological findings are summarized in Table 2.
Table 2.
Laboratory changes in COVID 19
| Laboratory parameter | Percentage |
|---|---|
| Low hemoglobin | 34 |
| Low platelets | 35.6 |
| Lymphocytopenia | 18.8 |
| Lymphocytosis | 12.3 |
| Leucocytosis | 19.1 |
| Increased CRP | 78 |
| Increased ferritin | 63 |
| Increased ESR | 18 |
| Increased LDH | 44 |
| Deranged renal function test | 46.2 |
| Elevated SGOT/SGPT | 56 |
| Decreased albumin | 46 |
| Decreased calcium | 54 |
| Hyponatremia | 6 |
| Hyperkalemia | 2 |
| Hypokalemia | 2 |
CRP: C reactive protein, ESR: Erythrocyte sedimentation rate, LDH: Lactate dehydrogenase, SGOT: Serum glutamic oxaloacetic transaminase, SGPT: Serum glutamic pyruvic transaminase
Comorbidities
Common comorbidities associated with our study were diabetes mellitus (DM), hypertension (HTN), cardiovascular diseases (CVD), chronic kidney diseases (CKD), and chronic liver disease (CLD) [Figure 1]. DM was the associated comorbidity in 38 patients (29%), of which 8 patients expired. Five patients of DM presented with diabetic ketoacidosis , of which 3 patients were well managed, but two patients could not survive. HTN was present in 37 patients (28.24%), out of which 08 patients expired. CKD was associated in 17 patients (12.98%), in which 13 patients were on maintenance hemodialysis (HD) and 03 patients of CKD could not survive. Chronic lung diseases such as chronic obstructive pulmonary disease, asthma, and old treated lung tuberculosis were found to be associated with nine cases of which four could not survive. Hypothyroidism was associated with eight patients (6.11%), two patients could not survive. Total six patients were admitted with a previous history of CVD while one patient presented with the acute coronary syndrome (ACS). This patient of ACS, along with one patient of rheumatic heart diseases, expired. CLD was associated with six females (4.58%) and two patients of CLD expired. Malignancy was associated with 04 females (3.05%) [Figure 2]. Other comorbidities found in this study were acute pancreatitis (1), cholelithiasis (2), fracture of lower limbs (2), and general tonic clinic seizure (GTCS) in one patient.
Figure 1.
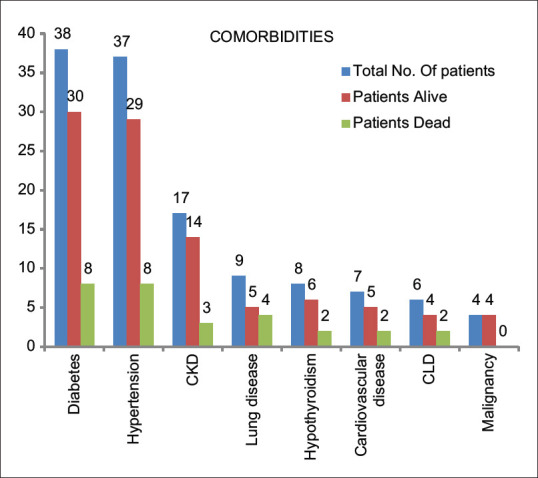
Prevalence of comorbidities. CKD: Chronic kidney disease, CLD: Chronic liver disease
Figure 2.
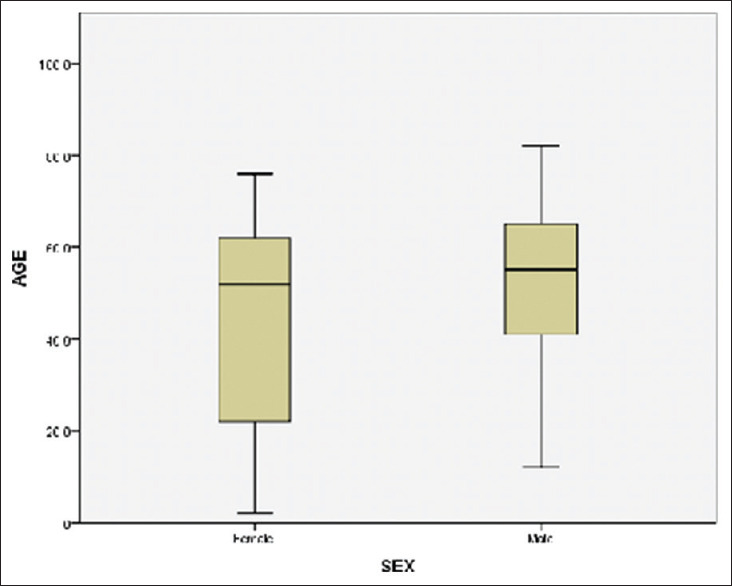
Comparisons of ages among males and female patients who died due to COVID-19
Treatment and outcome
Due to low oxygen saturation and other comorbidities, 32.82% (43/131) of patients were admitted in the intensive care unit (ICU). All patients admitted in ICU were given oxygen through appropriate type of masks, high flow nasal cannula , and bi-level positive airway pressure. Thirty-two patients required invasive mechanical ventilation. Antibiotics, steroids, paracetamol, and low-molecular-weight heparin were administered in maximum patients as per the treatment protocol. Remdesvir was given in 36 patients as per protocol designed by the All India Institute of Medical Sciences, New Delhi. Convalescent plasma was transfused in four patients, of which two survived. The patients who were admitted in wards and high definition unit were managed with standard of care treatment as when needed. Total of 14 patients were given renal replacement therapy or HD, including 13 patients already on HD. We also encouraged our patients to do yoga and lungs expanding exercises. We managed DM and steroid/stress-induced hyperglycemia with insulin. Other symptoms were managed accordingly. One case of cholelithiasis was managed surgically due to nonresponding pain. Fracture of lower limbs was managed conservatively with traction in both patients. In two patients of CLD paracentasis was done due to nonresponding ascites.
Total of 21 patients expired during hospitalization, 19 patients due to respiratory failure/ARDS and 02 patients due to sudden cardiac arrest. Maximum deaths were in patients who had associated with comorbidities such as DM, HTN, CKD, and chronic lung diseases. The median age of expired patients was 61.18 (48–76 years). Mortality was more in uncontrolled DM and HTN patients. Mortality was also associated with thrombocytopenia, increased CRP/ferritin and decreased calcium/albumin, and hyperglycemia at the time of admission. Oxygen requirement and involvement of lung parenchyma were also directly associated with poor outcome.
Gender and age-related differences in mortality
The mortality rate in females was 12.66%, whereas mortality in Males was 19.4%. In between-group analysis, 8.6% (16/185) females died in premenopausal group versus 19.4% (21/108) in postmenopausal age group [Figure 3]. The proportion of females who reported expired due to COVID significantly differ by postmenopausal status, X2 (1, n = 293) = 7.2, the P value is 0.007. The difference is statistically significant at P < 0.05. Postmenopausal women are more likely to expire due to COVID-19 [Figure 4].
Figure 3.
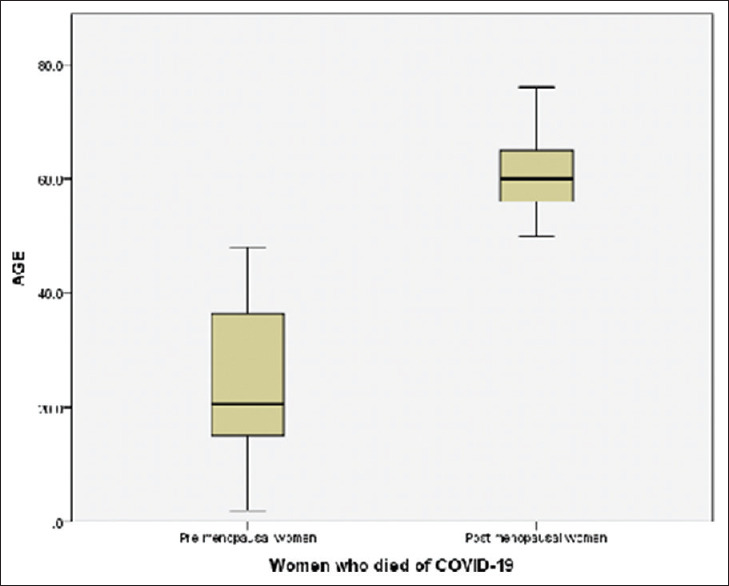
Comparisons of ages among pre- and post-menopausal women who died of COVID-19
Figure 4.
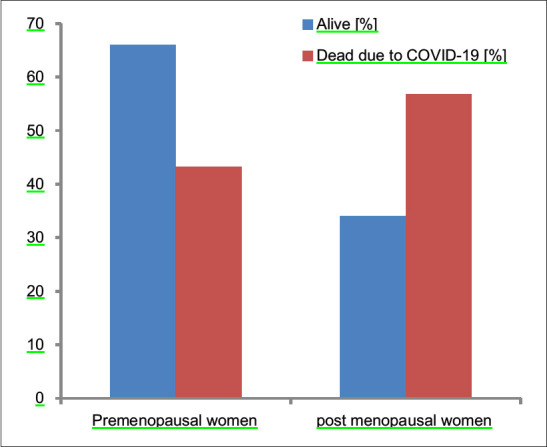
Comparison of mortality among pre- and post-menopausal women (P = 0.007)
Out of 211 males who were ≤48 years, 27 expired (12.8%). Whereas, 56 out of 216 (25.9%) males >48 years age expired due to COVID-19. The proportion of males who expired due to COVID significantly differ by age, cut off is 48 years, X2 (1, n = 427) = 11.7, The P value is. 001. The difference is statistically significant at P < 0.05. Older males are more likely to expire due to COVID-19 [Figure 5].
Figure 5.
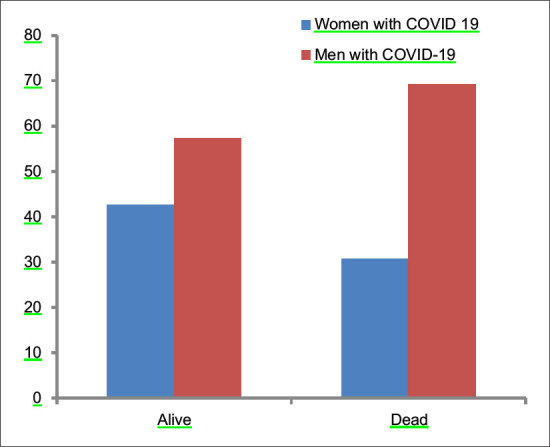
Comparison of mortality among males and females due to COVID-19 (P = 0.016)
The proportion of postmenopausal females who expired due to COVID-19 is higher than the age-matched males and this is not statistically significant at P < 0.05 [Figure 2]. The value of t is −0.55. The value of P is 0.58. In contrast, when compared, the proportion of premenopausal females who expired due to COVID19 with the age-matched males, the difference is statistically significant. The value of t is −5.2. The value of P is 0.00. The result is significant at P < 0.05. Symptoms such as fever, breathlessness, and cough were found to be statistically significantly more prevalent among postmenopausal women than the younger ones. Similarly, among males, breathlessness and myalgia were found to be significantly more prevalent among older males than their younger counterparts (P < 0.05).
DISCUSSION
This is the first descriptive Indian study of perimenopausal and postmenopausal females regarding their epidemiological, clinical characteristics, treatment, and outcome data to the best of our knowledge. Total of 720 patients (293 females and 427 males) were admitted in our facility during the study. Male preponderance could also be partly explained by the sex ratio in our country and usually, males are in occupation, therefore more exposed to COVID-19. High-Risk behaviors like occupational exposure, smoking, and alcohol are more in males adding to comorbities in them. Women are also not provided timely care in our country. Fever (56.49%) was the most prevalent symptom in our patients, followed by breathlessness (52.6%) and cough (35.11%) as in the study by Huang et al.[4] who reported fever in 98% cases and Wang et al.[5] (98.6%) and Guan et al.[6] (87.9%), where fever is the most common symptom. Cough was present in 76%, 59.4%, and 69.7% cases, respectively. Other symptoms were fatigue and dyspnoea.
Elevated CRP was the most common finding reported in 91% of cases by Zhang et al.[7] and 86% by Chen et al.[8] and Liu et al.[9] in 83% and 78% in our study. Coronavirus infection is associated with excessive inflammatory response, which explains elevated CRP in almost all the cases.[10] Thrombocytopenia (35.6%) in our study, 36.2% by Guan et al. and 41.7% by Liu et al. However, the prevalence of thrombocytopenia in severe cases was greater in the former study, whereas it was more common in nonsevere cases in the latter leukocytosis (19.1%) and lymphopenia (18.8%) were seen.
DM was the most commonly associated comorbidity seen in 29% of cases, followed by HTN (28.24%) and CKD (12.98%).
The result of our study matches with Garg et al.[10] as age is independent risk factor for mortality and is also reflected in our findings.
According to a study by Cai et al.[11] patients with deranged liver function tests have a higher risk of progressing to severe disease. In the study by Tezcan et al., it was concluded that electrolyte abnormalities, especially hyponatremia are a sign of poor prognosis in patients with COVID-19 disease.[12] Certain studies also showed hyperferritinemia in some critically ill patients.
Sex differences because of angiotensin-converting enzyme 2 expression
Angiotensin-converting enzyme 2 (ACE2) is a component of cell entry in airway epithelial cells. Sex-related differences in coronavirus pandemic have been found. More intensive care admissions have been found in males compared to females in COVID-19 as well as in MERS and SARS.[13] A protective role by estrogen has been documented in females. Estrogen downregulates ACE2 expression, and therefore, viral entry is reduced [Figure 1]. Marine studies have confirmed that males are more susceptible to a virus and when female mice were treated with estrogen receptor antagonist, females had more mortality compared with placebo males. In one study, estrogen treated normal human tracheal epithelial cells had lower levels of ACE2 versus vehicle treated controls.[14] ACE2 gene is present on the X chromosome. Its location has important implications which is yet to be studied. Males lack the alternative mechanism for protection after exposure to the SARS CoV-2 virus. The mortality rate was 0.5% in women versus 1.2% for males almost double in a study in China.[15] More males developed severe respiratory failure 89% versus 10.7% in females. In a study, it was found that myocardial injury was increased, but myocardial ACE2 expression was decreased after exposure of SARS CoV 2 virus.[16]
How estrogen might act
Estrogen receptors are also present in cardiomyocytes [Figure 6]. In males with cardiac injury have more chances of death versus males without cardiac injury. In severe COVID-19, there is coagulopathy, leading to thromboembolism. This is more common in men compared to women in premenopausal women. In premenopausal women,[17] beta-estradiol is the principal estrogen, where is in postmenopausal women estrone, which is less potent, is the predominant estrogen, leading to increased morbidity in SARS CoV-2 in postmenopausal females.[17,18]
Figure 6.
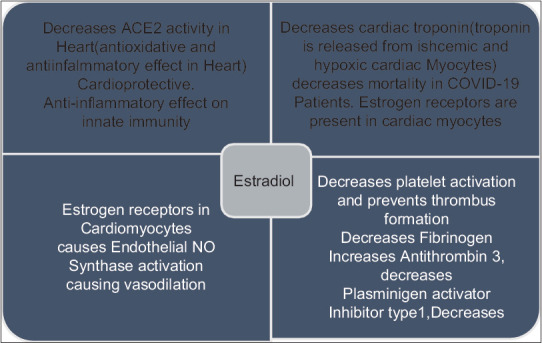
Mechanism by which estrogen may be protective against novel coronavirus disease 2019
Different immunological behaviors between the sexes
Immunogenic behavior in men and women is different because of estrogen, progesterone, and androgen hormones. Higher immunoglobulin levels and circulating CD4 CD 8 T-Lymphocytes ratio is found in females versus males.[18] Immune-related genes are present in high density in X chromosomes therefore, women have stronger innate and adaptive immunity than men leading to faster clearance of pathogens. Vaccine efficacy is also more in females.
In women, T helper 2 immune response is enhanced and Th1 is reduced. Selective estrogen receptor modulators Tormiphene Citrate and Tranexamic acid have been tried in SARS and MERS and it delayed virus infection.[19] Differences in the production of immunosuppressive interleukin 10 by cells of innate immunity might also explain gender-related differences in outcomes of COVID-19.
CONCLUSION
Postmenopausal women are at higher risk to succumb due to nCoV19 than males. The mortality rate in postmenopausal age group was greater than mortality in younger females emphasizing the protection that might be provided by estrogen in them. Postmenopausal women are a higher risk of severe disease. Mortality was greater in males compared to females further strengthening the role of estrogens. Estrogenic compounds and antiandrogenic drugs are being tried in various research trials for the treatment of coronavirus.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet (London, England) 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meeta S. Early age of natural menopause in India, a biological marker for early preventive health programs. Climacteric: The Journal of the International Menopause Society. 2012;15:581–6. doi: 10.3109/13697137.2011.643514. [DOI] [PubMed] [Google Scholar]
- 3.Ahuja M. Age of menopause and determinants of menopause age: A PAN India survey by IMS. J Midlife Health. 2016;7:126–31. doi: 10.4103/0976-7800.191012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS- CoV-2 in Wuhan, China. Allergy. 2020;75:1730–41. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Chinese J Tuber Respir Dis. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 9.Liu, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–74. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with Laboratory-confirmed coronavirus disease 2019-COVID- NET, 14 States, March 1-30, 2020. MMWR Morb Morta Wkly Rep. 2020;69:458–64. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–74. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tezcan ME, Gokce GD, Sen N, Kaymak NZ, Ozer RS. Baseline electrolyte abnormalities would be related to poor prognosis in hospitalized coronavirus disease 2019 patients. New microbes new Infect. 2020;37:100753. doi: 10.1016/j.nmni.2020.100753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stelzig KE, Canepa-Escaro F, Schiliro M, Berdnikovs S, Prakash YS, Chiarella SE. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2020;318:L1280–81. doi: 10.1152/ajplung.00153.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suba Z. Prevention and therapy of COVID-19 via exogenous estrogen treatment for both male and female patients: Prevention and therapy of COVID-19. J Pharm Pharm Sci. 2020;23:75–85. doi: 10.18433/jpps31069. [DOI] [PubMed] [Google Scholar]
- 15.Dudley JP, Lee NT. Disparities in age-specific morbidity and mortality from SARS-CoV-2 in China and the Republic of Korea. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2020;71:863–5. doi: 10.1093/cid/ciaa354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;7:618–25. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breithaupt-Faloppa AC, Correia CD, Prado CM, Stilhano RS, Ureshino RP, Moreira LF. 17β-Estradiol, a potential ally to alleviate SARS-CoV-2 infection. Clinics (Sao Paulo) 2020;75:e1980. doi: 10.6061/clinics/2020/e1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torcia MG, Nencioni L, Clemente AM, Civitelli L, Celestino I, Limongi D, et al. Sex Differences in the Response to Viral Infections: TLR8 and TLR9 Ligand Stimulation Induce Higher IL10 Production in Males. PLoS ONE. 2012;7(6):e39853. doi: 10.1371/journal.pone.0039853. doi:10.1371/journal.pone.0039853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyall J, Coleman CM, Hart BJ, Venkataraman T, Holbrook MR, Kindrachuk J, et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58:4885–93. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


