Abstract
Real‐time fMRI guided neurofeedback training has gained increasing interest as a noninvasive brain regulation technique with the potential to modulate functional brain alterations in therapeutic contexts. Individual variations in learning success and treatment response have been observed, yet the neural substrates underlying the learning of self‐regulation remain unclear. Against this background, we explored potential brain structural predictors for learning success with pooled data from three real‐time fMRI data sets. Our analysis revealed that gray matter volume of the right putamen could predict neurofeedback learning success across the three data sets (n = 66 in total). Importantly, the original studies employed different neurofeedback paradigms during which different brain regions were trained pointing to a general association with learning success independent of specific aspects of the experimental design. Given the role of the putamen in associative learning this finding may reflect an important role of instrumental learning processes and brain structural variations in associated brain regions for successful acquisition of fMRI neurofeedback‐guided self‐regulation.
Keywords: brain morphometry, instrumental learning, neurofeedback, real‐time fMRI, striatum
In the current study, we explored potential brain structural predictors for learning success with pooled data from three real‐time fMRI data sets that employed different paradigms during which different brain regions were trained. Our analysis revealed that gray matter volume of the right putamen could predict neurofeedback learning success across the three data sets (n = 66 in total), pointing to a general association with learning success independent of the experimental design variations. Given the role of the putamen in associative learning this finding may reflect an important role of instrumental learning processes and brain structural variations in associated brain regions for successful acquisition of fMRI neurofeedback‐guided self‐regulation.

1. INTRODUCTION
Real‐time fMRI‐based neurofeedback (rt‐fMRI NF) is a brain modulation technique that can non‐invasively modulate functional brain activity by allowing individuals to gain control over the neural signal (i.e., Blood Oxygenation Level Dependent, BOLD, activation). An increasing number of studies demonstrated that the successful self‐regulation of specific regions or networks can induce behavioral changes in domains associated with the trained neural systems in healthy subjects. Based on these promising findings, an increasing number of rt‐fMRI NF studies focused on clinical populations. These investigations demonstrated some promising therapeutic effects after single or repeated sessions of training in patients with mental disorders including anxiety disorder (Scheinost et al., 2013; Zilverstand, Sorger, Sarkheil, & Goebel, 2015), depression (Young et al., 2014; Young et al., 2017), attention deficit disorder (Alegria et al., 2017; Zilverstand et al., 2017), substance use disorder (Hanlon et al., 2013; Hartwell et al., 2016; Kirsch, Gruber, Ruf, Kiefer, & Kirsch, 2016; Li et al., 2013) and schizophrenia (Dyck et al., 2016; Okano et al., 2020; Orlov et al., 2018; Zweerings et al., 2019).
While these studies demonstrate that healthy subjects and patients can learn volitional control over brain activation and initial clinical studies reported beneficial effects on symptom improvement, the precise behavioral and neural mechanisms that underlie the acquisition of rt‐fMRI NF‐guided self‐regulation remain unclear. Within this context recent reviews in the field have proposed key questions that need to be addressed to promote a more conceptually‐based account to rt‐fMRI NF, such as to clarify which neural substrates support the acquisition of neural self‐regulation (Emmert et al., 2016) and how the regulation success can be translated into changes in specific behavioral domains and ultimately clinical responses (Hampson, 2017). A recent meta‐analysis aggregated results reported by 99 rt‐fMRI NF studies and found that only 57 of the 99 studies observed increased regulation in comparison to baseline, and less than half of the studies found overall improvements on the behavioral level (Thibault, MacPherson, Lifshitz, Roth, & Raz, 2018). A similar issue has been previously raised for electroencephalogram‐based neurofeedback (EEG NF) (Alkoby, Abu‐Rmileh, Shriki, & Todder, 2018). According to this review, 38% of the participants across the 11 EEG NF studies included were not able to gain regulatory control over their brain activity during training. Accordingly, a better understanding of critical aspects that determine regulation success is essential for the progression of the field.
Recent debates on optimizing rt‐fMRI NF training efficacy have been mostly focused on methodological aspects such as the number of training sessions, target region selection (Karch et al., 2015), novel brain‐computer interfaces (Lorenzetti et al., 2018; Mathiak et al., 2015), instructions and learning strategies (Sitaram et al., 2017; Stoeckel et al., 2014) or the specific form of feedback presentation (e.g., intermittent vs. continuous or implicit vs. explicit, see [Emmert et al., 2017; Stoeckel et al., 2014]). In addition to optimizing the efficacy of the training per se recently emerging frameworks conceptualizing a precision medicine approach for mental disorders (Insel, 2014) proposed that accounting for individual differences in the patients may represent a promising strategy to optimize treatment selection for specific patient populations or on the individual level. Extending this approach to neurofeedback training by determining factors that modulate or predict learning success could help to identify patients with the highest potential to benefit from the training and thus increase the training efficacy regarding symptom improvement. Moreover, the determination of neural predictors may generally help to further determine the complex processes underlying neurofeedback learning.
With the aim to determine the neural basis that underlies neurofeedback acquisition a recent meta‐analysis encompassing data from 12 rt‐fMRI NF studies revealed a brain network commonly engaged during training. The major nodes of this network included the dorsolateral and ventrolateral areas of the prefrontal cortex (dlPFC and vlPFC), basal ganglia, anterior insula cortex (AIC), anterior cingulate cortex (ACC), thalamus and visual associative areas (Emmert et al., 2016). A review by Sitaram et al. additionally proposed functional domains and associated brain systems that mediate neurofeedback learning. The authors proposed a network similar to that determined in the aforementioned meta‐analysis which supports the involvement of different cognitive systems including executive control, salience detection and reward processing during neurofeedback learning (Sitaram et al., 2017). In particular, the authors emphasized a key role of the dorsal and ventral striatum because of their central roles in instrumental and associative learning which are highly related to the acquisition of feedback‐dependent behavioral modification (Christoffersen & Schachtman, 2016; Gruart, Leal‐Campanario, Lopez‐Ramos, & Delgado‐Garcia, 2015; Yin et al., 2009).
NF training shares a strong learning‐related component with other forms of treatments that aim at modifying behavioral maladaptations, such as cognitive training elements of behavioral therapy. Cognitive‐behavioral therapy (CBT) for instance applies learning‐based strategies and has been shown efficient to attenuate behavioral and neural dysregulations in a range of mental disorders. Previous studies that combined the personalized medicine approach with neuroimaging in this context have reported that neural recruitment during baseline (Klumpp, Fitzgerald, & Phan, 2013) as well as individual variations in brain morphometry—in particular gray matter volumes (Bryant et al., 2008) in the dysregulated pathways—are predictive for the subsequent treatment response to CBT. When targeting patient populations, rt‐fMRI NF training is commonly focused on restoring the control over emotions using mental strategies which share similar components to CBT training (Linhartova et al., 2019). Results from previous EEG‐neurofeedback learning studies suggest that in particular brain structural variations may represent a promising candidate index for predicting learning success. These studies reported for instance that volumes of the cingulate cortex, AIC and putamen as measured by voxel‐based morphometry (VBM) were predictive for learning outcomes (Enriquez‐Geppert et al., 2013; Ninaus et al., 2015).
Given that highly similar cognitive processes may underlie the acquisition of self‐regulation by neurofeedback from EEG and fMRI modalities it is thus conceivable that brain structural indices may predict learning success in rt‐fMRI NF experiments. A previous meta‐analysis of NF studies aimed at determining whether pre‐training activation within the target regions of the training collected from more than 400 participants could predict subsequent learning success. However, this study did not find a common functional MRI‐based predictor for neurofeedback efficacy, suggesting an examination of more stable alternative predictors of neurofeedback learning success (Haugg et al., 2020). Against this background, the current study explored whether regional brain volume could predict learning success during rt‐fMRI NF training in healthy subjects. Based on the role of the dlPFC, ACC, AIC, and striatum in neurofeedback training as suggested by the previous literature (Emmert et al., 2016; Sitaram et al., 2017), we hypothesized that gray matter volumes (GMV) in these regions are associated with neurofeedback learning success in healthy individuals. Furthermore, to increase statistical power and generalize the association across different target regions our hypothesis was tested in data pooled from three data sets targeting different brain regions or pathways with NF. Given that the structural data in these data sets were collected prior to the completion of NF training, associations between individual variations in brain structure and subsequent learning success were considered as predictive relationships.
2. METHODS
2.1. Data sets and participants
The data sets reported in the current study were collected in healthy samples and were previously published in peer‐reviewed journals (Mathiak et al., 2015; Yao et al., 2016; Zhao et al., 2019; Zweerings et al., 2018). Only data from the experimental neurofeedback runs was included in our analysis (i.e., given the lack of learning success during the sham/control conditions the corresponding data was excluded from the current analyses; see Table 1 for demographics). The data sets are distinct from each other by the experimental design and the targeted training regions.
TABLE 1.
Demographic information of the data sets
| Paradigm | Number of participants | Age (SD) in years |
|---|---|---|
| AIC NF | 18 (9 females) | 22.22 (1.17) |
| vlPFC‐amygdala FC NF | 23 males | 22.74 (2.07) |
| ACC NF | 25 (18 females) | 30.80 (10.51) |
Abbreviations: ACC, anterior cingulate cortex; AIC, anterior insula cortex; FC, functional connectivity; NF, neurofeedback; vlPFC, ventrolateral prefrontal cortex.
Two out of the three data sets were collected on a 3‐Tesla MRI system (MR750, General Electric Medical System, Milwaukee, WI) in China. One of them trained upregulation of AIC activity (Yao et al., 2016) and the other study trained participants to up‐regulate functional connectivity between amygdala and ventrolateral prefrontal cortex (vlPFC) (Zhao et al., 2019). Both studies found significant changes in the trained neural activity after four runs of training. T1‐weighted brain structural images were collected immediately before the neurofeedback training. The third data set was collected on a 3‐Tesla Siemens MRI system (Magnetom TRIO, Siemens Medical Systems, Erlangen, Germany) at RWTH Aachen University in Germany. Participants in this data set were collected in two different studies (Mathiak et al., 2015; Zweerings et al., 2018) that shared the same training procedure during which participants received three sessions of neurofeedback training to increase brain activity in the anatomically defined ACC. The imaging parameters and further details of the data sets are provided in the Supporting Information.
2.2. Measurement of neurofeedback learning success
Significant training effects on neural indices on the group‐level have been previously demonstrated in all three upregulation training data sets. In the current study, we further explored whether variations in brain structure is linked to variations in the subsequent NF learning success on the individual level. Learning success was defined as changes in brain activity in the desired direction (as determined in the original studies) by subtracting the measurements in the early training runs/sessions from the ones in the late stages of the training. To this end, beta‐estimates within the trained regions of interest (ROI) were extracted from the generalized linear models provided by the authors of the original studies. For the Chinese data sets, learning success was previously determined by comparing differences in the targeted brain activity between the first two and last two neurofeedback runs (Yao et al., 2016; Zhao et al., 2019). This measurement was directly adopted in the current study. For the ACC regulation data, the learning success was calculated as the difference in mean ACC activation during regulation between the third and the first training sessions, a measure that approximates the approach in the other two data sets (Yao et al., 2016; Zhao et al., 2019). The data were extracted with the tailored ACC anatomical mask used during the original neurofeedback training (Mathiak et al., 2015; Zweerings et al., 2018). A previous study used z‐standardization to successfully explore a shared pattern of symptom improvements across neurofeedback studies (Rance et al., 2018). In line with this approach, we adopted standardization of the learning success by means of computing z‐scores within each sample using the zscore function incorporated in MATLAB (R2020a. Natick, Massachusetts: The MathWorks Inc.).
2.3. Preprocessing of the imaging data
The brain structural images were preprocessed with the VBM8 toolbox (http://dbm.neuro.uni-jena.de/wordpress/vbm/). Individual brain images were first segmented into gray matter, white matter and cerebrospinal fluid tissue probability maps by unified segmentation as implemented in SPM12 (“New Segment Toolbox”). After segmentation, the gray matter images were non‐linearly normalized to the MNI standard space with the DARTEL algorithm (Ashburner, 2007) while accounting for individual whole brain volume sizes (“Modulated normalization” in VBM8) to generate the gray matter volume (GMV) maps. Finally, these gray matter images were smoothed with a 4 mm full width at half maximum (FWHM) kernel. Total intracranial volume (TIV) was calculated and subsequently included as a covariate in the regression model. Before being subjected to further analysis, the quality of the GMV data were examined by visual inspection of the segmented and registered images. Additionally, the data quality tool in VBM8 was used to identify unsmoothed GMV maps with low covariance (below 2 SD) within each data set. Based on these quality assessments no data were excluded.
2.4. Structural predictors for neurofeedback learning
Associations between brain volume and neural learning success were examined by multiple regressions in SPM12 (www.fil.ion.ucl.ac.uk/spm/software/spm12/) using the gray matter images generated in the last step of the proprocessing as described above. In addition to the learning success measurement, data set, scanner, age, gender, and TIV were included in the regression model as control variables.
Our goal was to examine which brain structures proposed in the previous literature might contribute to individual differences in neurofeedback learning. To this end, the correlation between gray matter volume and learning success was separately tested for the core systems of the previously described neurofeedback network, specifically the bilateral dlPFC, ACC, AIC, and striatum (both dorsal and ventral division, details of the masks used for the region of interest, ROI, analysis are provided in SI) with a small volume correction (SVC) approach. Clusters that survived p < .05 while correcting for family‐wise errors (FWE) were considered significant. In order to further describe the determined regions associated with subsequent NF success on the functional network level, we additionally explored the intrinsic functional profile of the cluster that survived the correction. To this end we performed a resting‐state functional connectivity (rs‐FC) analysis in an independent sample of N = 252 healthy young adults. Briefly, the rs‐FC data were initially subjected to standard preprocessing steps including slice timing and head movement corrections, normalization to MNI space, removal of physiological noise and global signal intensity and finally, band‐pass filtering. The functional connectivity analysis employed the identified cluster associated with NF learning success from the VBM analysis as seed region to calculate whole brain resting state FC (details of the sample, preprocessing and analysis are additionally provided in the SI; for the further sample description see also [Liu et al., 2020]).
3. RESULTS
3.1. Demographics
During screening for data availability and quality, one subject from the ACC training sample was excluded due to missing age data. This resulted in a total of 66 participants included in the final analysis. The demographic information of these participants is reported in Table 1. None of them received control/sham feedback during the training.
3.2. Associations between brain structure and learning success
Applying region‐wise SVC on the statistical map revealed a significant positive correlation between the volume of a cluster located in the striatum and learning success in the pooled data (t = 4.56, p FWE = .039, voxels = 62). No significant associations within the other ROIs emerged. The cluster was located in the dorsal division of the right striatum, primarily in the putamen (x‐y‐z MNI‐coordinates of the peak: 27, 9, 8). No negative correlation between GMV and learning success was observed at the same threshold. Of note, because of the exploratory nature of the current work, no correction for multiple comparisons was performed for the number of tests/masks (N = 4 ROIs tested, see Discussion). The whole‐brain map of the structural correlation with learning success is shown in Figure 1, further suggesting an association between learning success and structural variations in the bilateral dorsal striatum at an uncorrected display level (p < .001, k > 20).
FIGURE 1.
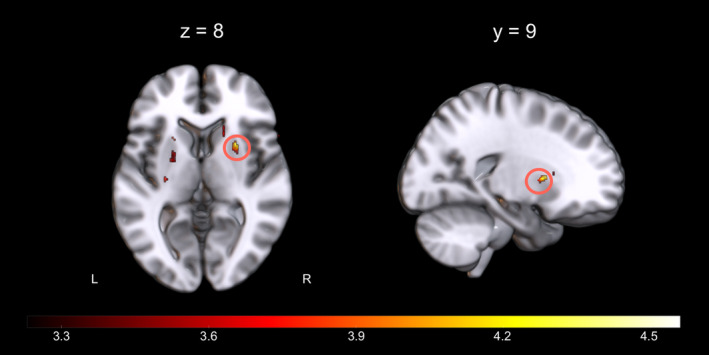
Brain clusters with a positive association between gray matter volume and neurofeedback learning success (uncorrected p < .001 with a cluster threshold of k > 20, whole brain level). The circle indicates the putamen region which survived small volume correction in the multiple regression analysis. Other clusters shown in this map are reported in Table S1
To separately explore the GMV‐learning success association pattern in each data set, regional volume was extracted from the putamen cluster within a 4 mm‐radius sphere centered at the peak coordinate. Extracted estimates were visualized with a scatter plot (Figure 2). Pearson correlation analysis revealed a consistent pattern of positive associations between larger gray matter volume in this region with better learning success across samples. Interestingly however the GMV estimates varied between the cohorts which may be explained by differences in the different gender ratios between the original studies. Such sex‐differences in the morphology of the basal ganglia have been reported previously (Ahsan et al., 2007). Together our results suggest an association between larger putamen volume with better neurofeedback learning success on the neural level across the studies.
FIGURE 2.
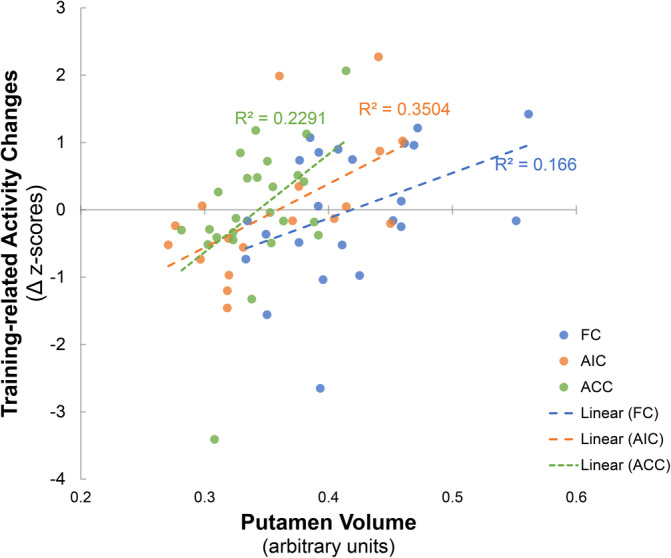
Association between gray matter volume in the putamen area and learning success in each data set with data points from each sample coded in different colors. The learning success (Y‐axis) was calculated as the z‐standardized changes (late vs. early‐stage training) in brain activity within each study sample. R 2 of the linear regressions were denoted. FC, functional connectivity training study; AIC, anterior insula cortex training study; ACC, anterior cingulate cortex training study
3.3. Network level characterization of the identified region
To facilitate a functional characterization of the identified putamen region we further examined rs‐FC of the putamen region (cluster after SVC correction) in a large independent sample (N = 252). The analysis revealed that the identified putamen region exhibited widespread positive intrinsic connectivity with a bilateral network encompassing supplementary motor regions, dlPFC, ACC, AIC, parietal lobe, thalamus, and cerebellum (Figure 3, see also Figure S2 for brain regions that exhibited negative connectivity with the putamen seed region). This network overlapped with the neurofeedback network proposed in the previous literature (Emmert et al., 2016).
FIGURE 3.
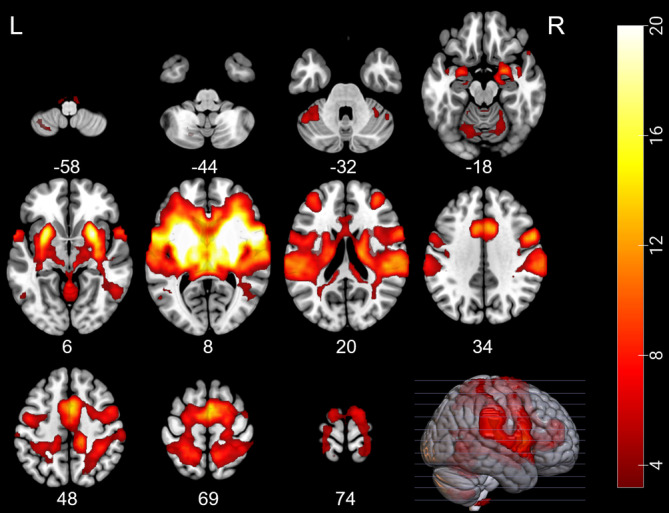
Rs‐FC network discovered in the independent sample (N = 252) with the putamen region that predicted neurofeedback learning as seed. The map is corrected at cluster level for family‐wise errors (p FWE < .05, cluster forming with height threshold at p < .001, k > 68)
4. DISCUSSION
In the current study, we investigated whether gray matter volume within the previously proposed neurofeedback training‐related brain network could be linked to individual variations in neural regulation success acquired during fMRI‐guided neurofeedback training. In our exploratory analyses, we separately examined brain regions previously suggested to underlie neurofeedback learning success, we found that in three independent samples, better neurofeedback learning success was associated with larger volumes of the dorsal striatum, despite differences in experimental designs and target regions employed in the original studies. Individual structural variations in this region before training prospectively associated with the changes in the targeted brain activity during training, suggesting that individual GM variations in this region may have the potential to predict subsequent learning success. Moreover, further functional characterization of the identified region by means of determining resting‐state functional connectivity between this region and the rest of the brain in an independent sample revealed strong intrinsic functional coupling between the identified dorsal striatum region and other major nodes of the neurofeedback network proposed in the literature (Emmert et al., 2016; Sitaram et al., 2017), which further reflects that the dorsal striatum may represent a core node within the networks that support the acquisition of neural self‐regulation via fMRI‐guided neurofeedback.
The underlying mechanisms and factors that contribute to individual differences in NF learning success have been rarely examined. A recent meta‐analysis that aimed at identifying functional brain markers has failed to find one that could reliably predict neurofeedback learning success across studies (Haugg et al., 2020). In the light of these efforts, we asked whether individual variations in brain structure could predict NF learning outcome in three samples and found a positive association between volume of the dorsal striatum, specifically the putamen, and learning success. The findings align with findings from initial studies that learning during rt‐fMRI NF has been linked to the recruitment of the striatal system (Mathiak et al., 2015), probably reflecting a feedback‐ and reward‐related teaching signal (Bray, Shimojo, & O'Doherty, 2007). A previous study further demonstrated that the failure to acquire fMRI‐guided neural self‐regulation in patients with schizophrenia was mediated by dysfunctional feedback‐related responses in the striatum (Dyck et al., 2016) and reduced putamen volumes have been repeatedly reported in this population (Gaser, Nenadic, Buchsbaum, Hazlett, & Buchsbaum, 2004). Additional support for an association between individual variations in brain structure and neurofeedback learning success comes from EEG‐neurofeedback studies which may share key mechanisms of the acquisition of neural self‐regulation with fMRI‐guided NF protocols. Using machine learning approaches a previous EEG‐neurofeedback study demonstrated that the fractional anisotropy (FA) value in local fiber structures including cingulum fibers was highly correlated with neurofeedback performance in a sample of 20 healthy participants (Halder et al., 2013). Two other studies reported that brain volumes of the cingulate area and the putamen along with regions including AIC and prefrontal cortex could predict participants' self‐regulation performance during EEG rhythm training (Enriquez‐Geppert et al., 2013; Ninaus et al., 2015). Partly resembling the previous findings our results suggest that the structural integrity of the putamen, a brain structure involved in feedback‐based and instrumental learning, is associated with neurofeedback learning success during fMRI‐guided NF in healthy individuals.
During a typical neurofeedback training setting, subjects gain regulatory control based on feedback that reflects the neural signal of the targeted region or network. The process of gaining control itself may be considered as intrinsically rewarding process. As such increasing regulatory control is followed by the presence of a reinforcing outcome (i.e., the provision of a rewarding feedback signal upon successful regulation efforts in the desired direction) which resembles a form of instrumental learning (Sitaram et al., 2017). Evidence from animal studies indicate a crucial role of the dorsal striatum in instrumental, associative, and procedural learning. This is especially supported by neuromodulatory effects observed on synapses in dorsal striatal regions during both skill learning and instrumental learning processes (Gruart et al., 2015; Lovinger, 2010). The dorsal striatum encompasses the caudate and putamen, and facilitates the integration of information input from the associative cortices (Haber, 2016). Furthermore, this region is strongly involved in the tracking of action‐outcome contingency during associative learning (Brovelli, Nazarian, Meunier, & Boussaoud, 2011; Yin & Knowlton, 2006). In humans, focal lesions of the putamen have found to be associated with impaired performance in punishment‐based avoidance learning which is in line with a large body of evidence showing the recruitment of this region during encoding of the outcome (Delgado, Nystrom, Fissell, Noll, & Fiez, 2000; O'Doherty et al., 2004; Palminteri et al., 2012; Seymour, Daw, Dayan, Singer, & Dolan, 2007). In line with recent overarching conceptualizations proposing the importance of instrumental and procedural learning mechanisms in NF learning, responses to NF‐guided neural signals reliably engage this region (Emmert et al., 2016; Shibata et al., 2019). In a study which trained upregulation of the right inferior frontal cortex in adolescents with attention deficit disorder, error‐monitoring related activity in the putamen region was increased in a stop signal task in the experimental group compared with the active control group. Additionally, this pre‐post change had trend‐level correlations with neurofeedback learning performance as well as the improvements in symptoms (Criaud et al., 2020; Lam et al., 2020). On the brain structural level, VBM studies have found that the gray matter volume of the putamen could be related to both the skill level of piano playing (Granert, Peller, Jabusch, Altenmuller, & Siebner, 2011) and the performance of neurofeedback learning in sensorimotor rhythm regulation (Ninaus et al., 2015), suggesting an association with procedural learning success. Taken together, our results may reflect that during neurofeedback learning, individuals with larger putamen volume might benefit from a better ability in adjusting mental strategies based on perceived feedback contingency or a better procedural learning ability which in turn may have promoted increased learning success.
The present exploratory data provides the first evidence that individual variations in the morphology of the dorsal striatum may predict NF learning success. The findings are based on three independent data sets with variation in the experimental design and the selected target regions, and hence suggest generalizability of the findings. However, the role of the putamen during neurofeedback training remains speculative due to the lack of direct support from behavioral data. As we have discussed previously, an overarching neurobiological model of neurofeedback learning is yet to be established. In this context, the exact mechanism of action needs to be further examined with tailored experimental designs and validated in larger samples. As a complex cognitive process, neurofeedback learning is presumably underpinned by the involvement of several brain structures or networks in addition to the region discovered in the current study. For instance, dlPFC and ACC are central for sustaining attention to the stimuli and error monitoring during feedback learning (van Duijvenvoorde, Zanolie, Rombouts, Raijmakers, & Crone, 2008). Findings from a recent closed‐loop training study indicated a role of the frontostriatal circuitry in learning to regulate high‐dimensional brain activity by bridging metacognition (prefrontal) and reinforcement learning (striatum) substrates which further suggested that neurofeedback learning relies on an integrated brain network rather than a single brain structure alone (Cortese, Lau, & Kawato, 2020). On the behavioral level, a meta‐analysis examining psychological factors for neurofeedback efficacy has highlighted the influence of attention, motivation, and mood on training outcome (Kadosh & Staunton, 2019). Therefore, it is worth examining whether the volume of the brain structures supporting these functions can be linked to neurofeedback efficacy in a similar way as demonstrated in the current study. Moreover, in the present study we focused on gray matter volume as a potential predictor of learning success in a sample of healthy subjects. Based on findings from a previous EEG‐NF study on a similar topic (Enriquez‐Geppert et al., 2013), it is likely that other brain structural measurements such as white matter volume or tracts also contribute to fMRI neurofeedback learning, which need to be examined by future studies. More importantly, since striatal gray matter volume reduction has been reported in a number of disorders such as addiction (Xiao et al., 2015) and attention deficit hyperactivity disorder (ADHD) (Ellison‐Wright, Ellison‐Wright, & Bullmore, 2008), the impact of the striatal structural deficit on the learning outcome needs to be assessed in patient populations. In clinical contexts, self‐efficacy has received increasing interest as another specific factor involved in the outcome of neurofeedback training, including in depression, eating disorders, ADHD and addiction (Mehler et al., 2018; Schmidt & Martin, 2016; Strehl et al., 2017; Weiss et al., 2020). Findings in the current study linked individual brain structural variations in the basal ganglia with neurofeedback learning. Of interest, in a healthy sample self‐efficacy scores were associated with lower mean diffusivity values in the right putamen (Nakagawa et al., 2017), a measurement that has been closely linked to the dopaminergic system which plays a central role in feedback‐guided learning processes (Takeuchi & Kawashima, 2018). Together with the current findings these previous studies suggest that future studies may explore associations between other brain structural indices, self‐efficacy and neurofeedback learning success in healthy individuals and patient populations. Finally, due to the substantial number of brain regions that may contribute to neurofeedback learning, four separate ROIs were tested with separate small volume correction. However, the multiple‐testing issues could not be resolved by simply applying Bonferroni correction on the number of tests as it would also inflate the false negatives rate. Due to this methodological limitation the present findings need to be interpreted as exploratory in nature, and associations between learning success and regional gray matter volume need to be interpreted cautiously. Replication studies and investigations of the aforementioned potential brain or behavioral factors are needed for further validating the present findings.
5. CONCLUSIONS
To the best of our knowledge, this is the first investigation showing that brain structure factor is predictive for rt‐fMRI neurofeedback learning success. The association between putamen volume and learning success may reflect the key role of instrumental learning processes during neurofeedback training. These findings await examination in further studies providing more comprehensive evidence.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Figure S1 The four regions of interest that were used in small volume correction overlaid on a MNI brain in different colors (see legends at bottom right corner). The striatum is further broken down into ventral and dorsal sections for illustration purpose.
Figure S2. Brain regions showed negative connectivity with the putamen region that predicted learning success. The statistical map is corrected for family‐wise errors at cluster level (pFWE < 0.05, k > 68 at height threshold of p < 0.001)
Table S1. Brain regions showed positive correlation with learning success at uncorrected threshold
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (2018YFA0701400) and the National Natural Science Foundation of China grants (NSFC grant number 31700998 and U1808204), the German Research Foundation (DFG; IRTG 2150), and the German Ministry for Education and Research (BMBF; APIC: 01EE1405A‐C). We thank Patrick Eisner for his help with data transferring.
Zhao Z, Yao S, Zweerings J, et al. Putamen volume predicts real‐time fMRI neurofeedback learning success across paradigms and neurofeedback target regions. Hum Brain Mapp. 2021;42:1879–1887. 10.1002/hbm.25336
Funding information Bundesministerium für Bildung und Forschung, Grant/Award Number: 01EE1405A‐C; Deutsche Forschungsgemeinschaft, Grant/Award Number: IRTG 2150; National Key Research and Development Program of China, Grant/Award Number: 2018YFA0701400; National Natural Science Foundation of China, Grant/Award Numbers: 31700998, U1808204
Contributor Information
Zhiying Zhao, Email: zhiying.zhao@yale.edu.
Shuxia Yao, Email: yaoshuxia@uestc.edu.cn.
Jana Zweerings, Email: jzweerings@ukaachen.de.
Xinqi Zhou, Email: xinqizhou.uestc@outlook.com.
Feng Zhou, Email: fengzhou.uestc@outlook.com.
Keith M Kendrick, Email: k.kendrick.uestc@gmail.com.
Huafu Chen, Email: chenhf@uestc.edu.cn.
Klaus Mathiak, Email: kmathiak@ukaachen.de.
Benjamin Becker, Email: ben_becker@gmx.de.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- Ahsan, R. L. , Allom, R. , Gousias, I. S. , Habib, H. , Turkheimer, F. E. , Free, S. , … Hammers, A. (2007). Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. NeuroImage, 38, 261–270. [DOI] [PubMed] [Google Scholar]
- Alegria, A. A. , Wulff, M. , Brinson, H. , Barker, G. J. , Norman, L. J. , Brandeis, D. , … Rubia, K. (2017). Real‐time fMRI neurofeedback in adolescents with attention deficit hyperactivity disorder. Human Brain Mapping, 38, 3190–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkoby, O. , Abu‐Rmileh, A. , Shriki, O. , & Todder, D. (2018). Can we predict who will respond to Neurofeedback? A review of the inefficacy problem and existing predictors for successful EEG Neurofeedback learning. Neuroscience, 378, 155–164. [DOI] [PubMed] [Google Scholar]
- Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38, 95–113. [DOI] [PubMed] [Google Scholar]
- Bray, S. , Shimojo, S. , & O'Doherty, J. P. (2007). Direct instrumental conditioning of neural activity using functional magnetic resonance imaging‐derived reward feedback. The Journal of Neuroscience, 27, 7498–7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovelli, A. , Nazarian, B. , Meunier, M. , & Boussaoud, D. (2011). Differential roles of caudate nucleus and putamen during instrumental learning. NeuroImage, 57, 1580–1590. [DOI] [PubMed] [Google Scholar]
- Bryant, R. A. , Felmingham, K. , Whitford, T. J. , Kemp, A. , Hughes, G. , Peduto, A. , & Williams, L. M. (2008). Rostral anterior cingulate volume predicts treatment response to cognitive‐behavioural therapy for posttraumatic stress disorder. Journal of Psychiatry & Neuroscience, 33, 142–146. [PMC free article] [PubMed] [Google Scholar]
- Christoffersen, G. R. J. , & Schachtman, T. R. (2016). Electrophysiological CNS‐processes related to associative learning in humans. Behavioural Brain Research, 296, 211–232. [DOI] [PubMed] [Google Scholar]
- Cortese, A. , Lau, H. , & Kawato, M. (2020). Unconscious reinforcement learning of hidden brain states supported by confidence. Nature Communications, 11, 4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud, M. , Wulff, M. , Alegria, A. A. , Barker, G. J. , Giampietro, V. , & Rubia, K. (2020). Increased left inferior fronto‐striatal activation during error monitoring after fMRI neurofeedback of right inferior frontal cortex in adolescents with attention deficit hyperactivity disorder. NeuroImage: Clinical, 27, 102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado, M. R. , Nystrom, L. E. , Fissell, C. , Noll, D. C. , & Fiez, J. A. (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84, 3072–3077. [DOI] [PubMed] [Google Scholar]
- Dyck, M. S. , Mathiak, K. A. , Bergert, S. , Sarkheil, P. , Koush, Y. , Alawi, E. M. , … Mathiak, K. (2016). Targeting treatment‐resistant auditory verbal hallucinations in schizophrenia with fMRI‐based neurofeedback–exploring different cases of schizophrenia. Frontiers in Psychiatry, 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison‐Wright, I. , Ellison‐Wright, Z. , & Bullmore, E. (2008). Structural brain change in attention deficit hyperactivity disorder identified by meta‐analysis. BMC Psychiatry, 8, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert, K. , Kopel, R. , Koush, Y. , Maire, R. , Senn, P. , Van De Ville, D. , & Haller, S. (2017). Continuous vs. intermittent neurofeedback to regulate auditory cortex activity of tinnitus patients using real‐time fMRI ‐ A pilot study. NeuroImage: Clinical, 14, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert, K. , Kopel, R. , Sulzer, J. , Bruhl, A. B. , Berman, B. D. , Linden, D. E. J. , … Haller, S. (2016). Meta‐analysis of real‐time fMRI neurofeedback studies using individual participant data: How is brain regulation mediated? NeuroImage, 124, 806–812. [DOI] [PubMed] [Google Scholar]
- Enriquez‐Geppert, S. , Huster, R. J. , Scharfenort, R. , Mokom, Z. N. , Vosskuhl, J. , Figge, C. , … Herrmann, C. S. (2013). The morphology of midcingulate cortex predicts frontal‐midline theta neurofeedback success. Frontiers in Human Neuroscience, 7, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser, C. , Nenadic, I. , Buchsbaum, B. R. , Hazlett, E. A. , & Buchsbaum, M. S. (2004). Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. The American Journal of Psychiatry, 161, 154–156. [DOI] [PubMed] [Google Scholar]
- Granert, O. , Peller, M. , Jabusch, H. C. , Altenmuller, E. , & Siebner, H. R. (2011). Sensorimotor skills and focal dystonia are linked to putaminal grey‐matter volume in pianists. Journal of Neurology, Neurosurgery, and Psychiatry, 82, 1225–1231. [DOI] [PubMed] [Google Scholar]
- Gruart, A. , Leal‐Campanario, R. , Lopez‐Ramos, J. C. , & Delgado‐Garcia, J. M. (2015). Functional basis of associative learning and its relationships with long‐term potentiation evoked in the involved neural circuits: Lessons from studies in behaving mammals. Neurobiology of Learning and Memory, 124, 3–18. [DOI] [PubMed] [Google Scholar]
- Haber, S. N. (2016). Corticostriatal circuitry. Dialogues in Clinical Neuroscience, 18, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder, S. , Varkuti, B. , Bogdan, M. , Kubler, A. , Rosenstiel, W. , Sitaram, R. , & Birbaumer, N. (2013). Prediction of brain‐computer interface aptitude from individual brain structure. Frontiers in Human Neuroscience, 7, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson, M. (2017). Identifying potential mechanisms of action underlying neurofeedback treatment response in depression. Biological Psychiatry, 82, 547–548. [DOI] [PubMed] [Google Scholar]
- Hanlon, C. A. , Hartwell, K. J. , Canterberry, M. , Li, X. , Owens, M. , Lematty, T. , … George, M. S. (2013). Reduction of cue‐induced craving through realtime neurofeedback in nicotine users: The role of region of interest selection and multiple visits. Psychiatry Research, 213, 79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell, K. J. , Hanlon, C. A. , Li, X. , Borckardt, J. J. , Canterberry, M. , Prisciandaro, J. J. , … Brady, K. T. (2016). Individualized real‐time fMRI neurofeedback to attenuate craving in nicotine‐dependent smokers. Journal of Psychiatry & Neuroscience, 41, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugg, A. , Sladky, R. , Skouras, S. , McDonald, A. , Craddock, C. , Kirschner, M. , … Scharnowski, F. (2020). Can we predict real‐time fMRI neurofeedback learning success from pretraining brain activity? Hum Brain: Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel, T. R. (2014). The NIMH research domain criteria (RDoC) project: Precision medicine for psychiatry. The American Journal of Psychiatry, 171, 395–397. [DOI] [PubMed] [Google Scholar]
- Kadosh, K. C. , & Staunton, G. (2019). A systematic review of the psychological factors that influence neurofeedback learning outcomes. NeuroImage, 185, 545–555. [DOI] [PubMed] [Google Scholar]
- Karch, S. , Keeser, D. , Hummer, S. , Paolini, M. , Kirsch, V. , Karali, T. , … Pogarell, O. (2015). Modulation of craving related brain responses using real‐time fMRI in patients with alcohol use disorder. PLoS One, 10, e0133034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch, M. , Gruber, I. , Ruf, M. , Kiefer, F. , & Kirsch, P. (2016). Real‐time functional magnetic resonance imaging neurofeedback can reduce striatal cue‐reactivity to alcohol stimuli. Addiction Biology, 21, 982–992. [DOI] [PubMed] [Google Scholar]
- Klumpp, H. , Fitzgerald, D. A. , & Phan, K. L. (2013). Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 45, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, S. L. , Criaud, M. , Alegria, A. , Barker, G. J. , Giampietro, V. , & Rubia, K. (2020). Neurofunctional and behavioural measures associated with fMRI‐neurofeedback learning in adolescents with attention‐deficit/hyperactivity disorder. NeuroImage: Clinical, 27, 102291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Hartwell, K. J. , Borckardt, J. , Prisciandaro, J. J. , Saladin, M. E. , Morgan, P. S. , … George, M. S. (2013). Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: A preliminary real‐time fMRI study. Addiction Biology, 18, 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhartova, P. , Latalova, A. , Kosa, B. , Kasparek, T. , Schmahl, C. , & Paret, C. (2019). fMRI neurofeedback in emotion regulation: A literature review. NeuroImage, 193, 75–92. [DOI] [PubMed] [Google Scholar]
- Liu, C. , Xu, L. , Li, J. , Zhou, F. , Yang, X. , Zheng, X. , … Becker, B. (2020). Serotonin and early life stress interact to shape brain architecture and anxious avoidant behavior – A TPH2 imaging genetics approach. Psychological Medicine, 1–9. 10.1017/S0033291720002809. [DOI] [PubMed] [Google Scholar]
- Lorenzetti, V. , Melo, B. , Basílio, R. , Suo, C. , Yücel, M. , Tierra‐Criollo, C. J. , & Moll, J. (2018). Emotion regulation using virtual environments and real‐time fMRI neurofeedback. Frontiers in Neurology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger, D. M. (2010). Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology, 58, 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiak, K. A. , Alawi, E. M. , Koush, Y. , Dyck, M. , Cordes, J. S. , Gaber, T. J. , … Mathiak, K. (2015). Social reward improves the voluntary control over localized brain activity in fMRI‐based neurofeedback training. Frontiers in Behavioral Neuroscience, 9, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler, D. M. A. , Sokunbi, M. O. , Habes, I. , Barawi, K. , Subramanian, L. , Range, M. , … Linden, D. E. J. (2018). Targeting the affective brain‐a randomized controlled trial of real‐time fMRI neurofeedback in patients with depression. Neuropsychopharmacology, 43, 2578–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, S. , Takeuchi, H. , Taki, Y. , Nouchi, R. , Kotozaki, Y. , Shinada, T. , … Kawashima, R. (2017). Lenticular nucleus correlates of general self‐efficacy in young adults. Brain Structure & Function, 222, 3309–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninaus, M. , Kober, S. E. , Witte, M. , Koschutnig, K. , Neuper, C. , & Wood, G. (2015). Brain volumetry and self‐regulation of brain activity relevant for neurofeedback. Biological Psychology, 110, 126–133. [DOI] [PubMed] [Google Scholar]
- O'Doherty, J. , Dayan, P. , Schultz, J. , Deichmann, R. , Friston, K. , & Dolan, R. J. (2004). Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science, 304, 452–454. [DOI] [PubMed] [Google Scholar]
- Okano, K. , Bauer, C. C. , Ghosh, S. S. , Lee, Y. J. , Melero, H. , de los Angeles, C. , … Whitfield‐Gabrieli, S. (2020). Real‐time fMRI feedback impacts brain activation, results in auditory hallucinations reduction: Part 1: Superior temporal Gyrus‐preliminary evidence. Psychiatry Research, 286, 112862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlov, N. D. , Giampietro, V. , O'Daly, O. , Lam, S. L. , Barker, G. J. , Rubia, K. , … Allen, P. (2018). Real‐time fMRI neurofeedback to down‐regulate superior temporal gyrus activity in patients with schizophrenia and auditory hallucinations: A proof‐of‐concept study. Translational Psychiatry, 8, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palminteri, S. , Justo, D. , Jauffret, C. , Pavlicek, B. , Dauta, A. , Delmaire, C. , … Pessiglione, M. (2012). Critical roles for anterior insula and dorsal striatum in punishment‐based avoidance learning. Neuron, 76, 998–1009. [DOI] [PubMed] [Google Scholar]
- Rance, M. , Walsh, C. , Sukhodolsky, D. G. , Pittman, B. , Qiu, M. , Kichuk, S. A. , … Hampson, M. (2018). Time course of clinical change following neurofeedback. NeuroImage, 181, 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost, D. , Stoica, T. , Saksa, J. , Papademetris, X. , Constable, R. T. , Pittenger, C. , & Hampson, M. (2013). Orbitofrontal cortex neurofeedback produces lasting changes in contamination anxiety and resting‐state connectivity. Translational Psychiatry, 3, e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, J. , & Martin, A. (2016). Neurofeedback against binge eating: A randomized controlled trial in a female subclinical threshold sample. European Eating Disorders Review, 24, 406–416. [DOI] [PubMed] [Google Scholar]
- Seymour, B. , Daw, N. , Dayan, P. , Singer, T. , & Dolan, R. (2007). Differential encoding of losses and gains in the human striatum. The Journal of Neuroscience, 27, 4826–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata, K. , Lisi, G. , Cortese, A. , Watanabe, T. , Sasaki, Y. , & Kawato, M. (2019). Toward a comprehensive understanding of the neural mechanisms of decoded neurofeedback. NeuroImage, 188, 539–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram, R. , Ros, T. , Stoeckel, L. , Haller, S. , Scharnowski, F. , Lewis‐Peacock, J. , … Sulzer, J. (2017). Closed‐loop brain training: The science of neurofeedback. Nature Reviews. Neuroscience, 18, 86–100. [DOI] [PubMed] [Google Scholar]
- Stoeckel, L. E. , Garrison, K. A. , Ghosh, S. , Wighton, P. , Hanlon, C. A. , Gilman, J. M. , … Evins, A. E. (2014). Optimizing real time fMRI neurofeedback for therapeutic discovery and development. NeuroImage: Clinical, 5, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehl, U. , Aggensteiner, P. , Wachtlin, D. , Brandeis, D. , Albrecht, B. , Arana, M. , … Holtmann, M. (2017). Neurofeedback of slow cortical potentials in children with attention‐deficit/hyperactivity disorder: A multicenter randomized trial controlling for unspecific effects. Frontiers in Human Neuroscience, 11, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, H. , & Kawashima, R. (2018). Mean diffusivity in the dopaminergic system and neural differences related to dopaminergic system. Current Neuropharmacology, 16, 460–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault, R. T. , MacPherson, A. , Lifshitz, M. , Roth, R. R. , & Raz, A. (2018). Neurofeedback with fMRI: A critical systematic review. NeuroImage, 172, 786–807. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde, A. C. , Zanolie, K. , Rombouts, S. A. , Raijmakers, M. E. , & Crone, E. A. (2008). Evaluating the negative or valuing the positive? Neural mechanisms supporting feedback‐based learning across development. The Journal of Neuroscience, 28, 9495–9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, F. , Aslan, A. , Zhang, J. , Gerchen, M. F. , Kiefer, F. , & Kirsch, P. (2020). Using mind control to modify cue‐reactivity in AUD: The impact of mindfulness‐based relapse prevention on real‐time fMRI neurofeedback to modify cue‐reactivity in alcohol use disorder: A randomized controlled trial. BMC Psychiatry, 20, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, P. , Dai, Z. , Zhong, J. , Zhu, Y. , Shi, H. , & Pan, P. (2015). Regional gray matter deficits in alcohol dependence: A meta‐analysis of voxel‐based morphometry studies. Drug and Alcohol Dependence, 153, 22–28. [DOI] [PubMed] [Google Scholar]
- Yao, S. , Becker, B. , Geng, Y. , Zhao, Z. , Xu, X. , Zhao, W. , … Kendrick, K. M. (2016). Voluntary control of anterior insula and its functional connections is feedback‐independent and increases pain empathy. NeuroImage, 130, 230–240. [DOI] [PubMed] [Google Scholar]
- Yin, H. H. , & Knowlton, B. J. (2006). The role of the basal ganglia in habit formation. Nature Reviews. Neuroscience, 7, 464–476. [DOI] [PubMed] [Google Scholar]
- Yin, H. H. , Mulcare, S. P. , Hilario, M. R. , Clouse, E. , Holloway, T. , Davis, M. I. , … Costa, R. M. (2009). Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nature Neuroscience, 12, 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, K. D. , Siegle, G. J. , Zotev, V. , Phillips, R. , Misaki, M. , Yuan, H. , … Bodurka, J. (2017). Randomized clinical trial of real‐time fMRI amygdala neurofeedback for major depressive disorder: Effects on symptoms and autobiographical memory recall. The American Journal of Psychiatry, 174, 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, K. D. , Zotev, V. , Phillips, R. , Misaki, M. , Yuan, H. , Drevets, W. C. , & Bodurka, J. (2014). Real‐time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One, 9, e88785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z. , Yao, S. , Li, K. , Sindermann, C. , Zhou, F. , Zhao, W. , … Becker, B. (2019). Real‐time functional connectivity‐informed Neurofeedback of amygdala‐frontal pathways reduces anxiety. Psychotherapy and Psychosomatics, 88, 5–15. [DOI] [PubMed] [Google Scholar]
- Zilverstand, A. , Sorger, B. , Sarkheil, P. , & Goebel, R. (2015). fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Frontiers in Behavioral Neuroscience, 9, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand, A. , Sorger, B. , Slaats‐Willemse, D. , Kan, C. C. , Goebel, R. , & Buitelaar, J. K. (2017). fMRI Neurofeedback training for increasing anterior cingulate cortex activation in adult attention deficit hyperactivity disorder. An exploratory randomized, single‐blinded study. PLoS One, 12, e0170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerings, J. , Hummel, B. , Keller, M. , Zvyagintsev, M. , Schneider, F. , Klasen, M. , & Mathiak, K. (2019). Neurofeedback of core language network nodes modulates connectivity with the default‐mode network: A double‐blind fMRI neurofeedback study on auditory verbal hallucinations. NeuroImage, 189, 533–542. [DOI] [PubMed] [Google Scholar]
- Zweerings, J. , Pflieger, E. M. , Mathiak, K. A. , Zvyagintsev, M. , Kacela, A. , Flatten, G. , & Mathiak, K. (2018). Impaired voluntary control in PTSD: Probing self‐regulation of the ACC with real‐time fMRI. Frontiers in Psychiatry, 9, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The four regions of interest that were used in small volume correction overlaid on a MNI brain in different colors (see legends at bottom right corner). The striatum is further broken down into ventral and dorsal sections for illustration purpose.
Figure S2. Brain regions showed negative connectivity with the putamen region that predicted learning success. The statistical map is corrected for family‐wise errors at cluster level (pFWE < 0.05, k > 68 at height threshold of p < 0.001)
Table S1. Brain regions showed positive correlation with learning success at uncorrected threshold
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.


