Abstract
Procrastination is a prevalent and universal problematic behavior, largely impairing individual's health, wealth and well‐being. Substantial studies have confirmed that conscientiousness, one of the big five personality, showed markedly inverse relation with procrastination. However, it is hitherto unknown about the neural basis underlying the impact of conscientiousness on procrastination. To address this issue, we employed the voxel‐based morphometry (VBM) and resting‐state functional connectivity (RSFC) methods to explore the neural substrates of conscientiousness responsible for procrastination (N = 330). In line with previous findings, the behavioral results showed a strong negative correlation between conscientiousness and procrastination (r = −.75). The VBM analysis found that conscientiousness was positively correlated with gray matter (GM) volumes in the left dorsal‐lateral prefrontal cortex (dlPFC), right orbital frontal cortex (OFC) and right putamen, but negatively correlated with that in the left insula. Moreover, the RSFC results revealed that both dlPFC‐IPL (inferior parietal lobule) and dlPFC‐PCC (posterior cingulate gyrus) functional connectivity were positively associated with conscientiousness, while the functional connectivity of parahippocampal gyrus (PHC)‐putamen and insula‐IPL were negatively associated with conscientiousness. More importantly, the structural equation modeling (SEM) integrating RSFC results were well fitted for the influence process of conscientiousness on procrastination by both self‐control (i.e., dlPFC‐IPL, dlPFC‐PCC) and motivation pathways (i.e., PHC‐putamen, insula‐IPL). The current findings suggest that self‐control and motivation could be the two neural pathways underlying the impact of conscientiousness on procrastination, which provides a new perspective to understand the relationship between conscientiousness and procrastination.
Keywords: conscientiousness, procrastination, resting‐state functional connectivity, voxel‐based morphometry
Short abstract
Based on the reconstruction of the sub‐facets of conscientiousness, the VBM and RSFC methods were used to investigate the neural basis underlying the impact of conscientiousness on procrastination. Moreover, the SEM analysis integrating RSFC results were well fitted for the influence process of conscientiousness on procrastination via self‐control (i.e., dlPFC‐IPL, dlPFC‐PCC) and motivation pathways (i.e., PHC‐putamen, insula‐IPL). In conclusion, the present study provided a neural underpinning perspective to promote the understanding of the relationship between conscientiousness and procrastination, which enlightened the researcher about interventions for procrastination.
1. INTRODUCTION
Procrastination refers to a voluntary but irrational delay of an intended course of actions (Gustavson, Miyake, Hewitt, & Friedman, 2014; Steel, 2007). As a chronic tendency of needless delaying things needed to be done, procrastination can lead to individuals' lower levels of health, well‐being, and social achievement (Ferrari, Díaz‐Morales, O'Callaghan, Díaz, & Argumedo, 2007; Gustavson et al., 2014; Kim & Seo, 2015; Dianne M Tice & Baumeister, 2018). Many studies have demonstrated that the stability of procrastination and indicated it can be affected by personality trait, especially conscientiousness (Schouwenburg & Lay, 1995; Watson, 2001). For example, the conscientiousness was suggested as a prominent predictor of procrastination (Lee, Kelly, & Edwards, 2006; Scher & Osterman, 2002; Watson, 2001), not only due to the correlation coefficient of them reached at ‐ 0.62 in a meta‐analysis (Steel, 2007) but also the finding that high consciousness could help inhibit the tendency to procrastinate (Lee et al., 2006). However, it still remains unclear that the neural substrates underlying the impact of conscientiousness on procrastination.
Conscientiousness is defined as individual differences in the propensity to organization, persistence, hard work, as well as motivation in the pursuit of goal accomplishment (DeYoung et al., 2010; H. Zhao & Seibert, 2006). Extensive previous researches mapped the hierarchically structure of conscientiousness at the facet level gathering on orderliness, industriousness, self‐control, responsibility, conventionality, punctuality and so on (DeYoung, Quilty, & Peterson, 2007; Jackson et al., 2010; Rueter, Abram, MacDonald III, Rustichini, & DeYoung, 2018). Specifically, orderliness represents the overarching tendency to be “prepared” for the future. The preparation consists of a series of actions which follow a meaningful sequence and ultimately lead to positive results (e.g., achieving goals). Individuals can choose current behaviors based on desired future outcomes (Baumeister, Vohs, & Oettingen, 2016; Seligman, Railton, Baumeister, & Sripada, 2013). Self‐control will be required to maintain the value of long‐term goals when tasks elicit motive conflicts between immediate, proximal motives and more abstract, distal motives (Fujita, 2011). Punctuality means attitudes towards “being on time” which reflects the degree of strictness to which deadlines are meted (Francis‐Smythe, 1999). Previous studies have indicated that these task‐related behavior selection and control are related to orderliness (Becker, 1998, 2011), self‐control (Luczynski & Hanley, 2013) and punctuality (Back, Schmukle, & Egloff, 2006). Thus, these three facets of conscientiousness (i.e., orderliness, self‐control and punctuality) are likely to be understood in terms of control, ensuring the selection, as well as the completion of goal‐directed tasks successfully. On the flip side, the remaining facets of conscientiousness also illuminated why people keep conscientious, which may be owing to the aspiration for excellence (industriousness), and following through with promises to others (responsibility) or upholding the rules and conventions in society (conventionality). Laboratory studies have found that manipulating incentive value, whether the small proximal rewards (e.g., positive feedback) or the distal outcome (e.g., high‐value reward), can reduce the aversion to industrious courses of actions (Krebs, Boehler, & Woldorff, 2010; Dianne M. Tice, Baumeister, Shmueli, & Muraven, 2007; Tyler & Burns, 2008). Likewise, study indicated responsibility often goes along with achievement motivation (Miyamoto, 1989), and its value judgment will arouse related emotional experience and internal motivation (Ming, Li & Haosheng, Ye, 2010). Conventionality refers to observance of social conventions that are widely accepted by given society (Hadarics, 2016). Being adhered to normative views and conventions is important for people with high conventional motivation in order to avoid insecurity and unpredictability. Thus, either the pursuit of excellence (industriousness), the avoidance of loss caused by dishonesty (responsibility) or the maintenance of the stability of existing rules (conventionality) are motivated by approaching reward and avoiding potential adverse effects. That is, these three facets are likely to be understood in terms of driving factors of conscientiousness, which maybe the another important motivational basis for individuals to overcome difficulties and complete given tasks. Taken together, we proposed that the structure of conscientiousness itself might contain two key components, namely self‐control and driving factors.
With regard to procrastination, previous findings suggested that procrastination was often considered as the product of self‐control failure, which deviated from goal achievement (Steel, 2007). Individuals showing self‐control deficiency were hard to resist the temptation deriving from pleasurable stimuli at present and thus procrastinate on tasks that should have been completed (Trope & Fishbach, 2000). Grund and Fries (2018) proposed that it was essential to consider the motivational basis of such impaired goal pursuits in terms of their congruence with personal values and action intentions (Grund & Fries, 2018). For example, studies revealed that the achievement‐oriented students reported lower academic procrastination scores than well‐being‐oriented students (Dietz, Hofer, & Fries, 2007). The former routinely preferred to study‐related over leisure‐related activities and experience less impairments during studying under a leisure temptation, presumably because personal values determine the valences of specific activities (Fries, Schmid, & Hofer, 2007). Meanwhile, the temporal decision model of procrastination also explains procrastination as a motivation trade‐off between task avoidance and task execution (S. Zhang, Liu, & Feng, 2019). In detail, perceived task aversiveness constitutes avoidance motivation and would hinder individual in taking action to complete the task at hand, while task incentives promoting task execution would motivate ones to initiate and maintain action to gain the reward. When the former outperforms the latter, individuals tend to procrastinate on the current tasks. Conversely, they will act immediately to get things done. Thus, both self‐control failure and the motivational trade‐offs seem to underlie the psychological process of procrastination. Taken together, individuals with high conscientiousness may have less procrastination due to their high self‐control and approach motivation. Therefore, based on our previous assumptions about conscientiousness structure, we can hypothesis that there might be similar pathways that further explained the intrinsic relationship between conscientiousness and procrastination.
Considerable neuroimaging evidence uncovered the neural substrates of conscientiousness. These studies have revealed that the involvement of the prefrontal cortex (PFC), the orbitofrontal cortex (OFC) and subcortical regions like basal ganglia (e.g., caudate, putamen) as well as insula in conscientiousness (Jackson et al., 2010; Kapogiannis, Sutin, Davatzikos, Costa Jr, & Resnick, 2013; W.‐Y. Liu et al., 2013). Specifically, the voxel‐based morphometry (VBM) studies indicated that regional gray matter (GM) volumes in the lateral prefrontal cortex (LPFC), including dorsal and medial regions, were positively correlated with conscientiousness (DeYoung et al., 2010; Jackson et al., 2010; Kapogiannis et al., 2013). Furthermore, a study in brain injury revealed that patients whose brain‐focal damage to the left dorsolateral prefrontal cortex (dlPFC) were linked to lower scores on conscientiousness, especially the self‐discipline facet (Forbes et al., 2014). Therefore, PFC might contribute to adaptive behavioral control of conscientious people and can help them maintain behavior consistent with goal‐pursuit. (Figner et al., 2010; Spiers, 2008). Meanwhile, the structural Magnetic Resonance Imaging (MRI) studies found that the brain areas, which played central roles in achievement or externally modulated motivation (i.e., OFC, putamen, insula and caudate) and episodic future thinking (i.e., parahippocampal gyrus [PHC]), also contributed to conscientiousness (W.‐Y. Liu et al., 2013; Takeuchi et al., 2014). Specifically, conscientiousness was negatively associated with GM volumes in the insula (W.‐Y. Liu et al., 2013), while positively correlated with the GM volumes of the caudate and PHC (Kapogiannis et al., 2013), as well as the cortical thickness of the medial OFC and PHC (Lewis et al., 2018). The ability episodic future thinking (represented by PHC) generally matters for the mental simulation of reward (represented by putamen and caudate) or loss (represented by insula), and this may be the motivational foundation of how people initiate and maintain action. Another functional imaging study found that the putamen activated strongly when individuals were highly motivated to learn (which means higher conscientious in academic areas) (Morgan, Mullen, & Skitka, 2010). Farrand and his colleagues (2012) also demonstrated that individuals scoring high on conscientiousness exhibited the increased dlPFC connectivity with insula (Farr, Hu, Zhang, & Chiang‐shan, 2012). Moreover, Rueter et al. (2018) employed the goal priority network (GPN) determined by independent component analysis (ICA) and uncovered the significant relationship among insula, dorsal anterior cingulate cortex (dACC) and conscientiousness (Rueter et al., 2018). Thus, these researches suggested that the prefrontal regions involved in top‐down control and subcortical regions supporting reward or motivation may be the neural basis of conscientiousness.
Moreover, the neural substrates of procrastination has been characterized into three brain networks: self‐control (i.e., dlPFC, ACC), emotion (i.e., OFC, insula) and episodic prospection (i.e., vmPFC, PHC) (Chen, Liu, Zhang, & Feng, 2019). For example, recent studies have identified the decreased GM volumes of left dlPFC in procrastinators and further explained it as a deficiency of self‐control ability (Y. Hu, Liu, Guo, & Feng, 2018; P. Liu & Feng, 2017). Previous research also revealed the close relation between the GM volumes of orbital frontal cortex (OFC), ventromedial prefrontal cortex (vmPFC), PHC and procrastination, indicating links of procrastination with emotion and future thinking capacities (Y. Hu et al., 2018; P. Liu & Feng, 2017; W. Zhang, Wang, & Feng, 2016). Furthermore, a resting‐state fMRI study revealed that procrastination was contributed to both the hyper‐activity of the default mode network (DMN) overriding the anterior prefrontal cortex (aPFC) and the failure of top‐down control exerted by aPFC on the DMN (W. Zhang et al., 2016). From a brain activity level, the reduced PHC‐striatal activities interactions during task construction could represent procrastinators' perception of task values and related incentives (Zhang, Becker, Chen, & Feng, 2019a). Summarily, procrastination mainly involved in the brain regions of self‐control (e.g, dlPFC, ACC) and future valued incentives (e.g., OFC, putamen, insula, PHC).
The voxel‐based morphometry (VBM) is considered as a highly reliable method to investigate the neuroanatomical basis of personality traits at a more microscopic level and can provide quantitative indicators for the analysis of personality traits (X. Hu et al., 2011; Mahoney, Rohrer, Omar, Rossor, & Warren, 2011). And the resting‐state functional connectivity (RSFC) can base on the VBM results as for in‐depth exploration when mapping complex neural functional coupling that might account for the underlying neuroanatomy (Andrews‐Hanna et al., 2007; Vincent et al., 2007). Thus, the structural and functional approaches can complement each other to improve understanding of the underlying neural correlates (Takeuchi et al., 2011; Watkins, 2008). On other hand, structural equation modeling (SEM), which seeks to fit the hypothesis model established according to theory, can be used as powerful predictors in neuroimaging studies (Boucard, Marchand, & Noguès, 2007). Hence, we employed the VBM and RSFC analyses to explore the neural basis of conscientiousness and integrated the results of RSFC using the SEM to disentangle and explore the structure pattern responsible for impacts of conscientiousness on procrastination.
As mentioned above, the present study aimed to explore the neural substrates of conscientiousness responsible for procrastination. We assessed conscientiousness and procrastination by the NEO Five Factor Inventory (NEO‐FFI) and General Procrastination Scale (GPS) respectively (Costa & MacCrae, 1992; Lay, 1986). Based on the reconstruction of the sub‐facets of conscientiousness, we performed both voxel‐based morphometry (VBM) to explore the gray matter volumes correlated with conscientiousness and resting‐state functional connectivity (RSFC) to reveal the connectivity patterns of that. Finally, the structural equation modeling (SEM) integrating the functional connectivity results was employed to fit the neural pathway underlying the impact of conscientiousness on procrastination.
2. METHODS
2.1. Participants and procedure
Three hundred and forty‐seven right‐handed volunteers from Southwest University, China, were recruited by convenient sampling in this study for payment. All participants were given the informed consent, and none of them reported a history of neurological or psychiatric disorder. The current study was approved by the Institutional Review Board of the Southwest University. Notably, due to the excessive head movement in the RSFC analysis (>2 mm translation in axis and > 2 angular rotation in axis), seventeen participants were removed, and 330 participants remained (93 males; 20.03 ± 1.67 years; see Table 1). All subjects completed the MRI scan before completing the behavioral measures, which contained the NEO Five Factor Inventory (NEO‐FFI) and General Procrastination Scale (GPS).
TABLE 1.
Demographic information (N = 330)
| Variable | Percentages |
|---|---|
| Demoographics | |
| Gender | |
| Male (n = 93) | 28.20% |
| Female (n = 273) | 71.80% |
| Undergraduate | 91.20% |
| Original family | 90.30% |
| Household disposable income/year(CNY) | |
| Less than 10,000 | 5.76% |
| 10,000‐20,000 | 20.00% |
| 20,000‐40,000 | 28.48% |
| 40,000‐60,000 | 18.48% |
| More than 60,000 | 27.27% |
| Health issues | |
| Body mass index (BMI) | |
| Less than 18.5 | 17.58% |
| 18.5–24.0 | 70.30% |
| 24.0–28.0 | 8.18% |
| More than 28.0 | 3.94% |
| Mental illness (i.e., depression) | 0.00% |
| Chronic disease (i.e., hypertension) | 0.00% |
| Infectious disease | 0.00% |
| Alcohol or smoke per day | 0.00% |
| Having operation in the past 1 year | 0.00% |
2.2. Measures
2.2.1. Conscientiousness assessment
The conscientiousness trait was assessed using the conscientiousness facet of the NEO‐Five‐Factors‐Inventory (NEO‐FFI) (Costa & MacCrae, 1992; McCrae & Costa Jr, 2010). The NEO‐FFI is composed of a subset of 60 items assessing five personality traits (i.e., Neuroticism, Extraversion, Openness, Agreeableness and Conscientiousness), and each trait facet of that has 12 items respectively. Ratings are made on a 5‐point Likert‐type scale ranging from 1 (strongly disagree) to 5 (strongly agree). Each individual was scored on each of the five facets and higher scores representing higher levels. It has been reported that the scale showed stability and consistency across time and culture (Cronbach's α = .82 in present study) (Kunnel John, Gaab, Xavier, Waldmeier, & Meyer, 2019; Magalhães et al., 2014; Terracciano, Costa Jr, & McCrae, 2006).
In order to explore the impacts of sub‐facets of conscientiousness on procrastination, we conducted the exploratory factor analysis (EFA) with conscientiousness subscale of NEO‐FFI. The results indicated that these items were adequate for factor analysis (KMO = .87, Bartlett = 982.94, p < .001). According to the Scree plot and the percentage of variance interpretation, there were three factors in conscientiousness accounting for 54.8% of the total variance (factor loadings > .40; see Table 2).
TABLE 2.
Rotation component matrix of conscientiousness subscale in NEO‐FFI No.: The item serial number of conscientiousness sub‐scale;R: items of reverse scoring
| No. | Factor | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Consci‐3R | .840 | ||
| Consci‐6R | .737 | ||
| Consci‐11R | .722 | ||
| Consci‐2 | .479 | ||
| Consci‐7 | .773 | ||
| Consci‐12 | .723 | ||
| Consci‐5 | .640 | ||
| Consci‐10 | .443 | ||
| Consci‐4 | .688 | ||
| Consci‐8 | .652 | ||
| Consci‐9R | .601 | ||
These three facets were supported by the EFA analysis of NEO‐FFI in 1998, which were named as orderliness, goal‐striving and dependability (Saucier, 1998). Factor1 (i.e., orderliness) converged the items of conscientious scale which made easily reminiscent of procrastination relating to time (e.g., Consci‐6 “Before working, I waste time”). Besides, Factor 2 (i.e., goal‐striving) and Factor 3 (i.e., dependability) seemed to describe conscientiousness from the perspective of motivation, such as pursuit goals (e.g., Consci‐7 “I work hard to achieve my goal”) and keep promise (e.g., Consci‐8 “Once I make a promise, I usually follow through”). Considering the inseparability between procrastination and time and according to the introduction statement, we renamed the conscientiousness sub‐facets from the perspective of time temporarily to further explore the neural basis for the impacts of sub‐facets of conscientiousness on procrastination in the follow‐up studies. Specifically, Time_consci facet included the factor1 of conscientiousness (orderliness), which represented the overarching tendency to be “prepared” based on time schedule (Cronbach's α = .75 in present study). Nontime_Consci facet included both the factor2 (i.e., goal‐striving, aspire to excellent) and factor3 (i.e., dependability, follow through with promises to others) (Cronbach's α = .72 in present study). Thus, in the following analyses, we explored the relationship between conscientiousness and procrastination based on this classification ‐ Time_Consci facet and Nontime_Consci facet respectively.
2.2.2. Procrastination assessment
The tendency to procrastinate was assessed with the General Procrastination Scale (GPS) designed by Lay involving the learning activities and daily life behavior (e.g., “I often find myself performing tasks that I had intended to do days before”, and “I do not do assignments until just before they are to be handed in”, etc.). It contains 20 items and has 5‐point Likert‐type response format ranging from 1 (strongly disagree) to 5 (strongly agree). The scores for each item were summed together to calculate the total score as an indicator of the propensity to procrastination. The higher score indicates the higher propensity to procrastination an individual has. Previous studies have demonstrated that the GPS have satisfactory internal consistencies, whose Cronbach's alpha coefficient is 0.82 (Lay, 1986). In current study, the Cronbach's alpha coefficient of GPS is .87.
2.3. fMRI data acquisition
All fMRI data including the T1‐ and T2‐weighted images were acquired from the Siemens 3 T MRI scanner (Siemens Magnetom Trio TIM, Erlangen, Germany). Magnetization‐Prepared Rapid‐Acquisition Gradient Echo (MPRAGE) sequence was adopted to collect the high‐resolution structural images (256 × 256 matrix; 128 slices; repetition time (TR), 2,530 ms; echo time (TE), 3.39 ms; flip angle, 7 °). In addition, functional images for each participant were acquired with a T2‐weighted echo‐planar imaging (EPI) sequence (resolution sequence = 64 × 64; voxel size = 3.1 × 3.1× 3.6; TR = 2000 ms; TE = 30 ms; flip angle = 90 °). Participants were asked to stay relaxed, think of nothing and open eyes during a resting scan. The resting scan acquired 360 brain volumes lasting for 12 min.
2.4. fMRI data analysis
2.4.1. VBM analysis
The structural data preprocessing and statistical analysis were performed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Preprocessing included the following steps. Firstly, through manually reoriented the front collocation coordinated with the origin (0, 0, 0), the image was manually reoriented so that the positioning approximated the MNI space. Secondly, the reoriented images were segmented into three parts ‐ white matter (WM), gray matter (GM), and Cerebrospinal fluid (CSF). The DARTEL algorithm, which automatically invoked the VBM8 toolkit by the SPM12, was then used to create a particular group template and a flow field that stored the deformation information. The GM image of the normal space was matched to the MNI space using affine spatial normalization in DARTEL toolbox. And then, the images were modulated using the Jacobian determinant to maintain the GM volume within a voxel. Finally, in order to improve the signal‐to‐noise ratio, the images were smoothed using 8 mm full‐width at half‐maximum (FWHM) Gaussian kernel.
Furthermore, the multiple linear regression models were performed to identify the brain regions where GM volumes were associated with individual difference in the two sub‐components of conscientiousness respectively. In this model, the score of single conscientiousness facet (e.g., Time_Consci facet) was included as the variable of interest, whereas the global GM volumes, age, gender, the other four personality traits of NEO‐FFI (i.e., Neuroticism, Extraversion, Openness and Agreeableness), especially another facet of conscientiousness (e.g., Nontime_Consci facet) were set as the covariates of no interest (Good et al., 2001; Kulynych, Vladar, Jones, & Weinberger, 1994; W.‐Y. Liu et al., 2013; Peelle, Cusack, & Henson, 2012). The global GM volumes were added as a global measure for proportional global scaling (Peelle et al., 2012). The global GM volumes or any ROIs' GM volumes of all participants were extracted by the MATLAB script “get_totals” provided by Ridgway (http://www.cs.ucl.ac.uk/staff/g.ridgway/vbm/get_totals.m). Then we applied the explicit masking instead of the absolute or relative threshold masking to decrease the risk of false negatives caused by the restrictive masking (Ridgway et al., 2009). Ultimately, the T contrasts were utilized to detect the voxels which significantly associated with the individuals' conscientiousness. Then, the multiple comparison was performed for the statistical maps using the Gaussian random filed (GRF, voxel p < .005; cluster p < .05). Notably, following a similar analytic procedure, another whole brain analysis was conducted for the detection of brain areas associated with the other facet of conscientiousness, which was considered as the variable of interest in this model.
2.4.2. RSFC analysis
The preprocessing of resting‐state fMRI data was performed with the software of Data Processing Assistant for resting‐state fMRI (DPARSF) (http://rfmri.org/DPARSF) (Yan & Zang, 2010). In order to avoid the effects of magnetization imbalance and to adapt to the scanning noise as much as possible, we discarded the first ten volumes of each participants. The remaining 350 volumes were corrected for temporal shifts between slices and corrected for motion. Functional images of all subjects were co‐registered with T1‐weighted anatomical images and then segmented into GM, WM, and CSF. Then, these images were normalized to MNI space in 3 × 3 × 3 mm3 voxel sizes and smoothed with a Gaussian kernel of 4 mm FWHM. Next, to reduce the head movement and nonneuronal BOLD fluctuations, we regressed out white matter, cerebrospinal fluid signal, global signal, and Friston 24‐parameters for head motion (Auer, 2008; Birn, Diamond, Smith, & Bandettini, 2006). And the residual signal was band‐pass time filtered (0.01–0.08 Hz) and linear detrended to obtain low‐frequency fluctuation from resting‐state fMRI data (Fox, Zhang, Snyder, & Raichle, 2009). Considering that motion‐related signal could not be removed fully by regression of motion estimates from the resting‐state fMRI data (Jonathan D. Power, Barnes, Snyder, Schlaggar, & Petersen, 2012), we performed framewise motion censoring after these steps. In detail, we removed volumes using a threshold of framewise displacement (FD) (Jonathan D. Power et al., 2012) > 0.2 mm as well as 1 back and 2 forward neighbors (Jonathan D Power, Barnes, Snyder, Schlaggar, & Petersen, 2013). As motion censoring can result in a removal of a large number of volumes and too few remaining volumes may lead to unreliable results, we set a 5‐min criterion and exclude subjects who had less than 5 min of data remaining after censoring (Jonathan D. Power et al., 2012). At the group‐level analyses, we further regressed out the mean FD for each subject. The resultant data were then subjected to functional connectivity analysis.
In addition, the statistical analysis of functional data was accomplished with the REST (Resting‐State fMRI Data Analysis Toolkit) toolbox (http://restfmri.net/forum/REST_V1.8) (Song, Schwarzkopf, Kanai, & Rees, 2011). Based on the VBM analysis, it revealed that the GM volumes of left dorsolateral prefrontal cortex (left dlPFC, MNI:−35, 29, 50) and right orbitofrontal cortex (right OFC, MNI: 18, 20, −15) were positively correlated with one facet of conscientiousness score, namely the Time_Consci facet. Besides, the other facet of conscientiousness (i.e., Nontime_Consci facet) was positively correlated with GM volumes in right putamen (MNI: 23, 20, 3) while negatively correlated with those in the left insula (MNI: −53, 12, −2). Therefore, the masks of these four brain regions were defined as seed regions of interests (ROIs) to calculate the whole brain voxel‐wise functional connectivity respectively in order to explore the functional connectivity correlated with conscientiousness. The steps to calculate the functional connectivity of these ROIs were as follows. In each participant the Pearson correlation coefficient between the average time courses of each ROI and each whole brain voxel was calculated to obtain the individual‐level correlation maps. These maps were then converted to normal z‐value maps by Fisher z‐transformation and was used in latter analysis. Subsequently, we calculated the correlation between individuals' z‐valued functional connectivity maps and the two facets of conscientiousness in the group‐level analyses respectively, which regions could survive with GRF correction (voxel p < .05; cluster p < .05). Eventually, the connectivity values (Fisher's z‐score) of each participant's seed ROIs connectivity map were extracted to calculate its correlation with procrastination.
2.5. The structural equation modeling analysis
In order to integrate the results of functional connectivity and explore their roles in the process of conscientiousness affecting procrastination, we employed the structural equation modeling (SEM) in software AMOS 23.0. The evaluation of model fit was based on chi‐squared plus recommended criteria for a set of fit indices. The normed chi‐square (χ2/df) less than three indicated well overall model fit (Chin & Todd, 1995). Comparative Fit Index (CFI) greater than .90 indicated a reasonable model fit (Brown, 2006; Weston & Gore Jr, 2006). The cut‐off value of root mean square error of approximation (RMSEA) was .05, whose values ranging from .05 to .08 indicated adequate fit (Brown, 2006). The Adjusted Goodness‐of‐fit Statistic (AGFI) greater than 0.9 was also acceptable (Bentler, 1990).
3. RESULTS
3.1. The behavioral results
We employed the Kolmogorov–Smirnov test and Pearson correlation analysis to test the normality and correlation between conscientiousness and GPS scores respectively. Results indicated that scores of conscientiousness and procrastination in our sample were normally distributed (Kolmogorov–Smirnov z = 1.15, p = .14; Kolmogorov–Smirnov z = 0.81, p = .53), and the conscientiousness score was negatively correlated with procrastination score (r = −.75, p < .01, 95%CI [−.79, −.70]; see Figure 1(a)). These results suggested that higher conscientiousness was associated with less procrastination.
FIGURE 1.
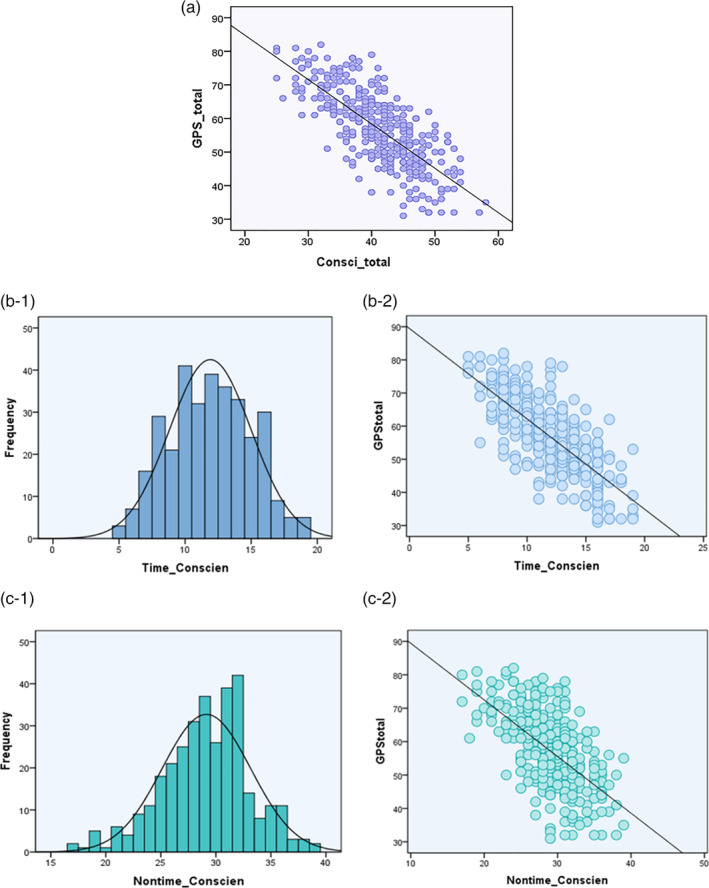
The behavior results. (a) The scores of conscientiousness were negatively correlated with procrastination (r = −.75, p < .01, 95%CI [−.79, −.70]); (b/c‐1) The normality distribution of Time_Consci/Nontime_Consci facet respectively; (b/c‐2) The scores of Time_Consci/Nontime_Consci facet were negatively correlated with procrastination (r Time = −.75, p < .001, 95%CI [−.80, −.70];r Nontime = − .61, p < .001, 95%CI [−.67, −.54])
Meanwhile, the Pearson correlation analysis between each facet score of conscientiousness (Time_consci facet and Nontime_Consci facet) and procrastination score were employed. Results indicated that both Time_consci facet score and Nontime_Consci facet score were negatively with GPS score (r Time = −.75, p < .001, 95%CI [−.80, −.70], see Figure 1(b);r Nontime = −.61, p < .001, 95%CI [−.67, −.54], see Figure 1(c)). Subsequently, we tested the prediction and explanatory power of these two facets of conscientiousness (Time_consci facet and Nontime_Consci facet)on GPS scores by the stepwise regression analysis. Results indicated that both independent variables entered the regression equation (β Time = −.60, β Nontime = −.25, p < .001) and together explained 60% of the dependent variables (R 2 = .60). That is, the two components of conscientiousness (Time_consci facet and Nontime_Consci facet) can explain the 60% variance of procrastination. Thus, in the following analyses of VBM and FC, we continued to adopt this classification ‐ Time_Consci facet and Nontime_Consci facet.
3.2. The VBM results
In order to explore the brain regions correlated with two components of conscientiousness respectively, we conducted a multiple regression model in VBM analysis. Results revealed that Time_Consci scores were positively correlated with GM volumes in the left dorsolateral prefrontal gyrus (dlPFC; x = −35, y = 29, z = 50; voxels = 208; voxel p < .005, cluster p < .05, GRF corrected; see Figure 2(a), Table 3) and right orbital frontal cortex (OFC; x = 18, y = 20, z = −15; voxels = 129; voxel p < .005, cluster p < .05, GRF corrected; see Figure 2(b), Table 3). Nontime_Consci scores were positively correlated with GM volumes in right putamen (x = 23, y = 20, z = 3; voxels = 113; voxel p < .005, cluster p < .05, GRF corrected; see Figure 2(c), Table 3),but negatively correlated with the left insula (x = −53, y = 12, z = −2; voxels = 131; voxel p < .005, cluster p < .05, GRF corrected; see Figure 2(d), Table 3).
FIGURE 2.
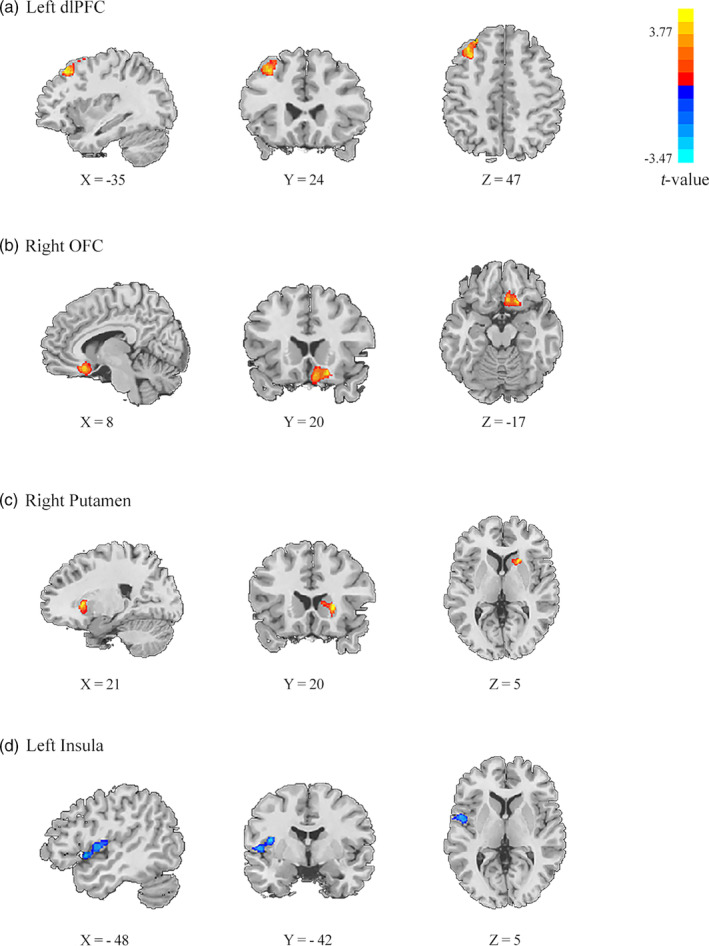
The VBM results. (a, b) The Time_Consci scores were positively correlated with the GM volumes of the left DLPFC and right OFC. (c, d) The Nontime_Consci scores were positively correlated with the GM volumes of the right putamen while negatively correlated with that of the left insula (GRF corrected; voxel p < .005; cluster p < .05)
TABLE 3.
GM volumes of brain areas significantly correlated with Conscientiousness +/−: the brain regions positively/negatively correlated with the conscientiousness (GRF corrected; voxel p < .005; cluster p < .05)
| Variables | Brain region | MNI | t | Voxels |
|---|---|---|---|---|
| Time_Consci facet | + dlPFC | ‐35, 29, 50 | 3.77 | 208 |
| + OFC | 18, 20, −15 | 3.65 | 129 | |
| Nontime_Consci facet | + putamen | 23, 20, 3 | 3.37 | 113 |
| ‐ insula | −53, 12, −2 | −3.47 | 131 |
3.3. The resting‐state functional connectivity results
In order to map the neural functional coupling reflecting the underlying neuroanatomy of conscientiousness, the brain regions deriving from VBM analysis (i.e., left dlPFC, right OFC, right putamen and left insula) were served as the regions of interests (ROIs) for the subsequent functional connectivity analysis. The results showed that Time_consci scores were positively correlated with dlPFC‐IPL (inferior parietal lobule) functional connectivity (see Figure 3(a), (b) and Table 4) and dlPFC‐PCC (posterior cingulate gyrus), while Nontime_Consci scores were negatively correlated with both putamen‐PHC (parahippocampal cortex) and insula‐IPL functional connectivity (see Figure 3(b), (C) and Table 4).
FIGURE 3.
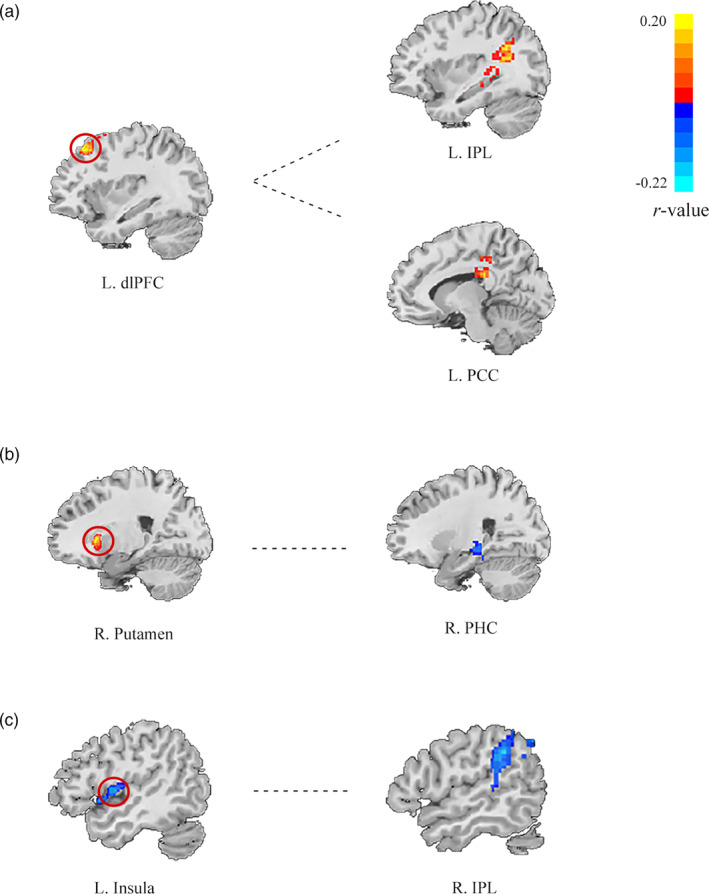
The resting‐state functional connectivity results. The left side presented defined seed regions, and the right side were the corresponding functional connectivity between seed regions and other regions which was significantly correlated with the component of conscientiousness respectively (GRF corrected; voxel p < .05; cluster p < .05)
TABLE 4.
Functional connectivity correlated with the component of conscientiousness
| Variables | Seed | Region | BA | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|
| Time_Consci | L.dlPFC |
L. Parietal lobe/ inferior parietal lobule L. Limbic lobe/ post cingulate Gyrus |
40 23 |
201 201 |
−33 −13 |
−53 −31 |
27 27 |
| Nontime_Consci | R.putamen | R. Limbic lobe/Parahippocampa Gyrus | 36 | 85 | 38 | −31 | −18 |
| L.insula | R. Parietal lobe/inferior parietal lobule | 40 | 388 | 53 | −42 | 36 |
To identify whether procrastination could be represented by the seed ROIs functional connectivity associated to two components of conscientiousness, we also extracted the connectivity values (Fisher's z‐score) of each participant's seed ROIs connectivity map to calculate its correlation with GPS score. Results showed that the DLPFC‐IPL and DLPFC‐PCC functional connectivity were negatively correlated with procrastination scores, respectively (r = −.27, p < .001; r = −.13, p < .05), while putamen‐PHC and insula‐IPL functional connectivity were positively correlated with procrastination scores, respectively (r = .14, p < .05; r = .11, p < .05). These results suggested that procrastination was also closely related to dlPFC‐IPL, dlPFC‐PCC, putamen‐PHC and insula‐IPL functional connectivity.
3.4. The structural equation modeling results
As mentioned earlier, we hypothesized that the impact of conscientiousness on procrastination can be explained by the self‐control and motivation pathways mainly. Furthermore, considering the inseparable relationship between procrastination and time, we named the two components of conscientiousness as time‐related (Time_Cosnci facet) and nontime‐related part (Nontime_Cosnci facet) based on the analysis of EFA. Subsequently, functional connectivity analysis results with VBM results as seed regions showed that Time_Consci facet was positively correlated with functional connectivity of dlPFC‐IPL and dlPFC‐PCC, while Nontime_Consci facet was negatively correlated with that of putamen‐PHC and insula‐IPL.
In order to further examine the neural basis of conscientiousness affecting procrastination, we established a structural equation model (SEM) graph and tested its fitting effect. The SEM results showed that the two components of conscientiousness mediated the relation between the functional couplings and GPS (χ2/df = 1.44; RMSEA = .037; AGFI = .965; CFI = .990). Furthermore, we performed the pathway analysis to verify the specific relationship among variables (see Figure 4). Although there was no direct effect among these functional connectivity and GPS, the indirect effect was especially worthy of attention. Specifically, on the one hand, the dlPFC‐IPL functional connectivity negatively and indirectly predicted GPS score through two components of conscientiousness (Total indirect effect = −0.27); it played a greater role in GPS score through Time_Consci facet (Indirect effect = −0.13). On the other hand, the dlPFC‐PCC functional connectivity negatively and indirectly predicted GPS score mainly through Time_Consci facet (Total indirect effect = −0.09), while the insula‐IPL functional connectivity could positively and indirectly predict GPS score mainly through Nontime_Consci facet (Total indirect effect = 0.12).
FIGURE 4.
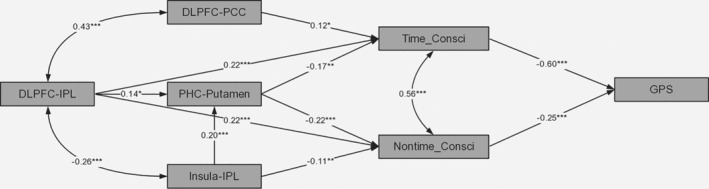
The structural model with standardized coefficients: *p < .05, **p < .01, *** p < .001
These results suggested that there could be two neural pathways explaining the relationship between conscientiousness and procrastination. Specifically, self‐control pathway was based on the dlPFC‐IPL and dlPFC‐PCC functional connectivity, which were the neural underpinnings of Time_Conscifacet, while motivation pathway was based on the putamen‐PHC and insula‐IPL functional connectivity, which were the neural substrates underlying the Nontime_Consci facet.
4. DISCUSSION
The present study investigated the neural underpinning responsible for the effect of conscientiousness on procrastination. The behavioral results revealed that conscientiousness was negatively associated with procrastination. The VBM analyses showed that conscientiousness was positively correlated with the GM volumes of the left dlPFC, right OFC, right putamen, but negatively correlated with those of the left insula. Moreover, the RSFC results revealed that the dlPFC‐IPL and dlPFC‐PCC functional connectivity were positively associated with Time_Consci facet of conscientiousness, while the PHC‐putamen and insula‐IPL functional connectivity were negatively associated with Nontime_Consci facet of conscientiousness. More importantly, the structural equation modeling (SEM) integrating RSFC results were well fitted for the influence process of conscientiousness on procrastination by both self‐control (i.e dlPFC‐IPL, dlPFC‐PCC) (which represented Time_consci facet) and motivation pathways (i.e., PHC‐putamen, insula‐IPL) (which represented Nontime_consci facet).
The current study showed higher conscientiousness was associated with lower procrastination. During the volitional implementation phase of action, whether making a prioritized list of tasks, controlling reasonable behavior, or simply showing compliance with the time, it reflected the individual control over time and personal behavior. That was the main reason we called the first component of conscientiousness as Time_Consci facet. In the case of procrastinators, however, they showed deficiency in those mentioned abilities thus postponing work to meet short‐term benefits (F. M. Sirois, 2014; J. Zhao et al., 2019). Moreover previous studies also found that conscientious individuals with adequate self‐control inhibited the desire for immediate or enjoyable temptation and can execute tasks timely (Pychyl, Lee, Thibodeau, & Blunt, 2000; Steel, 2007). As for the Nontime_Consci facet, it might be personal‐value‐related component that explained the relationship between conscientiousness and procrastination. On the one hand, the personal value of striving for excellence, avoiding loss of trust and maintaining current rules among conscientious people constituted the motivational basis to initiate or avoid some behavior in terms of goal pursuits (Schwartz, 2012). For example, achievement‐oriented students routinely preferred the study‐related over leisure‐related activities, and reported low procrastination compared with well‐being‐oriented students (Fries et al., 2007; Grund & Fries, 2018). On the other hand, conscientious people, who concerned more the value of future outcomes, were associated with the goal‐directed actions. Previous studies found that higher conscientious people tended to choose the larger and delayed options, while procrastinators preferred to choose the smaller and immediate options (Manning et al., 2014; Wu et al., 2016). The time perspective of the former was also more inclined to the future (F. M. Sirois, 2014). This goal orientation deemed as driving force might reduce the negative emotion experience during the execution of the task, which would contribute to completion of a task and then decrease procrastination (Cron, Slocum, John, VandeWalle, & Fu, 2005; Eum & Rice, 2011). Together, it seemed that there were two components of conscientiousness – self‐control (Time_Consci facet) and driving factor (Nontime_Consci facet), which could be the main pathways in the effect of conscientiousness on procrastination.
The VBM analysis revealed that Time_Consci facet was positively associated with the GM volumes in the left dlPFC and right OFC. Previous studies revealed the key roles of the gray volumetric changes in dlPFC and OFC for inhibitory functions and self‐control (Crews & Boettiger, 2009; Seo, Lacadie, & Sinha, 2016; Weygandt et al., 2015). And brain injury studies found that individuals with damaged dlPFC had lower self‐discipline (Forbes et al., 2014). It is worth noting that WM of dlPFC also shows BOLD signal in our results. Recently studies provided mounting evidence about the important meaning of blood oxygen level‐dependent (BOLD) singnals in white matter (WM) (Courtemanche, Sparrey, Song, Mackay, & Arcy, 2017; Li et al., 2019; Li, Biswal, Meng, Yang, & Liao, 2020). For instance, axonal injury involving the dlPFC would impair the executive function performance (Lipton et al., 2009). When combined, these findings suggested that self‐control implemented by dlPFC and OFC might promote individuals to behave in a conscientious manner. On the other hand, our results indicated that Nontime_Consci facet of conscientiousness was positively related to the GM volumes in the right putamen, while negatively correlated with that in the left insula. Converging fMRI studies confirmed the putamen and insula were involved in encoding subjective value of the rewards and aversion emotion, respectively (Bossaerts, 2010; Haruno & Kawato, 2006; Markett, Heeren, Montag, Weber, & Reuter, 2016; Sarinopoulos et al., 2009). Specifically, individuals with greater activation in putamen tended to have a higher value for positive reward (Mizuno et al., 2008; Schultz, 2000), which facilitated a strong driving force and made them work harder to achieve their goals and gain the expected rewards. Meanwhile, using positron emission tomography (PET) scanning, Treadway and his colleagues found that individuals with higher activation in the bilateral insula were more likely to avoid the task (Treadway et al., 2012). Together, these regions implicated in the value representation could trigger the approach (i.e., putamen) or avoidance (i.e., insula) motivation in the pursuit of goals (Wigfield & Eccles, 2000). Therefore, the VBM results suggested that the left dlPFC, right OFC, right putamen and left insula might be the neural underpinnings of conscientiousness, which supported the hypothesis for the self‐control and motivation components of conscientiousness.
The RSFC analysis showed that the dlPFC‐IPL and dlPFC‐PCC functional connectivity were positively associated with Time_Consci facet, while the insula‐IPL and PHC‐putamen functional connectivity were negatively correlated to Nontime_Consci facet. As what mentioned before, conscientiousness was inversely related to impulse behavior and the sensitivity of negative task process (Penley & Tomaka, 2002; Verplanken & Sato, 2011). Previous study have demonstrated in a probabilistic fiber tracking study that there are a direct anatomical connection between IPL and frontal areas (Caspers et al., 2011). The IPL, as a region involved in the frontoparietal network (FPCN), default mode network (DMN) and ventral attention network (VAN) (Igelstr? & Graziano, 2017), supported the processes requiring attention‐directed control (e.g., addictive behavior) (Anna, Huang, Nelly, & Goldstein, 2018). Moreover, neuroimaging studies have shown that the dlPFC, recruited in the frontoparietal control network, played key role in executive control and adaptive behavior by top‐down regulation of other brain regions as well as flexible encoding of task requirements and expected outcomes (Cole, Repovš, & Anticevic, 2014; Miller & Cohen, 2001). Therefore, the increased communication of dlPFC‐IPL in present study might have supported conscientious individuals to reorient attention away from distractions through the top‐down regulation from dlPFC to IPL, thereby focusing on the task object during goal pursuit. In addition, the activity of PCC which involves in DMN increased during episodic simulation of future events (D'Argembeau et al., 2010). A psychophysiological interaction (PPI) study has indicated that the FPCN will couple its activity with the DMN when engaged in introspective processes which regulated the balance between internally and externally as well as required simultaneously cognition control for the information in a goal‐directed manner (Gerlach, Spreng, Madore, & Schacter, 2014; Leech, Braga, & Sharp, 2012; Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010). Therefore, the dlPFC‐IPL and dlPFC‐PCC functional connectivity were positively related to Time_Consi facet in the present study, suggesting that conscientious people were prone to regulate internal and external cognition and then maintain attention on goal‐relevant tasks. Therefore, the dlPFC‐IPL and dlPFC‐PCC functional connectivity were positively related to Time_Consi facet in the present study, suggesting that conscientious people were prone to regulate internal and external cognition and then maintain attention on goal‐relevant tasks. On the other hand, previous studies revealed that insula and putamen could represent aversive emotion and enjoyable temptation respectively (Fujino et al., 2016; Haruno & Kawato, 2006; Simmons, Strigo, Matthews, Paulus, & Stein, 2006). The decreased attention regulation of IPL for insula meant that aversion emotion caused by negative task process was not alleviated in time and thus conscientiousness would be decreased. In addition, PHC was the neural underpinning of scene construction and episodic future thinking (Addis, Cheng, Roberts, & Schacter, 2011). With the imagination role of PHC, the putamen responsible for temptation was so attractive that weakened the individual's conscientious manner (i.e., avoidance motivation). However, these avoidance motivation would decrease as the aversive feelings to the negative task process decreases owning to the regulation role of IPL on insula. As a result, the enjoyable temptation which represented by PHC‐putamen functional connectivity would not seem so alluring as before. Therefore individuals may focus on the current task and engage in more conscientious behaviors to accomplish the goal, thereby reducing procrastination. Collectively, the RSFC results suggested that the functional connectivity involving self‐control and motivation might be the neural representation of conscientiousness.
Importantly, the structural equation modeling (SEM) results have shown how the functional connectivity plays key roles in the effect of conscientiousness on procrastination through two pathways. On one hand, the dlPFC‐IPL and dlPFC‐PCC functional connectivity of Time_Consci facet impacted procrastination negatively. As mentioned above, the frontoparietal network (e.g., dlPFC, IPL) and the default network (e.g., PCC) could interact to regulate the internal and external cognition so as to make conscientious individuals pay more attention to future goals in the goal‐directed task (Leech et al., 2012). Collectively, these results revealed that the self‐control pathway accounting for how conscientiousness affected procrastination. Specifically, the increased communication between regions supporting self‐control might suppress the behaviors that were not correlated with the goal tasks, which promoted the reduced occurrence of procrastination. On the other hand, procrastination was influenced by the insula‐IPL and PHC‐putamen functional connectivity of Nontime_Consci facet positively. As the temporal decision model of procrastination proposed, to procrastinate or not depended on the comparison between the strength of motivation to approach and avoid (SZhang, Liu, & Feng, 2019). Obviously, procrastinators are prone to delay a task in order to reduce aversion to negative task process (F. Sirois & Pychyl, 2013). In addition, the neuroimaging studies also revealed that the insula and putamen were involved in the simulation of the task's negative process and enjoyable temptation respectively (Fujino et al., 2016; Haruno & Kawato, 2006). Thus, when the IPL did not well regulate insula involving in the task aversion or the PHC concerning the distractions of temptation represented by putamen, the conscientious manner of individuals would be weakened and make procrastination aggravated. This might be the motivation pathway responsible for the effect of conscientiousness on procrastination. More importantly, the dlPFC‐IPL functional connectivity also participates in the process of this pathway, which suggests that it plays a key role in the process of conscientiousness. Together, self‐control and motivation could be the two pathways of conscientiousness, which both have impacts on the individual tendency of procrastination.
Future research could revolve around the following directions. Despite its advantage as well acknowledged for detecting procrastination level, the GPS, along with all self‐report instruments, may have been criticized for socially desirable responses or other biases. The current findings would be more ecologically valid if future researches were to probe the performance of procrastinated behaviors in the real‐life settings (Zhang, Becker, Chen, & Feng, 2019b). Moreover, although the effect of neural functional pattern of conscientiousness on procrastination has been integrated in the two pathways of self‐control and motivation, these results cannot be inferred to the causal effect. Findings interested in causal effects also needs to employ the task fMRI or noninvasive stimulation research in future (Francis & David, 1988). Finally, the present research revealed that how the potential two neurocognitive pathways of conscientiousness affected procrastination, which provided a new perspective for future intervention studies of procrastination. Specifically, it is well known that conscientiousness as a personality trait is characterized by stable across time and situations (Cobb‐Clark & Schurer, 2012; Linden, Nijenhuis, & Bakker, 2010). However, self‐control and motivation, as the main pathway of conscientiousness associated with procrastination, can be improved and enhanced to a certain extent (None, 2004; Oaten & Cheng, 2006; Smith, Panfil, Bailey, & Kirkpatrick, 2019), which would open up new possibilities for practical suggestions or interventions to reduce the harmful effects of procrastination.
In summary, based on the reconstruction of the sub‐facets of conscientiousness, the VBM and RSFC methods were used to investigate the neural basis underlying the impact of conscientiousness on procrastination. Moreover, the SEM analysis integrating RSFC results were well fitted for the influence process of conscientiousness on procrastination via self‐control (i.e., dlPFC‐IPL, dlPFC‐PCC) and motivation pathways (i.e., PHC‐putamen, insula‐IPL). In conclusion, the present study provided a neural underpinning perspective to promote the understanding of the relationship between conscientiousness and procrastination, which enlightened the researcher about interventions for procrastination.
CONFLICT OF INTEREST
All the authors report no potential competing conflicts.
ETHICS STATEMENT
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board (IRB) of Southwest University.
PATIENT CONSENT STATEMENT
Informed consent was obtained from all individual participants included in the study.
AUTHOR CONTRIBUTIONS
Kanxin Gao: Conceptualization, Methodology, Formal analysis, Data curation, Writing ‐ Original Draft Rong Zhang: Formal analysis, Writing ‐ Review & Editing Ting Xu: Data curation, Writing ‐ Review & Editing Fan Zhou: Software, Invastigation Tingyong Feng: Writing ‐ Review & Editing, Supervision, Project administration, Funding acquisition.
Gao K, Zhang R, Xu T, Zhou F, Feng T. The effect of conscientiousness on procrastination: The interaction between the self‐control and motivation neural pathways. Hum Brain Mapp. 2021;42:1829–1844. 10.1002/hbm.25333
Funding information Fundamental Research Funds for the Central Universities, Grant/Award Number: SWU2009104; National Natural Science Foundation of China, Grant/Award Number: 31971026
DATA AVAILABILITY STATEMENT
The data in this study is not available due to the following reasons: a) participants of this study did not agree for their data to be shared publicly; b) these data can be further researched, and the research group is currently digging deeply, which is not convenient for sharing. If any researchers are interested in our research and willing to make cooperation, please do not to hesitate to contact with us (fengty0@swu.edu.cn).
REFERENCES
- Addis, D. R. , Cheng, T. , Roberts P., R., & Schacter, D. L. (2011). Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus, 21(10), 1045–1052.doi: 10.1002/hipo.20870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna, J. R. , Snyder, A. Z. , Vincent, J. L. , Lustig, C. , Head, D. , Raichle, M. E. , & Buckner, R. L. (2007). Disruption of large‐scale brain systems in advanced aging. Neuron, 56(5), 924–935. 10.1016/j.neuron.2007.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anna, Z. , Huang, A. S. , Nelly, A. K. , & Goldstein, R. Z. (2018). Neuroimaging impaired response inhibition and salience attribution in human drug addiction: A systematic review. Neuron, 98(5), 886–903. 10.1016/j.neuron.2018.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer, D. P. (2008). Spontaneous low‐frequency blood oxygenation level‐dependent fluctuations and functional connectivity analysis of the 'resting' brain. Magnetic Resonance Imaging, 26(7), 1055–1064. 10.1016/j.mri.2008.05.008 [DOI] [PubMed] [Google Scholar]
- Back, M. D. , Schmukle, S. C. , & Egloff, B. (2006). Who is late and who is early? Big five personality factors and punctuality in attending psychological experiments. Journal of Research in Personality, 40(5), 841–848. 10.1016/j.jrp.2005.11.003 [DOI] [Google Scholar]
- Baumeister, R. F. , Vohs, K. D. , & Oettingen, G. (2016). Pragmatic prospection: How and why people think about the future. Review of General Psychology, 20(1), 3–16. 10.1037/gpr0000060 [DOI] [Google Scholar]
- Becker, P. (1998). Special feature: A multifacet Circumplex model of personality as a basis for the description and therapy of personality disorders. Journal of Personality Disorders, 12(3), 213–225. 10.1521/pedi.1998.12.3.213 [DOI] [PubMed] [Google Scholar]
- Becker, P. (2011). Special feature: A multifacet Circumplex model of personality as a bas. Journal of Personality Disorders, 12(3), 213–225. [DOI] [PubMed] [Google Scholar]
- Bentler, P. M. (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107(2), 238–246. 10.1037/0033-2909.107.2.238 [DOI] [PubMed] [Google Scholar]
- Birn, R. M. , Diamond, J. B. , Smith, M. A. , & Bandettini, P. A. (2006). Separating respiratory‐variation‐related fluctuations from neuronal‐activity‐related fluctuations in fMRI. NeuroImage, 31(4), 1536–1548. 10.1016/j.neuroimage.2006.02.048 [DOI] [PubMed] [Google Scholar]
- Bossaerts, P. (2010). Risk and risk prediction error signals in anterior insula. Brain Structure and Function, 214(5–6), 645–653. 10.1007/s00429-010-0253-1 [DOI] [PubMed] [Google Scholar]
- Boucard, A. , Marchand, A. , & Noguès, X. (2007). Reliability and validity of structural equation modeling applied to neuroimaging data: A simulation study. Journal of Neuroscience Methods, 166(2), 278–292. 10.1016/j.jneumeth.2007.07.011 [DOI] [PubMed] [Google Scholar]
- Brown, T. A. (2006). Confirmatory factor analysis for applied research, 156, New York, London: Guilford Publications. [Google Scholar]
- Caspers, S. , Eickhoff, S. B. , Rick, T. , Kapri, A. V. , Kuhlen, T. , Huang, R. , … Zilles, K. (2011). Probabilistic fibre tract analysis of cytoarchitectonically defined human inferior parietal lobule areas reveals similarities to macaques. NeuroImage, 58(2), 362–380. 10.1016/j.neuroimage.2011.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Liu, P. , Zhang, C. , & Feng, T. (2020). Brain morphological dynamics of procrastination: the crucial role of the self‐control, emotional, and episodic prospection network. Cerebral Cortex, 30(5), 2834–2853. 10.1093/cercor/bhz278. [DOI] [PubMed] [Google Scholar]
- Chin, W. W. , & Todd, P. A. (1995). On the use, usefulness, and ease of use of structural equation modeling in MIS research: A note of caution. MIS Quarterly, 19(2), 237–246. 10.2307/249690 [DOI] [Google Scholar]
- Cobb‐Clark, D. A. , & Schurer, S. (2012). The stability of big‐five personality traits. Economics Letters, 115(1), 11–15. 10.1016/j.econlet.2011.11.015 [DOI] [Google Scholar]
- Cole, M. W. , Repovš, G. , & Anticevic, A. (2014). The frontoparietal control system: A central role in mental health. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 20(6), 652–664. 10.1177/1073858414525995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, P. T. , & MacCrae, R. R. (1992). Revised NEO personality inventory (NEO PI‐R) and NEO five‐factor inventory, Odessa, Florida, FL. Psychological Assessment Resources Differences, 35, 1285–1292. [Google Scholar]
- Courtemanche, M. J. , Sparrey, C. J. , Song, X. , Mackay, A. , & D" Arcy, R. C. N. (2017). Detecting white matter activity using conventional 3 tesla fMRI: An evaluation of standard field strength and hemodynamic response function. Neuroimage , 169, 145–150.doi: 10.1016/j.neuroimage.2017.12.008 [DOI] [PubMed] [Google Scholar]
- Crews, F. T. , & Boettiger, C. A. (2009). Impulsivity, frontal lobes and risk for addiction. Pharmacology Biochemistry and Behavior, 93(3), 237–247. 10.1016/j.pbb.2009.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cron, W. L. , Slocum, J. , John, W. , VandeWalle, D. , & Fu, Q. (2005). The role of goal orientation on negative emotions and goal setting when initial performance falls short of one's performance goal. Human Performance, 18(1), 55–80. 10.1207/s15327043hup1801_3 [DOI] [Google Scholar]
- D'Argembeau, A. , Stawarczyk, D. , Majerus, S. , Collette, F. , Van der Linden, M. , Feyers, D. , … Salmon, E. (2010). The neural basis of personal goal processing when envisioning future events. Journal of Cognitive Neuroscience, 22(8), 1701–1713. 10.1162/jocn.2009.21314 [DOI] [PubMed] [Google Scholar]
- DeYoung, C. G. , Hirsh, J. B. , Shane, M. S. , Papademetris, X. , Rajeevan, N. , & Gray, J. R. (2010). Testing predictions from personality neuroscience: Brain structure and the big five. Psychological Science, 21(6), 820–828. 10.1177/0956797610370159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung, C. G. , Quilty, L. C. , & Peterson, J. B. (2007). Between facets and domains: 10 aspects of the big five. Journal of Personality and Social Psychology, 93(5), 880–896. 10.1037/0022-3514.93.5.880 [DOI] [PubMed] [Google Scholar]
- Dietz, F. , Hofer, M. , & Fries, S. (2007). Individual values, learning routines and academic procrastination. British Journal of Educational Psychology, 77(4), 893–906. 10.1348/000709906X169076 [DOI] [PubMed] [Google Scholar]
- Eum, K. , & Rice, K. G. (2011). Test anxiety, perfectionism, goal orientation, and academic performance. Anxiety, Stress, & Coping, 24(2), 167–178. 10.1080/10615806.2010.488723 [DOI] [PubMed] [Google Scholar]
- Farr, O. M. , Hu, S. , Zhang, S. , & Chiang‐shan, R. L. (2012). Decreased saliency processing as a neural measure of Barratt impulsivity in healthy adults. NeuroImage, 63(3), 1070–1077. 10.1016/j.neuroimage.2012.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari, J. R. , Díaz‐Morales, J. F. , O'Callaghan, J. , Díaz, K. , & Argumedo, D. (2007). Frequent behavioral delay tendencies by adults: International prevalence rates of chronic procrastination. Journal of Cross‐Cultural Psychology, 38(4), 458–464. 10.1177/0022022107302314 [DOI] [Google Scholar]
- Figner, B. , Knoch, D. , Johnson, E. J. , Krosch, A. R. , Lisanby, S. H. , Fehr, E. , & Weber, E. U. (2010). Lateral prefrontal cortex and self‐control in intertemporal choice. Nature Neuroscience, 13(5), 538–539. 10.1038/nn.2516 [DOI] [PubMed] [Google Scholar]
- Forbes, C. E. , Poore, J. C. , Krueger, F. , Barbey, A. K. , Solomon, J. , & Grafman, J. (2014). The role of executive function and the dorsolateral prefrontal cortex in the expression of neuroticism and conscientiousness. Social Neuroscience, 9(2), 139–151. 10.1080/17470919.2013.871333 [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , Zhang, D. , Snyder, A. Z. , & Raichle, M. E. (2009). The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology, 101(6), 3270–3283. 10.1152/jn.90777.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, D. J. (1988). An introduction to structural equation models. Journal of Clinical and Experimental Neuropsychology, 10(5), 623–639. 10.1080/01688638808402800. [DOI] [PubMed] [Google Scholar]
- Fries, S. , Schmid, S. , & Hofer, M. (2007). On the relationship between value orientation, valences, and academic achievement. European Journal of Psychology of Education, 22(2), 201–216. 10.1007/BF03173522 [DOI] [Google Scholar]
- Fujita, K. (2011). On conceptualizing self‐control as more than the effortful inhibition of impulses. Personality and Social Psychology Review, 15(4), 352–366. 10.1177/1088868311411165. [DOI] [PubMed] [Google Scholar]
- Fujino, J. , Fujimoto, S. , Kodaka, F. , Camerer, C. F. , Kawada, R. , Tsurumi, K. , … Sugihara, G. (2016). Neural mechanisms and personality correlates of the sunk cost effect. Scientific Reports, 6, 33171. 10.1038/srep33171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach, K. D. , Spreng, R. N. , Madore, K. P. , & Schacter, D. L. (2014). Future planning: Default network activity couples with frontoparietal control network and reward‐processing regions during process and outcome simulations. Social Cognitive and Affective Neuroscience, 9(12), 1942–1951. 10.1093/scan/nsu001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good, C. D. , Johnsrude, I. S. , Ashburner, J. , Henson, R. N. , Friston, K. J. , & Frackowiak, R. S. (2001). A voxel‐based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14(1), 21–36. 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- Grund, A. , & Fries, S. (2018). Understanding procrastination: A motivational approach. Personality and Individual Differences, 121, 120–130. 10.1016/j.paid.2017.09.035 [DOI] [Google Scholar]
- Gustavson, D. E. , Miyake, A. , Hewitt, J. K. , & Friedman, N. P. (2014). Genetic relations among procrastination, impulsivity, and goal‐management ability: Implications for the evolutionary origin of procrastination. Psychological Science, 25(6), 1178–1188. 10.1177/0956797614526260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadarics, M. (2016). Motivational and ideological underpinnings of welfare preferences in eastern and Western Europe. Europe's Journal of Psychology, 12(1), 169–190. 10.5964/ejop.v12i1.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno, M. , & Kawato, M. (2006). Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus‐action‐reward association learning. Journal of Neurophysiology, 95(2), 948–959. 10.1152/jn.00382.2005 [DOI] [PubMed] [Google Scholar]
- Hu, X. , Erb, M. , Ackermann, H. , Martin, J. A. , Grodd, W. , & Reiterer, S. M. (2011). Voxel‐based morphometry studies of personality: Issue of statistical model specification—Effect of nuisance covariates. NeuroImage, 54(3), 1994–2005. 10.1016/j.neuroimage.2010.10.024 [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Liu, P. , Guo, Y. , & Feng, T. (2018). The neural substrates of procrastination: A voxel‐based morphometry study. Brain and Cognition, 121, 11–16. 10.1016/j.bandc.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Igelström, K. M. , & Graziano, M. S. A. (2017). The inferior parietal lobule and temporoparietal junction: A network perspective. Neuropsychologia, 105, 70–83. 10.1016/j.neuropsychologia.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Jackson, J. J. , Wood, D. , Bogg, T. , Walton, K. E. , Harms, P. D. , & Roberts, B. W. (2010). What do conscientious people do? Development and validation of the behavioral indicators of conscientiousness (BIC). Journal of Research in Personality, 44(4), 501–511. 10.1016/j.jrp.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis, D. , Sutin, A. , Davatzikos, C. , Costa, P., Jr. , & Resnick, S. (2013). The five factors of personality and regional cortical variability in the Baltimore longitudinal study of aging. Human Brain Mapping, 34(11), 2829–2840. 10.1002/hbm.22108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. R. , & Seo, E. H. (2015). The relationship between procrastination and academic performance: A meta‐analysis. Personality and Individual Differences, 82, 26–33. 10.1016/j.paid.2015.02.038 [DOI] [Google Scholar]
- Krebs, R. M. , Boehler, C. N. , & Woldorff, M. G. (2010). The influence of reward associations on conflict processing in the Stroop task. Cognition, 117(3), 341–347. 10.1016/j.cognition.2010.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulynych, J. J. , Vladar, K. , Jones, D. W. , & Weinberger, D. R. (1994). Gender differences in the normal lateralization of the supratemporal cortex: MRI surface‐rendering morphometry of Heschl's gyrus and the planum temporale. Cerebral Cortex, 4(2), 107–118. 10.1093/cercor/4.2.107 [DOI] [PubMed] [Google Scholar]
- Kunnel John, R. , Gaab, J. , Xavier, B. , Waldmeier, A. , & Meyer, A. H. (2019). Psychometric evaluation of the BFI‐10 and the NEO‐FFI‐3 in Indian adolescents. Frontiers in Psychology, 10, 1057. 10.3389/fpsyg.2019.01057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay, C. H. (1986). At last, my research article on procrastination. Journal of Research in Personality, 20(4), 474–495. 10.1016/0092-6566(86)90127-3 [DOI] [Google Scholar]
- Lee, D.‐G. , Kelly, K. R. , & Edwards, J. K. (2006). A closer look at the relationships among trait procrastination, neuroticism, and conscientiousness. Personality and Individual Differences, 40(1), 27–37. 10.1016/j.paid.2005.05.010 [DOI] [Google Scholar]
- Leech, R. , Braga, R. , & Sharp, D. J. (2012). Echoes of the brain within the posterior cingulate cortex. Journal of Neuroscience, 32(1), 215–222. 10.1523/JNEUROSCI.3689-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, G. J. , Dickie, D. A. , Cox, S. R. , Karama, S. , Evans, A. C. , Starr, J. M. , … Deary, I. J. (2018). Widespread associations between trait conscientiousness and thickness of brain cortical regions. NeuroImage, 176, 22–28. 10.1016/j.neuroimage.2018.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Biswal, B. B. , Meng, Y. , Yang, S. , & Liao, W. (2020). A neuromarker of individual general fluid intelligence from the white‐matter functional connectome. Translational Psychiatry, 10(1), 147. 10.1038/s41398-020-0829-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Biswal, B. B. , Wang, P. , Duan, X. , Cui, Q. , Chen, H. , & Liao, W. (2019). Exploring the functional connectome in white matter. Human Brain Mapping, 40(15), 4331–4344. 10.1002/hbm.24705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden, D. V. D. , Nijenhuis, J. T. , & Bakker, A. B. (2010). The general factor of personality: A meta‐analysis of big five intercorrelations and a criterion‐related validity study. Journal of Research in Personality, 44(3), 315–327. 10.1016/j.jrp.2010.03.003 [DOI] [Google Scholar]
- Lipton, M. L. , Gulko, E. , Zimmerman, M. E. , Friedman, B. W. , Kim, M. , Gellella, E. , … Branch, C. A. (2009). Diffusion‐tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. International Journal of Medical Radiology, 252(3), 816–824. 10.1007/s00125-004-1392-9 [DOI] [PubMed] [Google Scholar]
- Liu, P. , & Feng, T. (2017). The overlapping brain region accounting for the relationship between procrastination and impulsivity: A voxel‐based morphometry study. Neuroscience, 360, 9–17. 10.1016/j.neuroscience.2017.07.042 [DOI] [PubMed] [Google Scholar]
- Liu, W.‐Y. , Weber, B. , Reuter, M. , Markett, S. , Chu, W.‐C. , & Montag, C. (2013). The big five of personality and structural imaging revisited: A VBM–DARTEL study. Neuroreport, 24(7), 375–380. 10.1097/WNR.0b013e328360dad7 [DOI] [PubMed] [Google Scholar]
- Luczynski, K. C. , & Hanley, G. P. (2013). Prevention of problem behavior by teaching functional communication and self‐control skills to preschoolers. Journal of Applied Behavior Analysis, 46(2), 355–368. 10.1002/jaba.44 [DOI] [PubMed] [Google Scholar]
- Magalhães, E. , Salgueira, A. , Gonzalez, A.‐J. , Costa, J. J. , Costa, M. J. , Costa, P. , & Lima, M. P. d. (2014). NEO‐FFI: Psychometric properties of a short personality inventory in Portuguese context. Psicologia: Reflexão e Crítica, 27(4), 642–657. 10.1590/1678-7153.201427405 [DOI] [Google Scholar]
- Mahoney, C. J. , Rohrer, J. D. , Omar, R. , Rossor, M. N. , & Warren, J. D. (2011). Neuroanatomical profiles of personality change in frontotemporal lobar degeneration. The British Journal of Psychiatry, 198(5), 365–372. 10.1192/bjp.bp.110.082677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, J. , Hedden, T. , Wickens, N. , Whitfield‐Gabrieli, S. , Prelec, D. , & Gabrieli, J. D. (2014). Personality influences temporal discounting preferences: Behavioral and brain evidence. NeuroImage, 98, 42–49. 10.1016/j.neuroimage.2014.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markett, S. , Heeren, G. , Montag, C. , Weber, B. , & Reuter, M. (2016). Loss aversion is associated with bilateral insula volume. A Voxel Based Morphometry Study. Neuroscience letters, 619, 172–176. 10.1016/j.neulet.2016.03.029 [DOI] [PubMed] [Google Scholar]
- McCrae, R. R. , & Costa, P., Jr. (2010). NEO inventories professional manual for the NEO personality Inventory‐3, NEO five‐factor Inventory‐3, and NEO personality inventory‐revised. Lutz, FL: Par. [Google Scholar]
- Miller, E. K. , & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Ming, L. , & Haosheng, Y. (2010). A multi‐cultural view and the theoretical re‐integration of responsibility. Psychological Science, 33(3), 643–645. [Google Scholar]
- Miyamoto, M. (1989). The concept of achievement motive among Japanese college students through the behavior characteristics. Japanese Psychological Research, 31(2), 80–91. 10.1080/00207598908247841 [DOI] [Google Scholar]
- Mizuno, K. , Tanaka, M. , Ishii, A. , Tanabe, H. C. , Onoe, H. , Sadato, N. , & Watanabe, Y. (2008). The neural basis of academic achievement motivation. NeuroImage, 42(1), 369–378. 10.1016/j.neuroimage.2008.04.253 [DOI] [PubMed] [Google Scholar]
- Morgan, G. S. , Mullen, E. , & Skitka, L. J. (2010). When values and attributions collide: Liberals' and conservatives' values motivate attributions for alleged misdeeds. Personality and Social Psychology Bulletin, 36(9), 1241–1254. 10.1177/0146167210380605 [DOI] [PubMed] [Google Scholar]
- None . (2004). Motivation and reward. Science, 304(5668), 167m–167m. 10.1126/science.304.5668.167m [DOI] [Google Scholar]
- Oaten, M. , & Cheng, K. (2006). Improved self‐control: The benefits of a regular program of academic study. Basic & Applied Social Psychology, 28(1), 1–16. 10.1207/s15324834basp2801_1 [DOI] [Google Scholar]
- Peelle, J. E. , Cusack, R. , & Henson, R. N. (2012). Adjusting for global effects in voxel‐based morphometry: Gray matter decline in normal aging. NeuroImage, 60(2), 1503–1516. 10.1016/j.neuroimage.2011.12.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penley, J. A. , & Tomaka, J. (2002). Associations among the big five, emotional responses, and coping with acute stress. Personality and Individual Differences, 32(7), 1215–1228. 10.1016/S0191-8869(01)00087-3 [DOI] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2013). Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to carp. NeuroImage, 76, 439–441. 10.1016/j.neuroimage.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pychyl, T. A. , Lee, J. M. , Thibodeau, R. , & Blunt, A. (2000). Five days of emotion: An experience sampling study of undergraduate student procrastination. Journal of Social Behavior and Personality, 15(5), 239–271. 10.1080/00926230152035859 [DOI] [Google Scholar]
- Ridgway, G. R. , Omar, R. , Ourselin, S. , Hill, D. L. , Warren, J. D. , & Fox, N. C. (2009). Issues with threshold masking in voxel‐based morphometry of atrophied brains. NeuroImage, 44(1), 99–111. 10.1016/j.neuroimage.2008.08.045 [DOI] [PubMed] [Google Scholar]
- Rueter, A. R. , Abram, S. V. , MacDonald, A. W., III , Rustichini, A. , & DeYoung, C. G. (2018). The goal priority network as a neural substrate of conscientiousness. Human Brain Mapping, 39(9), 3574–3585. 10.1002/hbm.24195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarinopoulos, I. , Grupe, D. , Mackiewicz, K. , Herrington, J. , Lor, M. , Steege, E. , & Nitschke, J. (2009). Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral Cortex, 20(4), 929–940. 10.1093/cercor/bhp155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucier, G. (1998). Replicable item‐cluster subcomponents in the NEO five‐factor inventory. Journal of Personality Assessment, 70(2), 263–276. 10.1207/s15327752jpa7002_6 [DOI] [PubMed] [Google Scholar]
- Scher, S. J. , & Osterman, N. M. (2002). Procrastination, conscientiousness, anxiety, and goals: Exploring the measurement and correlates of procrastination among school‐aged children. Psychology in the Schools, 39(4), 385–398. 10.1002/pits.10045 [DOI] [Google Scholar]
- Schouwenburg, H. C. , & Lay, C. H. (1995). Trait procrastination and the big‐five factors of personality. Personality and Individual Differences, 18(4), 481–490. 10.1016/0191-8869(94)00176-S [DOI] [Google Scholar]
- Schultz, W. (2000). Multiple reward signals in the brain. Nature Reviews Neuroscience, 1(3), 199–207. 10.1038/35044563 [DOI] [PubMed] [Google Scholar]
- Schwartz, S. H. (2012). An overview of the Schwartz theory of basic values. Online Readings in Psychology and Culture, 2(1), 2307–0919.1116. 10.9707/2307-0919.1116 [DOI] [Google Scholar]
- Seligman, M. E. P. , Railton, P. , Baumeister, R. F. , & Sripada, C. (2013). Navigating into the future or driven by the past. Perspectives on Psychological Science, 8(2), 119–141. 10.1177/1745691612474317 [DOI] [PubMed] [Google Scholar]
- Seo, D. , Lacadie, C. M. , & Sinha, R. (2016). Neural correlates and connectivity underlying stress‐related impulse control difficulties in alcoholism. Alcoholism: Clinical and Experimental Research, 40(9), 1884–1894. 10.1111/acer.13166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, A. , Strigo, I. , Matthews, S. C. , Paulus, M. P. , & Stein, M. B. (2006). Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety‐prone subjects. Biological Psychiatry, 60(4), 402–409. 10.1016/j.biopsych.2006.04.038 [DOI] [PubMed] [Google Scholar]
- Sirois, F. , & Pychyl, T. (2013). Procrastination and the priority of short‐term mood regulation: Consequences for future self. Social & Personality Psychology Compass, 7(2), 115–127. 10.1111/spc3.12011 [DOI] [Google Scholar]
- Sirois, F. M. (2014). Out of sight, out of time? A meta‐analytic investigation of procrastination and time perspective. European Journal of Personality, 28(5), 511–520. 10.1002/per.1947 [DOI] [Google Scholar]
- Smith, T. , Panfil, K. , Bailey, C. , & Kirkpatrick, K. (2019). Cognitive and behavioral training interventions to promote self‐control. Journal of Experimental Psychology Animal Learning & Cognition, 45(3), 259–279. 10.1037/xan0000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, C. , Schwarzkopf, D. S. , Kanai, R. , & Rees, G. (2011). Reciprocal anatomical relationship between primary sensory and prefrontal cortices in the human brain. Journal of Neuroscience, 31(26), 9472–9480. 10.1523/JNEUROSCI.0308-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers, H. J. (2008). Keeping the goal in mind: Prefrontal contributions to spatial navigation. Neuropsychologia, 46(7), 2106–2108. 10.1016/j.neuropsychologia.2008.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N. , Stevens, W. D. , Chamberlain, J. P. , Gilmore, A. W. , & Schacter, D. L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal‐directed cognition. NeuroImage, 53(1), 303–317. 10.1016/j.neuroimage.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel, P. (2007). The nature of procrastination: A meta‐analytic and theoretical review of quintessential self‐regulatory failure. Psychological Bulletin, 133(1), 65–94. 10.1037/0033-2909.133.1.65 [DOI] [PubMed] [Google Scholar]
- Takeuchi, H. , Taki, Y. , Nouchi, R. , Sekiguchi, A. , Kotozaki, Y. , Miyauchi, C. M. , … Nakagawa, S. (2014). Regional gray matter density is associated with achievement motivation: Evidence from voxel‐based morphometry. Brain Structure and Function, 219(1), 71–83. 10.1007/s00429-012-0485-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, H. , Taki, Y. , Sassa, Y. , Hashizume, H. , Sekiguchi, A. , Fukushima, A. , & Kawashima, R. (2011). Regional gray matter density associated with emotional intelligence: Evidence from voxel‐based morphometry. Human Brain Mapping, 32(9), 1497–1510. 10.1007/s00429-012-0485-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano, A. , Costa, P. T., Jr. , & McCrae, R. R. (2006). Personality plasticity after age 30. Personality and Social Psychology Bulletin, 32(8), 999–1009. 10.1177/0146167206288599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice, D. M. , & Baumeister, R. F. (1997). Longitudinal study of procrastination, performance, stress, and health. Psychological Science, 8(6), 454–458. 10.1111/j.1467-9280.1997.tb00460.x. [DOI] [Google Scholar]
- Tice, D. M. , Baumeister, R. F. , Shmueli, D. , & Muraven, M. (2007). Restoring the self: Positive affect helps improve self‐regulation following ego depletion. Journal of Experimental Social Psychology, 43(3), 379–384. 10.1016/j.jesp.2006.05.007 [DOI] [Google Scholar]
- Treadway, M. T. , Buckholtz, J. W. , Cowan, R. L. , Woodward, N. D. , Li, R. , Ansari, M. S. , … Zald, D. H. (2012). Dopaminergic mechanisms of individual differences in human effort‐based decision‐making. Journal of Neuroscience, 32(18), 6170–6176. 10.1523/JNEUROSCI.6459-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trope, Y. , & Fishbach, A. (2000). Counteractive self‐control in overcoming temptation. Journal of Personality and Social Psychology, 79(4), 493–506. 10.1037//0022-3514.79.4.493 [DOI] [PubMed] [Google Scholar]
- Tyler, J. M. , & Burns, K. C. (2008). After depletion: The replenishment of the Self's regulatory resources. Self & Identity, 7(3), 305–321. 10.1080/15298860701799997 [DOI] [Google Scholar]
- Verplanken, B. , & Sato, A. (2011). The psychology of impulse buying: An integrative self‐regulation approach. Journal of Consumer Policy, 34(2), 197–210. 10.1007/s10603-011-9158-5 [DOI] [Google Scholar]
- Vincent, J. L. , Patel, G. H. , Fox, M. D. , Snyder, A. Z. , Baker, J. T. , Van Essen, D. C. , … Raichle, M. E. (2007). Intrinsic functional architecture in the anaesthetized monkey brain. Nature, 447(7140), 83–86. 10.1038/nature05758 [DOI] [PubMed] [Google Scholar]
- Watkins, E. R. (2008). Constructive and unconstructive repetitive thought. Psychological Bulletin, 134(2), 163–206. 10.1037/0033-2909.134.2.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, D. C. (2001). Procrastination and the five‐factor model: A facet level analysis. Personality and Individual Differences, 30(1), 149–158. 10.1016/S0191-8869(00)00019-2 [DOI] [Google Scholar]
- Weston, R. , & Gore, P. A., Jr. (2006). A brief guide to structural equation modeling. The Counseling Psychologist, 34(5), 719–751. 10.1177/0011000006286345 [DOI] [Google Scholar]
- Weygandt, M. , Mai, K. , Dommes, E. , Ritter, K. , Leupelt, V. , Spranger, J. , & Haynes, J.‐D. (2015). Impulse control in the dorsolateral prefrontal cortex counteracts post‐diet weight regain in obesity. NeuroImage, 109, 318–327. 10.1016/j.neuroimage.2014.12.073 [DOI] [PubMed] [Google Scholar]
- Wigfield, A. , & Eccles, J. S. (2000). Expectancy–value theory of achievement motivation. Contemporary Educational Psychology, 25(1), 68–81. 10.1006/ceps.1999.1015 [DOI] [PubMed] [Google Scholar]
- Wu, H. , Gui, D. , Lin, W. , Gu, R. , Zhu, X. , & Liu, X. (2016). The procrastinators want it now: Behavioral and event‐related potential evidence of the procrastination of intertemporal choices. Brain and Cognition, 107, 16–23. 10.1016/j.bandc.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Yan, C. , & Zang, Y. (2010). DPARSF: A MATLAB toolbox for" pipeline" data analysis of resting‐state fMRI. Frontiers in Systems Neuroscience, 4, 13. 10.3389/fnsys.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Becker, B. , Chen, Q. , & Feng, T. (2019a). Insufficient task‐outcome association promotes task procrastination through a decrease of hippocampal–striatal interaction. Human Brain Mapping, 40(2), 597–607. 10.1002/hbm.24397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Liu, P. , & Feng, T. (2019). To do it now or later: The cognitive mechanisms and neural substrates underlying procrastination. Wiley Interdisciplinary Reviews: Cognitive Science, 10(4), e1492. 10.1002/wcs.1492 [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Wang, X. , & Feng, T. (2016). Identifying the neural substrates of procrastination: A resting‐state fMRI study. Scientific Reports, 6, 33203. 10.1038/srep33203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , & Seibert, S. E. (2006). The big five personality dimensions and entrepreneurial status: A meta‐analytical review. Journal of Applied Psychology, 91(2), 259–271. 10.1037/0021-9010.91.2.259 [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Meng, G. , Sun, Y. , Xu, Y. , Geng, J. , & Han, L. (2019). The relationship between self‐control and procrastination based on the self‐regulation theory perspective: the moderated mediation model. Current Psychology, 1, 10.1007/s12144-019-00442-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study is not available due to the following reasons: a) participants of this study did not agree for their data to be shared publicly; b) these data can be further researched, and the research group is currently digging deeply, which is not convenient for sharing. If any researchers are interested in our research and willing to make cooperation, please do not to hesitate to contact with us (fengty0@swu.edu.cn).


