Abstract
Although fibrotic disorders are frequently assumed to be linked to TH2 cells, quantitative tissue interrogation studies have rarely been performed to establish this link, and certainly many fibrotic diseases do not fall within the Type 2/allergic disease spectrum. We have previously linked two human autoimmune fibrotic diseases, IgG4-related disease and systemic sclerosis to the clonal expansion and lesional accumulation of cytotoxic CD4+ T cells. In both these diseases TH2 cell accumulation was found to be sparse. Fibrosing mediastinitis linked to Histoplasma capsulatum infection histologically resembles IgG4-related disease in terms of the inflammatory infiltrate and fibrosis and it provides an example of a fibrotic disease of infectious origin in which the potentially pro-fibrotic T cells may be induced and re-activated by fungal antigens. We show here that in this human disease, CD4+cytotoxic T cells accumulate in the blood, are clonally expanded, infiltrate into disease lesions and can be re-activated in vitro by H. capsulatum antigens. TH2 cells are relatively sparse at lesional sites. These studies support a general role for CD4+ cytotoxic T cells in inflammatory fibrosis and suggest that fibrosing mediastinitis is an antigen-driven disease that may provide important mechanistic insights into the pathogenesis of idiopathic fibrotic diseases.
Introduction
Fibrosis occurs in the context of autoimmune, allergic and infectious diseases, and is also prominent in some cancers. In most chronic fibrotic inflammatory diseases, including fibrosing mediastinitis (FM), the underlying mechanism of fibrosis is poorly understood (1–4). FM histologically resembles IgG4 related disease (IgG4-RD), a chronic inflammatory disorder with characteristic tumescent lesions that exhibit storiform fibrosis and are infiltrated by activated T and B lymphocytes with an abundance of IgG4 expressing plasma cells and/or plasmablasts (5). In some of our recent studies on IgG4-RD, we have established that cytotoxic CD4+ T cells (CD4+CTLs) are clonally expanded in the blood, enter tissue sites where they secrete pro-fibrotic cytokines including IL-1β and TGF-β, and may contribute to apoptosis followed by overexuberant repair processes (6–8). TH2 cells are relatively sparse and are not clonally expanded in active IgG4-RD (6). In addition, we have also observed clonal expansions of activated B cells, including plasmablasts, that infiltrate disease tissues, interact with CD4+ T cells and secrete pro-fibrotic products (9–11). More recently we have established that in systemic sclerosis tissues as well, TH2 cells are relatively sparse and CD4+CTLs are the dominant CD4+ T cells in tissue lesions suggesting that broadly similar pathogenic mechanisms may drive these two distinct autoimmune fibrotic diseases (12–14). In systemic sclerosis, prominent apoptosis of endothelial cells and subsequent overexuberant tissue remodeling likely results in fibrosis, but the specific antigenic peptides that are recognized by CD4+CTLs remain to be identified (12). It is therefore likely that the process of inflammatory fibrosis in IgG4-RD, systemic sclerosis and possibly other autoimmune and infection-linked fibrotic disorders may be linked to the expansion of antigen-specific CD4+CTLs that induce apoptosis and secrete pro-fibrotic products. Although many descriptions of the underlying basis of fibrosis invariably mention TH2 cells, the role of TH2 cells in fibrosis may be restricted to a relatively limited spectrum of disorders.
A number of autoantibodies have been described in IgG4-RD (15–18) but while the antigen or antigens recognized by CD4+CTLs in this disease are likely to be self-antigens, no specific antigen that triggers these expanded T cells has so far been identified in patients with IgG4-RD. Similarly, while specific autoantibodies have long been described in systemic sclerosis, antigens that drive T cells in this disease have not been characterized (13). Confounding this issue, CD4+CTL expansions have been associated with ubiquitous human viral infections such as EBV and CMV; one of the reasons for our studying FM is to ask whether indeed CD4+ CTLs are abundant in this disorder and if fungal antigens can activate CD4+ CTLs in this disease.
Despite being the rarest manifestation of Histoplasma capsulatum infection, FM is arguably its most severe presentation. Mortality is very high in the twenty percent of FM patients in whom vessels of both lungs are affected (19). Histoplasma capsulatum-associated FM represents the vast majority of FM cases in North America (20), and presumably results from an excessive fibroinflammatory response to H. capsulatum antigen in the vicinity of mediastinal lymph nodes and surrounding mediastinal tissues. As dictated by the geographic distribution of this dimorphic fungus, the original infection is acquired in the central portions of the United States, but progression of fibrosis is slow and silent for a long period (5–10 years) before vascular or airway stenosis reaches the critical levels that cause symptoms, which happen wherever the patient resides at that time (3).
The cellular and molecular mechanisms leading to FM development are currently poorly understood. H. capsulatum generally replicates in macrophages, typically induces a TH1 type response in immunocompetent individuals and is cleared. Dominant TH2 cells and regulatory T cells expansion are known to impede clearance (2). It is understood that, following H. capsulatum inhalation, in most individuals, this dimorphic fungus disseminates to the mediastinal lymph nodes and then throughout the body, as commonly evidenced by multiple splenic microcalcifications. H. capsulatum is unique, as remnants of cell wall persist indefinitely in the lungs and mediastinal lymph nodes in most persons after infection, and it is the only organism shown to cause FM in the US, so the hypothesis is that seepage of fungal antigens into the mediastinum trigger a hypersensitivity reaction coupled to a local inflammatory response and exuberant fibrosis in genetically predisposed individuals (21). Collagen deposition by activated fibroblasts which invade inside critical vessels or airways leads to total obstruction, with reduced blood flow or airflow that is the basis for the various clinical manifestations of FM (20).
Despite the strong association with H. capsulatum, the fungus itself is rarely grown from FM tissues so the organism is likely dead, and in endemic areas the fungal link is established by serologic testing and pathognomonic clinical features. Adaptive immune involvement in the pathophysiology of FM has been suggested by the abundance of CD20+ B cells infiltrating the lesion (3). Those B cells surround areas of fibrosis or form poorly organized secondary lymphoid structures without germinal centers. The lesions are also marked by an abundance of both CD3+ CD8+ and CD3+ CD8- T cells, the latter most likely representing CD4+ T cells. Overall the similarities to IgG4-RD (and systemic sclerosis) are quite striking. FM linked to H. capsulatum may therefore provide us with an infectious “model”, with defined antigens, that has the potential to further our understanding of idiopathic inflammatory fibrotic disorders such as IgG4-RD and systemic sclerosis.
In this study we have demonstrated that CD4+CTLs are expanded in the blood of patients with FM and that these cells also infiltrate disease lesions. The CD4+CD45RO+CD27-SLAMF7+CD28loCD57hi effector population of CD4+CTLs in the blood is clonally expanded. Purified effector CD4+CTLs can be reactivated in vitro following exposure to H. capsulatum antigens, suggesting that fungal-specific CD4+CTLs likely contribute to the pathogenesis of fibrosing mediastinitis.
Material and methods
Patient Cohorts
Patients with FM were evaluated and diagnosed at Vanderbilt University and anticoagulated blood was shipped to Boston where PBMCs were isolated by Ficoll and preserved in gas-phase liquid nitrogen until the time of use. Mediastinum biopsies were performed at Vanderbilt University and paraformaldehyde-fixed paraffin-embedded tissue slides were shipped to Boston for staining and analysis. Blood from healthy donors was obtained at Massachusetts General Hospital. Healthy donors were defined by lacking any current or prior history of malignancy, autoimmune disease or recurrent/chronic infections. Clinical and demographic data on all subjects used for these studies is displayed in Table S1 & S2. Data on sex, age, ethnicity, clinical manifestations and H. capsulatum serology were extracted from the medical records of all patients.
Study Approval
These studies were approved by the Institutional Review Boards at the Massachusetts General Hospital and Vanderbilt University School of Medicine. All patients provided written informed consent prior to inclusion in the study.
Flow cytometry and sorting
Aliquot of frozen PBMCs were rapidly thawed and washed twice in complete DMEM. To avoid non-specific binding of the antibodies used in the staining procedure, Fc receptors were blocked using Human TruStain FcX (BioLegend, 422302) following manufacturer recommendations. Cells were then stained for 30 minutes on ice and protected from light to minimize any photobleaching. T cells immunophenotype was assessed by staining (10 million cells/mL) using the following antibody panel (manufacturer, clone, concentration used): anti-human CD3-BUV395 (BD Biosciences, Clone SK7, 1:40), anti-human CD45RO-APC-Cy7 (BioLegend, Clone UCHL1, 1:300), CD27-BV510 (Biolegend, Clone M-T271, 1:20), (CD4-BUV805 (BD Biosciences, Clone SK3, 1:80), anti-human SLAMF7-AF648 (BD Biosciences, Clone 235614, 1:10), CD28-PerCP-Cy5,5 (Biolegend, clone CD28.2, 1:20), CD57-PE (Biolegend, Clone NK-1, 1:100). After 30 minutes in the staining buffer at 4 degrees, cells were washed 3 times with cold 1% BSA in PBS and resuspended in 500μl for analysis. Immediately prior to flow cytometry, cells were further stained with SYTOX AADvanced Dead Cell Stain (Thermo Fisher Scientific, S10274) to exclude dead cell. Flow cytometry was then performed on a BD LSRFortessa (BD Biosciences, San Jose, CA). Rainbow calibration particles (8 peaks) were used to monitor instrument performances and ensure consistency between analysis. The FCS files generated were analyzed using FlowJo software (version 10.6).
Multi-color immunofluorescence staining
Tissue samples were fixed in formalin, embedded in paraffin, and sectioned. These specimens were incubated with antibodies: CD4 (clone: CM153A; Biocare), GATA3 (clone: CM405A; Biocare) and GZMA (clone: LS-C312742; LSBio) followed by incubation with secondary antibody using an OpalTM Multiplex Kit (Perkin Elmer). The samples were mounted with ProLong™ Diamond Antifade mountant containing DAPI (Invitrogen).
Microscopy and Quantitative Image Analysis
Images of the tissue specimens were acquired using the TissueFAXS platform (TissueGnostics). For quantitative analysis, the entire area of the tissue was acquired as digital grayscale images in five channels with filter settings for FITC, Cy3 and Cy5 in addition to DAPI. Cells of a given phenotype were identified and quantitated using the TissueQuest software (TissueGnostics), with cut-off values determined relative to the positive controls. This microscopy-based multicolor tissue cytometry software permits multicolor analysis of single cells within tissue sections similar to flow cytometry. The principle of the method and the algorithms used have been described in detail elsewhere (22).
TCR Repertoire Studies
In TCR repertoire studies, PBMC were handled and stained exactly as for the flow cytometry experiments, above. CD4+CTLs were defined using the following markers: DUMP(CD20-CD8)- CD3+ CD4+ CD45RO+ CD27- SLAMF7+. CD4+CTLs were further divided using CD28 and CD57 to delineate “effector” and a “memory like” phenotype within the CD4+CTLs. Our previous studies have indeed showed that CD28loCD57hi CD4+ CTLs exhibit increased markers of cytotoxicity while CD28hiCD57lo CD4+ CTLs harbor a phenotype resembling memory CD4+ T cells (26). Cells were sorted directly into 350 μL of RLT-BME lysis buffer, vortexed briefly and flash-frozen on dry ice and kept at −80C until RNA extraction. Cells were sorted on a FACSAria II operating with FACSDiva version 7. RNA isolation was performed from lysates using RNeasy Plus Micro Kits (Qiagen, 74034 processed through 5’RACE RT-PCR, as previously described (23) For gene-specific amplification of the TCRβ gene, the following reverse primer was used: 5’ TGCTTCTGATGGCTCAAACACAGCGACCT 3’.
Antigens
The antigen used for this study was a cell wall/cell membrane extract of the G217B strain of H. capsulatum. Preparation of this extract has been described elsewhere (24). For in vitro stimulation, the final concentration in a well was 1 μg/ml.
Activation of CD4+CTLs
Fresh PBMCs from FM patient and healthy controls were plated in a 96 well plate at a density of 1 million cells per ml. Cells were incubated in the presence of CD40L blocking antibody and either vehicle, anti-CD3/CD28 beads 1:500 or the H. Capsulatum antigen mixture at a dilution of 1 μg/ml for 24h. Cells were then washed and stained to assay for the induction of various activation markers. Briefly, to detect activation of CD4+CTLs, the flow cytometry panel was performed with the following additional markers: CD69-BV421 (Biolegend, Clone FN50, 1:20), CD25-BV515 (BD Pharmingen, clone 2A3, 1:20); CD40L-Pe-Dazzle 594 (Biolegend, clone 24–31, 1:20), OX40-BV711 (BD Pharmingen, clone ACT35, 1:20). Results were analyzed using Fluorescence minus one control for every activation marker and a healthy control.
Statistics
Flow cytometry and clinical correlations.
GraphPad Prism version 8 was used for statistical analysis, curve fitting and linear regression. A two-tailed Mann-Whitney U test was used to calculate p-values for continuous, non-parametric variables and a p-value of < 0.05 was considered significant.
Results
Expansions of CD4+CTLs in the peripheral blood of FM patients
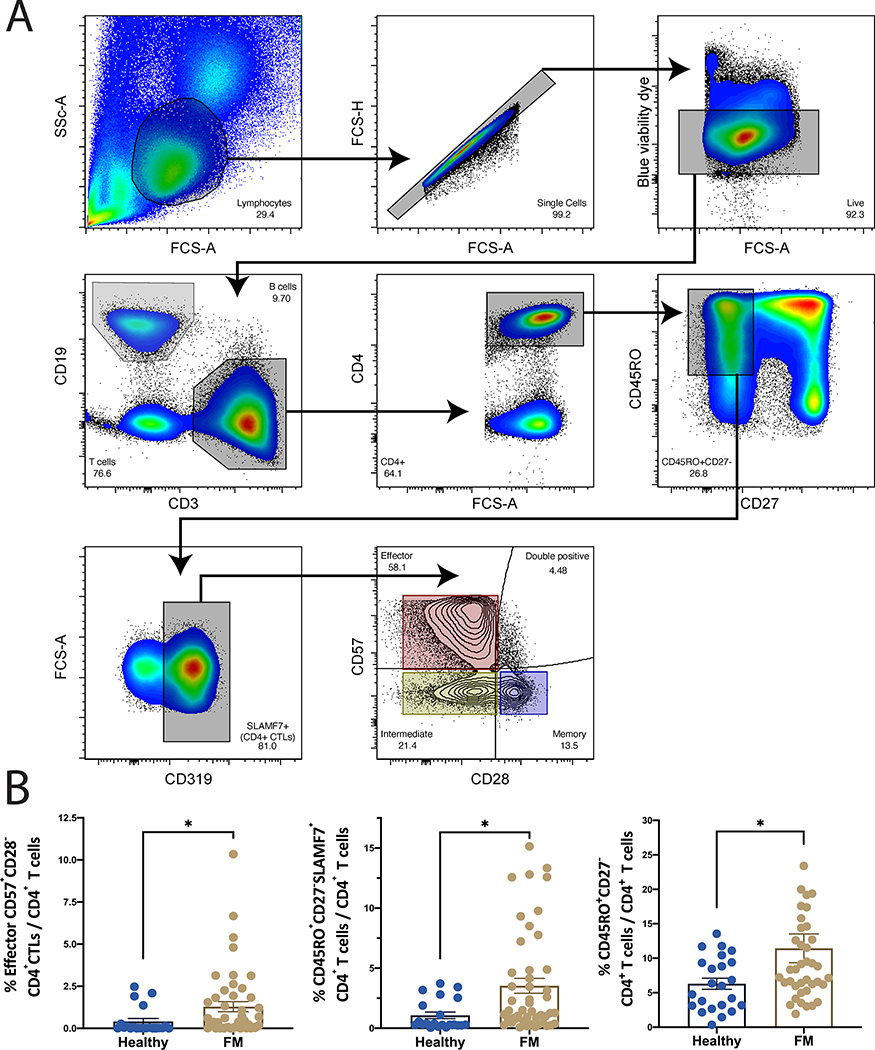
Based on our earlier studies on IgG4-RD, we quantified circulating CD4+CTLs by gating on SLAMF7+ CD4+ TEM cells as defined by CD27- CD45RO+ in PBMCs from both FM patients (n=39) and healthy controls (n= 24). Subsets of CD4+CTLs were additionally determined based on surface expression of CD28 and CD57 (Figure 1A) (25, 26). We observed expansions of total CD4+ TEM cells, CD4+CTLs and specifically, of CD28lo CD57hi CD4+CTLs in the blood of FM patients (Figure 1B). The CD28lhiCD57lo CD4+CTL memory subset was decreased in the blood of FM patients while the intermediate (CD28loCD57lo) and unspecified (CD28hiCD57hi) CD4+CTLs did not vary significantly (Supplemental Figure 1).
Figure 1. CD4+CTLs are expanded in fibrosing mediastinitis.
(A) Flow cytometry gating strategy to identify effector CD4+ T cells (CD4+CD45Ro+CD27-), CD4+CTLs (CD4+CD45Ro+CD27-SLAMF7+), and effector CD4+CTLs (CD4+CD45Ro+CD27-SLAMF7+CD28loCD57hi) (top). (B) Comparison of the frequency of effector CD4+ T cells (right), CD4+CTLs (center), and effector CD4+CTLs (left). Quantified as % of total CD4+ T cells and expressed as means +/− SE (Control n=21, FM n= 47). *P<0.05 by two-tailed Mann-Whitney t-test.
Clonal expansion of Effector CD4+CTLs
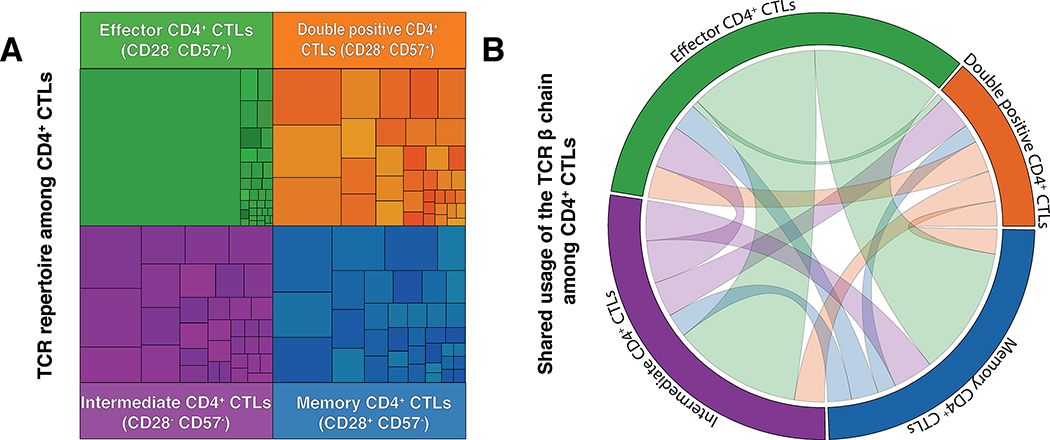
Next Generation Sequencing of the rearranged T cell receptor β chain gene was undertaken on the four different subsets of CD4+ SLAMF7+ T cells based on the expression of CD28 and CD57 (Figure 2A). We identified large clonal expansions among the effector CD28lo CD57hi CD4+CTL subset in FM patients indicating a dominant response of these T cells to some defined antigen. Moreover, effector CD4+CTLs showed a high degree of clonal connectivity with the remaining CD4+CTLs (CD28loCD57lo, CD28hiCD57hi and CD28hiCD57lo) but with markedly increased clonal expansion with the top 5 clones accounting for more than 90% of the total clonal populations (Figure 2B). Among the 5 FM patients whose CD4+CTLs were studied by TCR repertoire, we observed no shared Vβ gene usage in the dominantly expanded clones across these patients. In contrast to the CD28loCD57hi effector CD4+CTLs, the other subsets of CD4+SLAMF7+ T cells demonstrated a low degree of clonal expansion. TCRβ sequence and distribution from a representative patient are provided in Table S3.
Figure 2. CD28- CD57+ CD4+ CTLs are clonally restricted in fibrosing mediastinitis.
(A) Analysis of the diversity of the TCR β usage among the 4 subsets of CD4+CTLs. The surface of each square in treemap graph represent the relative frequency of the use of a single TCR β chain in the population. (B) Shared usage of the TCR β chain between the different populations of CD4+CTLs. In order to assess the phylogeny of the different T cells subsets, we looked at the frequency of shared clones expressed in each CD4+ CTLs subset (Effector[Green], Intermediate[Purple], Unspecified[Orange] and Memory[Blue]) using a circos plot. The shared TCR β clonotype are illustrated by a ribbon linking the 2 subsets in which the clone was detected. The thickness of the ribbon represents the frequency of the shared clonotype between the various subsets of CD4 CTLs.
(n = 5; representative patient shown).
Infiltration of FM tissues by CD4+CTLs
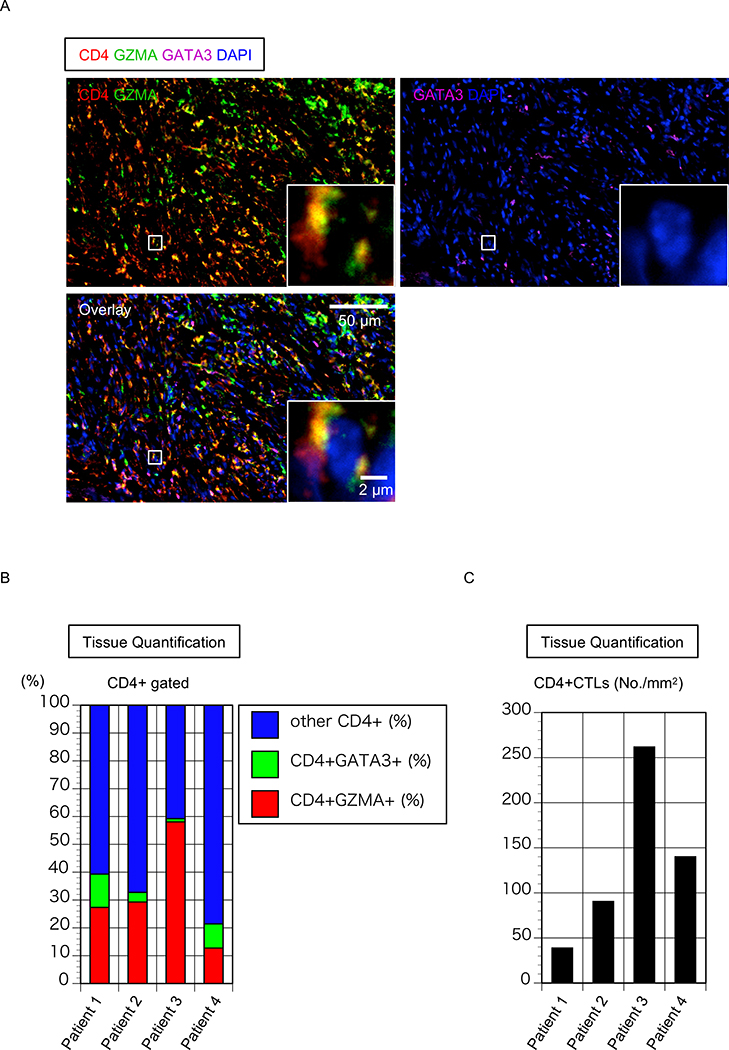
Our previous studies have suggested that the fibrosis in IgG4-RD and SSc occurs in a non-TH2 driven inflammatory rather than allergic context and that CD4+CTLs are a prominent component of the inflammatory infiltrate but TH2 cells are not. We used antigenicity restoration and multi-color immunofluorescence on formalin fixed paraffin embedded tissue sections from 4 FM patients and quantitated CD4+GATA3+ TH2 cells and Granzyme-A (GZMA) +CD4+CTLs in each patient. An example of the staining performed is depicted in Figure 3A.
Figure 3. CD4+CTLs are an important part of the CD4+ T cells infiltrating tissue from patient with FM.
(A) Representative multicolor immunofluorescence image of cells co-expressing CD4 (red) and Granzyme A (GZMA)(green) that infiltrate the mediastinum in FM. (B) Relative proportions of GZMA+CD4+CTLs, GATA3+TH2 cells and other cells (Each column represents a patient; n = 4). (C) Absolute number of GZMA+CD4+CTLs in mediastinal biopsy of 4 patients with FM.
Nuclei were stained with DAPI (Blue) CD4 was in red, GZMA was green and GATA3 was purple. The inset shows a single CD4+CTL with extranuclear CD4 and GZMA.
Paralleling our results in IgG4-RD and systemic sclerosis, in FM tissues CD4+CTLs were more prominent than TH2 cells (31.86 ± 18.97% vs 6.33 ± 4.9% of total T cells) (Figures 3B and C).
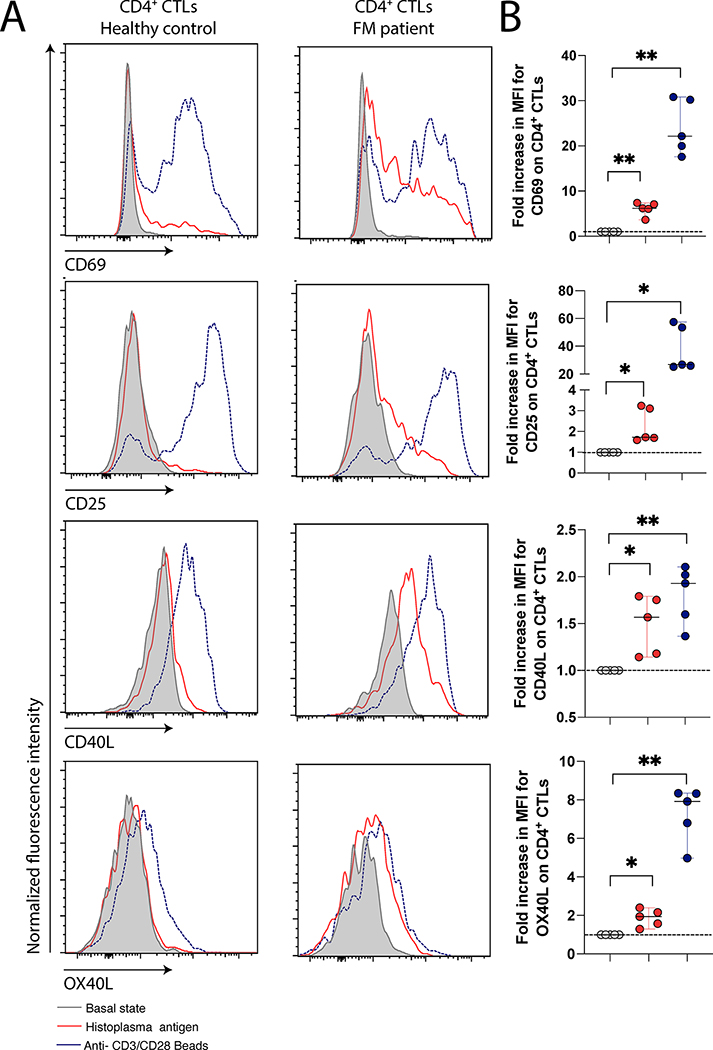
Effector CD4+ CTLs are specific for Histoplasma capsulatum antigens
PBMCs from healthy controls and from patients with FM were either left untreated or exposed to anti-CD3 and anti-CD28 beads (as a positive control) or exposed to H. capsulatum antigens at a concentration of 1μg/ml for 24 hours. CD4+ CTLs were gated on and levels of makers of recent activation (CD69, CD25, CD40L and OX40L) subsequently compared. As seen in Figures 4A and B, a large proportion of the CD4+CTLs in FM patients responded to H. capsulatum antigens and were activated. The response was minimal in healthy controls although their CD4+CTLs were responsive to the non-specific anti-CD3 and anti-CD28 stimulation. Response to H. capsulatum was further objectively measured in five patients by assessing the variation of the median fluorescence intensity difference between CD4+CTLs exposed to vehicle or H. capsulatum antigen (Fig 4B). Significant increase in the expression of the early activation markers CD69 (p=0.0046), CD25 (p=0.0157), CD40L (p=0.0201) and OX40L (p=0.0065), were observed.
Figure 4. CD4+CTLs from fibrosing mediastinitis can be activated by Histoplasma capsulatum antigens.
Activation of T cells by exposure to H. capsulatum antigen. (A) Comparison of the T cells activation profile difference in patient with fibrosing mediastinitis and heathy controls. (B) Quantification of the increase in median fluorescence intensity after antigen exposure in FM subjects. *P<0.05; *P<0.01 by two-tailed Mann-Whitney t-test. (n=5)
Discussion
Convincing evidence exists for the role of TH2 type immune responses in fibrosis caused by nematodes such as Schistosoma mansoni (28), and clearly TH2 cells may be relevant to some infections that result in fibrosis. However, there are numerous infectious diseases that result in fibrosis and these include many diseases like tuberculosis, amebiasis and some fungal infections in which Type 2 immune responses are not known to be prominent. Our studies on IgG4-RD and systemic sclerosis have provided support for non-Type 2 adaptive immune responses that drive autoimmune fibrosis (6, 26, 27). In many infections, as in autoimmune fibrotic conditions, induction of cell death at sites of infections by CD4+CTLs possibly sustained by activated pathogenic B cells may result in apoptosis, overexuberant tissue remodeling and organ damage resulting from the ensuing fibrosis. CD28- CD4+CTLs are considered especially active among CD4+CTLs subsets as they exhibit increased markers of cytotoxicity (Granzyme A and perforin) (6, 26). This report therefore raises two important issue relevant to inflammatory fibrotic diseases. It suggests that many fibrotic diseases both of autoimmune and infectious origin may be driven by CD4+CTLs and not by TH2 cells. It also provides evidence for antigen-specific CD4+CTLs in the context of any fibrotic inflammatory disease.
Very little is known about the pathogenesis of FM beyond the strong link to. H capsulatum. FM is an important clinical problem because it has high mortality, especially when both lungs are affected. Based on our current understanding of IgG4-RD and systemic sclerosis we surmise that FM may be driven by H.capsulatum specific CD4+CTLs and (possibly CD8+ CTLs as well), which recognize H. capsulatum antigens possibly on macrophages and inflamed stromal cells. These activated T cells may induce apoptosis in the mediastinal space as well and also contribute to overexuberant tissue remodeling and activation of macrophages and myofibroblasts. Activated B cells likely also play some role in reactivating CD4+CTLs at tissue sites as they could display peptides on HLA class II molecules (6, 13) and may themselves secrete pro-fibrogenic molecules like LOXL2 and PDGF (11, 13, 14).
The studies described here have focused on CD4+CTLs in FM and have established that at least some of these CD4+CTLs were induced by H. Capsulatum antigens. Given that there is a known linkage of this disease to HLA-DQβ1*0402 (29), future studies may focus on identifying the specific H.capsulatum antigenic peptide/s that are presented on this MHC class II molecule in order to potentially generate and activate CD4+CTLs. However, this HLA association was modest and based on a relatively small sample size, so it likely that in any population susceptibility may be linked to a number of HLA class II alleles. We will also seek to more thoroughly dissect all infiltrating adaptive and innate immune cells and to explore the contributions of CD8+ T cells and especially B cells since, as for IgG4-RD, there is hope that B cell depletion might contribute to clinical improvement in FM (30).
Supplementary Material
Key Points:
In Fibrosing mediastinitis CD4+cytotoxic T cells accumulate in lesions
CD4+CTLs in the blood of patients respond to H.capsulatum antigens in vitro
Acknowledgments
This work was supported by National Institutes of Health Grant U19 AI 110495 to SP
References
- 1.Strock SB, Mason W, and Loyd JE. 2015. Further Progress in Understanding Fibrosing Mediastinitis. Am J Respir Crit Care Med 192:767–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horwath MC, Fecher RA, and Deepe GS Jr. 2015. Histoplasma capsulatum, lung infection and immunity. Future Microbiol 10:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peikert T, Colby TV, Midthun DE, Pairolero PC, Edell ES, Schroeder DR, and Specks U. 2011. Fibrosing mediastinitis: clinical presentation, therapeutic outcomes, and adaptive immune response. Medicine (Baltimore) 90:412–423. [DOI] [PubMed] [Google Scholar]
- 4.Peikert T, Shrestha B, Aubry MC, Colby TV, Ryu JH, Sekiguchi H, Smyrk TC, Specks U, and Yi ES. 2012. Histopathologic Overlap between Fibrosing Mediastinitis and IgG4-Related Disease. Int J Rheumatol 2012:207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamisawa T, Zen Y, Pillai S, and Stone JH. 2015. IgG4-related disease. Lancet 385:1460–1471. [DOI] [PubMed] [Google Scholar]
- 6.Mattoo H, Mahajan VS, Maehara T, Deshpande V, Della-Torre E, Wallace ZS, Kulikova M, Drijvers JM, Daccache J, Carruthers MN, Castelino FV, Stone JR, Stone JH, and Pillai S. 2016. Clonal expansion of CD4(+) cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol 138:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maehara T, Mattoo H, Ohta M, Mahajan VS, Moriyama M, Yamauchi M, Drijvers J, Nakamura S, Stone JH, and Pillai S. 2017. Lesional CD4+ IFN-gamma+ cytotoxic T lymphocytes in IgG4-related dacryoadenitis and sialoadenitis. Ann Rheum Dis 76:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Della-Torre E, Bozzalla-Cassione E, Sciorati C, Ruggiero E, Lanzillotta M, Bonfiglio S, Mattoo H, Perugino CA, Bozzolo E, Rovati L, Arcidiacono PG, Balzano G, Lazarevic D, Bonini C, Falconi M, Stone JH, Dagna L, Pillai S, and Manfredi AA. 2018. A CD8alpha- Subset of CD4+SLAMF7+ Cytotoxic T Cells Is Expanded in Patients with IgG4-Related Disease and Decreases Following Glucocorticoid Treatment. Arthritis Rheumatol 70:1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattoo H, Mahajan VS, Della-Torre E, Sekigami Y, Carruthers M, Wallace ZS, Deshpande V, Stone JH, and Pillai S. 2014. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol 134:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maehara T, Mattoo H, Mahajan VS, Murphy SJ, Yuen GJ, Ishiguro N, Ohta M, Moriyama M, Saeki T, Yamamoto H, Yamauchi M, Daccache J, Kiyoshima T, Nakamura S, Stone JH, and Pillai S. 2018. The expansion in lymphoid organs of IL-4(+) BATF(+) T follicular helper cells is linked to IgG4 class switching in vivo. Life Sci Alliance 1: doi: 10.26508/lsa.201800050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Della-Torre E, Rigamonti E, Perugino C, Baghai-Sain S, Sun N, Kaneko N, Maehara T, Rovati L, Ponzoni M, Milani R, Lanzillotta M, Mahajan V, Mattoo H, Molineris I, Deshpande V, Stone JH, Falconi M, Manfredi AA, and Pillai S. 2019. B lymphocytes directly contribute to tissue fibrosis in patients with IgG4-related disease. J Allergy Clin Immunol 145: 968–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maehara T, Kaneko N, Perugino CA, Mattoo H, Kers J, Allard-Chamard H, Mahajan VS, Liu H, Murphy SJ, Ghebremichael M, Fox DA, Payne AS, Lafyatis R, Stone JH, Khanna D, and Pillai S. 2020. Cytotoxic CD4+ T lymphocytes may induce endothelial cell apoptosis in systemic sclerosis. J Clin Invest doi: 10.1172/JCI131700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillai S 2019. T and B lymphocytes in fibrosis and systemic sclerosis. Curr Opin Rheumatol 31:576–581. [DOI] [PubMed] [Google Scholar]
- 14.Pillai S, Perugino C, and Kaneko N. 2020. Immune mechanisms of fibrosis and inflammation in IgG4-related disease. Curr Opin Rheumatol 32:146–151. [DOI] [PubMed] [Google Scholar]
- 15.Hubers LM, Vos H, Schuurman AR, Erken R, Oude Elferink RP, Burgering B, van de Graaf SFJ, and Beuers U. 2018. Annexin A11 is targeted by IgG4 and IgG1 autoantibodies in IgG4-related disease. Gut 67:728–735. [DOI] [PubMed] [Google Scholar]
- 16.Perugino CA, AlSalem SB, Mattoo H, Della-Torre E, Mahajan V, Ganesh G, Allard-Chamard H, Wallace Z, Montesi SB, Kreuzer J, Haas W, Stone JH, and Pillai S. 2019. Identification of galectin-3 as an autoantigen in patients with IgG4-related disease. J Allergy Clin Immunol 143:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiokawa M, Kodama Y, Sekiguchi K, Kuwada T, Tomono T, Kuriyama K, Yamazaki H, Morita T, Marui S, Sogabe Y, Kakiuchi N, Matsumori T, Mima A, Nishikawa Y, Ueda T, Tsuda M, Yamauchi Y, Sakuma Y, Maruno T, Uza N, Tsuruyama T, Mimori T, Seno H, and Chiba T. 2018. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci Transl Med 10: [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Perugino CA, Ghebremichael M, Wallace ZS, Montesi SB, Stone JH, and Pillai S. 2019. Disease severity is linked to an increase in autoantibody diversity in IgG4-related disease. Arthritis Rheumatol 72, 687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponamgi SP, DeSimone CV, Lenz CJ, Coylewright M, Asirvatham SJ, Holmes DR, and Packer DL. 2015. Catheter-based intervention for pulmonary vein stenosis due to fibrosing mediastinitis: The Mayo Clinic experience. Int J Cardiol Heart Vasc 8:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loyd JE, Tillman BF, Atkinson JB, and Des Prez RM. 1988. Mediastinal fibrosis complicating histoplasmosis. Medicine (Baltimore) 67:295–310. [DOI] [PubMed] [Google Scholar]
- 21.Mathisen DJ, and Grillo HC 1992. Clinical manifestation of mediastinal fibrosis and histoplasmosis. Ann Thorac Surg 54: 1053–1057. [DOI] [PubMed] [Google Scholar]
- 22.Ecker RC, and Steiner GE. 2004. Microscopy-based multicolor tissue cytometry at the single-cell level. Cytometry A 59:182–190. [DOI] [PubMed] [Google Scholar]
- 23.Britanova OV, Shugay M, Merzlyak EM, Staroverov DB, Putintseva EV, Turchaninova MA, Mamedov IZ, Pogorelyy MV, Bolotin DA, Izraelson M, Davydov AN, Egorov ES, Kasatskaya SA, Rebrikov DV, Lukyanov S, and Chudakov DM. 2016. Dynamics of Individual T Cell Repertoires: From Cord Blood to Centenarians. J Immunol 196:5005–5013. [DOI] [PubMed] [Google Scholar]
- 24.Gomez AM, Rhodes JC, and Deepe GS Jr. 1991. Antigenicity and immunogenicity of an extract from the cell wall and cell membrane of Histoplasma capsulatum yeast cells. Infect Immun 59:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patil VS, Madrigal A, Schmiedel BJ, Clarke J, O’Rourke P, de Silva AD, Harris E, Peters B, Seumois G, Weiskopf D, Sette A, and Vijayanand P. 2018. Precursors of human CD4(+) cytotoxic T lymphocytes identified by single-cell transcriptome analysis. Sci Immunol 3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perugino CA, Kaneko N, Maehara T, Mattoo H, Kers J, Allard-Chamard H, Mahajan VS, Liu H, Della-Torre E, Murphy SJH, Ghebremichael M, Wallace ZS, Bolster MB, Harvey LM, Mylvaganam G, Tuncay Y, Liang L, Montesis SB, Zhang X, Tinju A, Mochizuki K, Munemura R, Sakamoto M, Moriyama M, Nakamura S, Yosef N, Stone JH JH, and Pillai S. 2020. CD4(+) and CD8(+) cytotoxic T lymphocytes may induce mesenchymal cell apoptosis in IgG4-related disease. J Allergy Clin Immunol. S0091–6749(20)30741–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maehara T, Kaneko N, Perugino CA, Mattoo H, Kers J, Allard-Chamard H, Mahajan VS, Liu H, Murphy SJH, Ghebremichael M, Fox D, Payne AS, Lafyatis R, Stone JH, Khanna D, and Pillai S. 2020CD4+ cytotoxic T lymphocytes and apoptosis of HLA-DR expressing endothelial cells in systemic sclerosis J Clin Invest. 130: 2451–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wynn TA 2004. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 4:583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strock SB, Gaudieri S, Mallal S, Yu C, Mitchell D, Cogan J, Mason W, Crowe D, and Loyd JE. 2015. Fibrosing mediastinitis complicating prior histoplasmosis is associated with human leukocyte antigen DQB1*04:02 - a case control study. BMC Infect Dis 15:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerly BD, Johnson GB, Maldonado F, Utz JP, Specks U, and Peikert T. 2014. Targeting B lymphocytes in progressive fibrosing mediastinitis. Am J Respir Crit Care Med 190:1069–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.