Abstract
BACKGROUND AND PURPOSE: Widespread cerebral changes are observed in advanced stages of Parkinson disease (PD), suggesting that PD is a multisystem disorder. We investigated with MR imaging whether global brain changes are present in early clinical stages of PD and correlated the findings with the type of clinical presentation.
MATERIALS AND METHODS: T1-weighted images and mean diffusivity and fractional anisotropy (FA) maps calculated from diffusion tensor imaging (DTI) were obtained in 27 patients with de novo drug-naïve PD, who were classified according to the clinical features in tremor-dominant type (n = 13), akinetic-rigid type (n = 11), and mixed type (n = 3). Sixteen healthy subjects provided control data. With SIENAX software, total brain, gray matter (GM), and white matter (WM) volumes were computed from T1-weighted images, whereas brain histograms were obtained from mean diffusivity and FA maps.
RESULTS: Total brain, GM and WM volumes were not significantly different in patients as a whole or subgroups and controls. As compared with controls, patients with PD as a whole and patients with the akinetic-rigid type showed an increase (P ≤ .01) of the twenty-fifth percentile of the FA histogram. In patients with the akinetic-rigid type, there also was a trend toward an increase of the mean and fiftieth and seventy-fifth percentiles, and a reduction of the skewness of the FA histogram. Patients with tremor-dominant type showed a trend toward an increase of the twenty-fifth percentile of the FA histogram.
CONCLUSIONS: In patients with de novo PD, there is an increase of FA values, more pronounced in patients with the akinetic-rigid type, probably reflecting diffuse subtle GM loss. This is in line with the hypothesis that widespread neurodegeneration is already present at the time of the clinical onset.
The neuropathologic hallmark of Parkinson Disease (PD) is neural depletion, replacement gliosis, and intraneural formation of Lewy bodies and dystrophic Lewy neurites in the substantia nigra and other subcortical brain nuclei. However, PD is a multisystem disorder because inclusion bodies ultimately affect the entire cerebral cortex.1,2 Because the pathologic process requires years to reach its greatest severity and topographic extent, it has been hypothesized that the course of the disease can be subdivided into presymptomatic and symptomatic phases, the latter corresponding to the advanced phase of the degenerative process.1,2 Moreover, although the core features for diagnosing PD are resting tremor, bradykinesia, rigidity, and postural instability, there is great variability in the clinical presentation of individual patients, suggesting the existence of subtypes of the disease with differences in clinical pattern, pathogenesis, and disease progression.3–6 In particular, a tremor-dominant type is usually distinguished from an akinetic-rigid type.5–7
Neuroimaging techniques, including voxel-based morphometry,8–12 proton MR spectroscopy,13,14 positron-emission tomography (PET),15–18 and single-photon emission tomography19–20 demonstrate widespread changes in the cerebral hemispheres in patients in advanced clinical stages of PD. Recently, several studies pointed out the capability of the histogram analysis of the apparent diffusion coefficient computed from diffusion-weighted images and of the mean diffusivity and fractional anisotropy (FA) computed from diffusion tensor imaging (DTI) to reveal brain-tissue damage in neurodegenerative diseases with predominant involvement of the gray matter (GM), such as Alzheimer disease,21 Huntington disease,22 and progressive ataxias.23
We hypothesized that global measurements of brain volume and structure, such those possible with SIENAX software (structural image evaluation using normalization of atrophy, single time-point version; available at http://www.fmrib.ox.ac.uk/fsl),24 and histogram analysis of DTI could reveal subtle tissue changes in the early clinical phase of PD. Accordingly, we investigated with SIENAX and DTI a group of patients with drug-naïve de novo PD and a group of healthy controls. Moreover, in the patients, the measurements of brain volume and structure were correlated with the type of clinical presentation.
Materials and Methods
Subjects
Twenty-seven patients (9 women and 18 men; mean age, 61 ± 9.7 years) with drug-naïve de novo PD were consecutively referred to our neurology unit for the treatment or diagnostic evaluation of parkinsonian symptoms from January 2004 to November 2006. All patients satisfied the UK Brain Bank criteria for the diagnosis of PD.25
A clinical examination was conducted by one of the authors (C.L.). A levodopa challenge test was performed in all the patients as a supportive criterion for the diagnosis of idiopathic PD. Moreover, all patients were screened for cardiovascular autonomic dysfunction and cognitive impairment. Signs of autonomic failure, Mini-Mental State Examination scores ≤24/30,26 significant medical conditions, and previous therapies with antiparkinsonian drugs were considered as exclusion criteria.
Sixteen healthy volunteers (7 women and 9 men; mean age, 59.4 ± 9.4 years), recruited among personnel working at the MR imaging unit and their relatives, served as controls. They were of the same socioeconomic and educational level, and significant (P < .05) differences between patients and controls for sex (χ2 test) and age (Mann Whitney U test) were not observed. All patients and healthy subjects gave their informed consent to participate in the study, which was approved by our institutional review board.
Motor Evaluation.
Severity of parkinsonism was evaluated by the Unified Idiopathic Parkinson's Disease Rating Scale (UPDRS),27 and the Hoehn and Yahr staging.28 On the basis of the predominant motor features in UPDRS, patients were subtyped into 1 of 3 clinical groups, namely tremor-dominant type, akinetic-rigid type, and mixed type, following the method proposed by Kang et al,6 a modification of Schiess classification system.7 Thirteen patients were typed as tremor-dominant type, 11 as akinetic-rigid type, and 3 as mixed type. The data concerning the clinical subtypes of patients with PD are reported in Table 1. Differences in sex (χ2 test), disease duration, and age (Mann Whitney U test) between patients with akinetic-rigid type and tremor-dominant type were not significant. Patients with akinetic-rigid type had higher UPDRS II (P = .007) and UPDRS III (P = .005) scores and Hoehn and Yahr stages (P = .011) than patients with tremor-dominant type (all, Mann Whitney U test).
Table 1:
Demographic and clinical data in patients with de novo Parkinson disease
| Whole PD (n = 27) | TDT (n = 13) | ART (n = 11) | MT (n = 3) | |
|---|---|---|---|---|
| Age at exam (years ± SD) | 60.9 ± 9.7 | 57.9 ± 10.4 | 62.2 ± 8.3 | 69.3 ± 7.7 |
| Sex | ||||
| Male | 18 | 8 | 8 | 2 |
| Female | 9 | 5 | 3 | 1 |
| Disease duration (months ± SD) | 17 ± 7.4 | 16.5 ± 5 | 17.5 ± 9.3 | 18 ± 10.3 |
| UPDRS (mean ± SD) | ||||
| Subitem II | 6.0 ± 4.0 | 3.6 ± 2.0 | 7.0 ± 3.4* | 13.0 ± 3.0 |
| Subitem III | 12.7 ± 8.3 | 7.3 ± 3.6 | 15.8 ± 8.0* | 24.3 ± 8.5 |
| Hoehn and Yahr (mean ± SD) | 1.2 ± 0.4 | 1.0 ± 0.1 | 1.4 ± 0.4* | 1.8 ± 0.2 |
Note:—TDT indicates tremor-dominant type; ART, akinetic-rigid type; MT, mixed type; UPDRS, Unified Idiopathic Parkinson's Disease Rating Scale.
Significantly different (P < .05) with respect to patients with TDT.
MR Imaging Acquisition Protocol
All examinations were performed on a 1.5T system (Magnetom Symphony; Siemens, Erlangen, Germany) equipped with 30 mT/m gradients and a quadrature head coil.
MR Imaging.
After scouts, the examination protocol included axial high-resolution contiguous 3D T1-weighted images, which were obtained with a magnetization-prepared rapid acquisition of gradient echo sequence (TR = 2500 ms, TE = 3.7 ms, TI = 730 ms, flip angle = 15°, section thickness = 1 mm, FOV = 256 mm, matrix size = 256 × 256, NEX = 1), and axial T2-weighted images, which were obtained with a fluid-attenuated inversion recovery (FLAIR) sequence (TR = 9000 ms, TE = 114 ms, TI = 2500 ms, section thickness = 4 mm, FOV = 230 mm, matrix size = 256 × 256, turbo factor = 21, NEX = 1).
DTI.
A diffusion-weighted single-shot echo-planar imaging sequence (TR = 6000 ms, TE = 95 ms, section thickness = 5 mm, FOV = 256 mm, matrix size = 128 × 128) was acquired on an axial plane with diffusion-sensitizing gradients applied along 6 noncollinear directions [(1,0,1), (−1,0,1), (0,1,1), (0,1,−1), (1,1,0), (−1,1,0)] by using a b-value of 0 (b0 image) and 1000 s/mm2. To improve the signal-to-noise ratio, we obtained 8 averaged acquisitions for each DT image. Maps of mean diffusivity and FA were calculated from the DTI after both automatic segmentation of the brain from the nonbrain tissue and eddy currents correction by means of FDT 1.0 (FMRIB's Diffusion Toolbox 1.0)29 part of FSL 3.3 (FMRIB Image Analysis Group, Oxford, UK).30
Image Analysis
Visual Assessment.
Two operators with more than 10 years of clinical experience (C.T. and M.M.) jointly evaluated T1-weighted images and FLAIR MR images to exclude patients with extensive white matter (WM) signal-intensity changes, defined as a score >2 on a 0–6 visual scale,31 and those with secondary parkinsonism. Moreover, they excluded from further analysis patients with overt motion artifacts in the DTI.
Total Brain, GM, and WM Volumes.
Brain volumes were measured by using the SIENAX software extensively described elsewhere.24 Briefly, brain was first segmented from nonbrain tissue by using a brain-extraction tool of FSL 3.3. The brain images were affine-registered to MNI152 (Montreal Neurological Institute, Montreal, Canada) space (by using the skull image to determine the registration scaling); this is primarily to obtain a volumetric scaling factor to be used as a normalization for head size. Finally, brain images were segmented, and the total brain, GM, and WM volume values, normalized for head size of the subject, were calculated.
Whole-Brain Histograms of Mean Diffusivity and FA.
The methods for histogram analysis were reported previously.32 Preliminarily, motion artifacts and eddy current distortions in the source DTIs were corrected by using the FTD software implemented in the FSL package.30 Then, to segment brain parenchyma from CSF, for each b0 image we created a binary brain mask by using the FAST (FMRIB's Automated Segmentation Tool 3.53, part of FSL 3.3)33 2 classes segmentation function. This mask was applied in MRIcro34 on mean diffusivity and FA maps for voxel-by-voxel data extraction. We generated histograms of mean diffusivity and FA, normalizing each bin to the total number of voxels contributing to the histogram. The mean; twenty-fifth, fiftieth (median), and seventy-fifth percentile values; skewness and kurtosis of the whole-brain mean diffusivity; and FA histograms were computed by using a custom-made Matlab (Matlab 6.5 R13; MathWorks, Natick, Mass) program.
Statistical Methods
The nonparametric Mann Whitney U test was used to compare the total brain, GM, and WM volumes and histogram-derived metrics of mean diffusivity and FA between patients with PD as a whole and controls and between patient clinical subgroups and controls. Because of the small number of patients with mixed type of clinical presentation, they were excluded from the subgroups analysis.
To correct for multiple comparisons, we considered a P value ≤ .01 statistically significant, a P value between .01 and .05 was considered as a trend, and P values > .05 were considered not significant.
Correlations between the parameters that resulted in a significant difference or showed a trend, and the clinical motor scores (UPDRS item II and III) and disease duration were assessed by using the statistical Spearman rank testing.
Results
Few small focal lesions of the cerebral WM were observed in 11 patients and 7 controls.
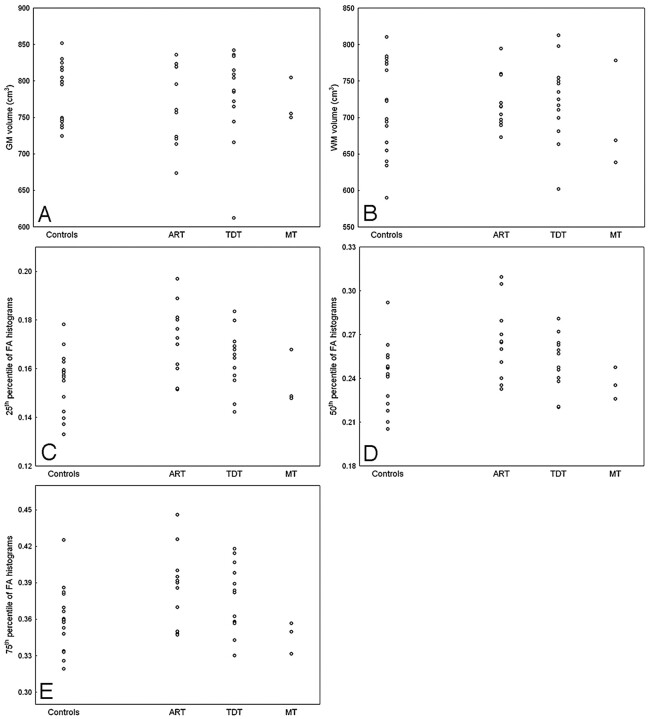
Brain volumes and mean diffusivity and FA histogram data in patients and controls are detailed in Tables 2–4 and shown in Fig 1. No significant differences were found for total brain, GM, and WM volumes and histogram-derived mean diffusivity metrics between controls and the whole group of patients with PD or any subgroup of patients with PD. The twenty-fifth percentile of the FA histograms was significantly (P = .009) higher in the whole group of patients than in controls (Fig 2). A significant (P = .006) increase of the twenty-fifth percentile of the FA histograms as compared with controls was also observed in patients with akinetic-rigid type PD, which was accompanied by a trend for an increase of the mean (P = .043), fiftieth percentile (P = .026), and seventy-fifth percentile (P = .029) and for a reduction of the skewness (P = .033) of the FA histograms. In patients with tremor-dominant type PD, there was a trend (P = .045) for an increase of the twenty-fifth percentile of the FA histograms as compared with controls.
Table 2:
Normalized GM, WM, and total brain volumes*
| GM | WM | Total | |
|---|---|---|---|
| Controls | 782 ± 41 | 713 ± 65 | 1495 ± 81 |
| PD Patients | 773 ± 55 | 718 ± 49 | 1491 ± 89 |
| ART-PD | 767 ± 54 | 720 ± 36 | 1487 ± 79 |
| TDT-PD | 778 ± 62 | 722 ± 55 | 1500 ± 104 |
Note:—TDT indicates tremor-dominant type; ART, akinetic-rigid type; GM, gray matter; WM, white matter.
Volume values (cubic centimeters) are reported as mean ± SD.
Table 4:
FA histogram parameters*
| Percentile |
Mean | SD | Skewness | Kurtosis | |||
|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | |||||
| Controls | 0.155 ± 0.012 | 0.241 ± 0.021 | 0.356 ± 0.026 | 0.285 ± 0.018 | 0.190 ± 0.009 | 1.97 ± 0.17 | 8.59 ± 0.86 |
| PD Patients | 0.166 ± 0.013† | 0.256 ± 0.023 | 0.377 ± 0.030 | 0.297 ± 0.020 | 0.190 ± 0.006 | 1.87 ± 0.20 | 8.14 ± 0.95 |
| ART-PD | 0.172 ± 0.015† | 0.265 ± 0.026‡ | 0.386 ± 0.031‡ | 0.303 ± 0.022‡ | 0.190 ± 0.008 | 1.82 ± 0.16‡ | 7.98 ± 0.85 |
| TDT-PD | 0.164 ± 0.012‡ | 0.253 ± 0.020 | 0.377 ± 0.028 | 0.296 ± 0.017 | 0.191 ± 0.004 | 1.86 ± 0.21 | 8.01 ± 0.97 |
Note:—TDT indicates tremor-dominant type; ART, akinetic-rigid type.
FA values (unitless) are reported as mean ± SD.
Significantly different (P ≤ .01) with respect to control subjects.
Trend for an increase/decrease (.01 < P ≤ .05) with respect to control subjects.
Fig 1.
Scatterplots showing the distribution of the GM (A) and WM (B) volumes and of the twenty-fifth (C), fiftieth (D), and seventy-fifth (E) percentiles of the whole-brain FA histogram in the healthy controls and in the subgroups of patients with PD including those with tremor-dominant type (TDT), akinetic-rigid type (ART), and mixed type (MT).
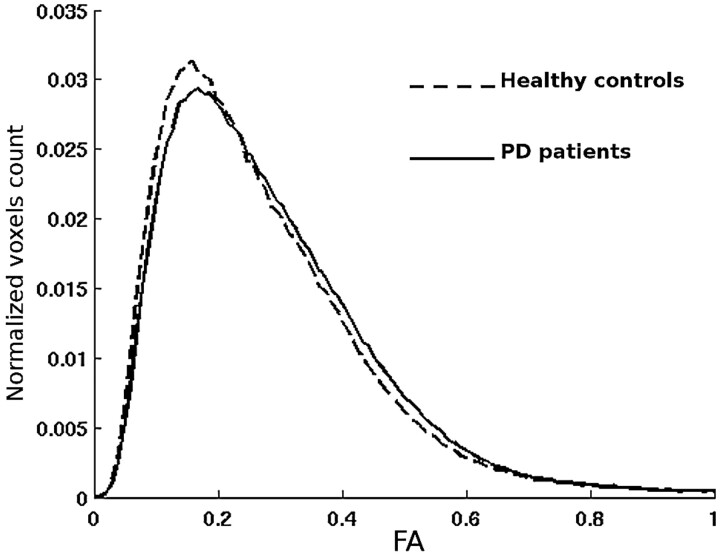
Fig 2.
Averaged whole-brain FA histograms in healthy controls (dashed line) and the whole group of patients with PD (continuous line). Note the right shift of the FA histogram curve in patients with PD, implying higher anisotropy values, and its lower asymmetry (skewness) with respect to the curve of the controls.
Correlations
No significant correlation between FA histogram-derived metrics and clinical scores and disease duration was found in the whole group of patients with PD or in any PD subgroup.
Discussion
Despite several cross-sectional and longitudinal MR imaging studies,8–12,35–41 the distribution and progression of brain atrophy in PD are not yet established. In particular, early cross-sectional MR imaging studies by using visual assessment found no evidence of generalized atrophy in PD.35 However, later studies by using volumetry or visual assessment reported hippocampal or medial temporal lobe atrophy in patients with PD with and without dementia.36–38 Hippocampal and prefrontal atrophy, evaluated by means of a visual score, were also described in nonmedulated patients with PD.39 Cross-sectional voxel-based morphometry studies showed extensive clusters of GM volume reduction in the cerebral cortex, which were more pronounced in patients with dementia. In particular, Burton et al8 reported large clusters of GM loss in frontal lobes, which were accompanied by similar clusters in the temporal, parietal, and occipital lobes as well as in some subcortical regions in patients with PD and dementia.
Nagano-Saito et al10 reported atrophy in the limbic-paralimbic areas and the prefrontal cortex in patients with PD without dementia. Atrophy was more widespread in patients with dementia, in whom GM reduction was found widely in the limbic-paralimbic system, including the anterior cingulate gyrus and hippocampus as well as the temporal lobe, prefrontal cortex, thalamus, and caudate nucleus. Summerfield et al11 reported that patients with PD with dementia showed GM volume decrease in the putamen, accumbens nuclei, thalamus, hippocampus, parahippocampal region, and anterior cingulate gyrus. Patients with PD also showed GM reductions in the hippocampus, anterior cingulate gyrus, and superior temporal gyrus. Finally, in a recent voxel-based morphometry study, widespread areas of cortical atrophy were found in the temporal, frontal, and parietal lobes in patients with PD with dementia and in the frontal and temporal lobes in patients with PD with mild cognitive impairment.12 Two longitudinal volumetric MR imaging studies demonstrated a significant increase in the rate of total brain atrophy in patients with PD40 and in patients with PD and dementia,41 compared with controls. In a longitudinal study, voxel-based morphometry demonstrated a significant loss of GM volume in patients with PD with and without dementia with disease progression.9
Most MR imaging studies of diffusion of water protons in patients with PD to date adopted region of interest analysis and focused on subcortical gray nuclei, mainly in search of features useful for differential diagnosis with other parkinsonism.42–46 In most of these studies, no significant differences in apparent diffusion coefficient values in the subcortical GM between patients with PD and controls were found. Also in a recent study that used voxel-based analysis of the mean apparent diffusion coefficient, no evidence of brain-tissue damage was found in patients with PD except an isolated increase of diffusivity in the olfactory tracts.47
Overall, previous MR imaging studies assessing global or regional atrophy were performed on patients in relatively advanced stages of the disease. To the best of our knowledge, this is the first study in which cross-sectional global measurements of atrophy and mean diffusivity and FA of water protons were performed to detect possible subtle changes of the brain in patients with de novo PD.
For assessment of brain volumes, we used SIENAX, which is the cross-sectional version of SIENA (Structural Image Evaluation, by using Normalization, of Atrophy; available at http://www.fmrib.ox.ac.uk/fsl) software, a fully automated, robust, and accurate method of longitudinal analysis designed to estimate global brain atrophy. It provides measures of total brain, GM, and WM volumes normalized for head size of the subject and has been used in normal aging,48 multiple sclerosis,49 and neurodegenerative50 and vascular disease.51 In recent years, histogram analysis of whole-brain MR images exploring different tissue parameters reflecting microstructure of the nervous tissue, including apparent diffusion coefficient calculated from diffusion-weighted imaging or mean diffusivity and FA maps calculated from DTI, was proposed to get a global assessment of brain damage in diffuse or multifocal diseases, such as multiple sclerosis, neurodegenerative disorders, and leukoaraiosis.21–23,52,53 The whole-brain volume and mean diffusivity and FA analyses imply loss of information about the spatial distribution of any abnormality but offer several advantages as compared with region-of-interest or voxel-based analyses, which are tailored to detect local differences. These advantages include the simplicity and automatic nature of the procedure with high reproducibility of results,24,52 the possibility to reveal subtle diffuse changes that could remain undetected by regional measurements, and the capability to provide a robust measurement of the global changes associated with a disease condition of potential interest in longitudinal studies.
In our study, global atrophy measurements and histogram analysis of mean diffusivity failed to detect significant differences between patients with PD as a whole or subgroups and controls. This seems to indicate that overt generalized atrophy or tissue changes are not present in the early symptomatic phase of the disease. On the contrary, histogram analysis of FA maps revealed an increase of the twenty-fifth percentile in patients with PD as a whole, which was more pronounced in patients with akinetic-rigid type. FA histogram analysis also showed a trend for an increase of the mean and fiftieth and seventy-fifth percentiles and for a reduction of the skewness in patients with akinetic-rigid type and for an increase of the twenty-fifth percentile in patients with tremor-dominant type.
We submit the following explanation for this unexpected result. FA is an index that reflects the degree of alignment of cellular structures as well as their structural integrity. Accordingly, FA is lower in normal GM than in WM, can show variations in normal WM structures on a regional basis, and usually decreases in WM disease processes, reflecting the destruction of regularly ordered WM fiber tracts. Moreover, although the whole-brain mean diffusivity histogram is composed of a single curve, the FA histogram results from the superimposition of 2 different curves representing the distribution of FA values of GM (lower values) and WM (higher values) in isolation.52 The FA increase could thus be due to subtle GM loss and a percentage increase of WM content. The reduction of the GM component could also have determined an increase of the symmetry, namely reduction of the skewness, of the whole-brain histogram of the FA curve (Fig 2), associated with a proportional increase of the contribution to the curve of the WM, with higher anisotropy values and its typical symmetric gaussian shape. Moreover, as in most previous studies of diffusion in PD, we did not observe significant differences in histogram analysis of mean diffusivity maps, and this seems to indicate that our results are not related to changes in diffusivity properties.
We hypothesize that SIENAX failed to demonstrate significant GM loss because the spatial normalization and the GM/WM segmentation steps, which are part of the SIENAX procedure but are absent in the histogram analysis of mean diffusivity or FA maps, can reduce the sensitivity of the method. Obviously, the presumed superiority of whole-brain FA histogram analysis as compared with global atrophy measurements for detecting subtle changes of the relative volumes of GM and WM deserves further investigations.
To the best of our knowledge, so far no study evaluated differences in MR imaging findings between clinical subtypes of PD. Evidence of differences in pathophysiology, disease severity, and outcome between clinical subtypes of PD are increasing.3,4 The akinetic-rigid type shows more severe cell loss in the ventrolateral part of the zona compacta of the substantia nigra that projects to the dorsal putamen. On the contrary, the tremor-dominant type shows predominant degeneration in the medial pars compacta of the substantia nigra and in the retrorubral field A8,54,55 which project to the dorsolateral striatum and ventromedial thalamus. Furthermore, clinical and epidemiologic studies have demonstrated that patients with akinetic-rigid type PD have faster motor and cognitive decline than patients with tremor-dominant type PD and that the akinetic-rigid type may be considered a risk factor for development of dementia in PD.4,56,57 In our sample, patients with akinetic-rigid type showed higher UPDRS and Hoehn and Yahr scores than patients with the tremor-dominant type. Hence, it is possible that our results merely reflect the severity of the clinical scores rather than differences between subtypes. However FA values did not correlate with UPDRS and Hoehn and Yahr scores in our series. On the other hand, in large epidemiologic studies,3,6 patients with new-onset akinetic-rigid type tended to have higher UPDRS and Hoehn and Yahr scores than patients with tremor-dominant type, confirming a more severe impairment already at this early clinical stage. Our results seem to indicate that patients with akinetic-rigid type might have a more severe brain involvement than patients with tremor-dominant type in an early stage of the disease. Further longitudinal studies in larger sample sizes are required to address this issue and to ascertain whether these abnormalities are predictive of the clinical-pathologic evolution, and notably of the development of cognitive impairment.
We recognize some limitations of our study. First, we examined a relatively small number of patients in whom no correlation between the MR imaging measurements and severity of the clinical motor features was found. Hence, our results have to be considered as preliminary to future studies on larger samples of patients whose motor and cognitive performances need to be evaluated and correlated with the MR imaging data. Second, we used a DTI protocol with relatively thick sections and only 6 directions for computation of DT. Optimized protocols with thinner sections and more directions could improve quantification of the tissue structure evaluation with DTI. Finally, with the present approach, we could not establish whether the observed subtle loss of GM in patients with PD was due to generalized or regional changes.
Conclusion
Our study indicates that total, GM, and WM volumes as calculated with SIENAX are not significantly decreased in patients with de novo PD. However as compared with age-matched controls, patients with PD as a whole and patients with akinetic-rigid type show an increase of the twenty-fifth percentile of the FA histogram. This finding is consistent with the hypothesis that subtle GM loss is present in patients with PD since the early clinical phases and that this feature is more pronounced in patients with akinetic-rigid type.
Table 3:
Mean diffusivity histogram parameters*
| Percentile |
Mean | SD | Skewness | Kurtosis | |||
|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | |||||
| Controls | 0.732 ± 0.032 | 0.825 ± 0.043 | 0.968 ± 0.058 | 0.884 ± 0.048 | 0.311 ± 0.021 | 1.75 ± 0.15 | 9.88 ± 1.22 |
| PD Patients | 0.734 ± 0.030 | 0.824 ± 0.053 | 0.971 ± 0.062 | 0.888 ± 0.045 | 0.315 ± 0.024 | 1.70 ± 0.21 | 9.42 ± 1.32 |
| ART-PD | 0.737 ± 0.029 | 0.829 ± 0.072 | 0.986 ± 0.076 | 0.900 ± 0.050 | 0.326 ± 0.024 | 1.66 ± 0.30 | 9.01 ± 1.74 |
| TDT-PD | 0.722 ± 0.027 | 0.809 ± 0.028 | 0.943 ± 0.037 | 0.866 ± 0.031 | 0.301 ± 0.015 | 1.74 ± 0.10 | 9.88 ± 0.79 |
Note:—TDT indicates tremor-dominant type; ART, akinetic-rigid type.
Mean diffusivity values (10−3 mm2/s) are reported as mean ± SD.
Footnotes
This work was supported in part by research grants of the Italian Ministry of Research to Mario Mascalchi (PRIN 2007).
References
- 1.Braak H, Ghebremedhin E, Rüb U, et al. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res 2004;318:121–34 [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Rüb U, Jansen Steur EN, et al. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 2005;64:1404–10 [DOI] [PubMed] [Google Scholar]
- 3.Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort—The Parkinson Study Group. Neurology 1990;40:1529–34 [DOI] [PubMed] [Google Scholar]
- 4.Jankovic J, Kapadia AS. Functional decline in Parkinson disease. Arch Neurol 2001;58:1611–15 [DOI] [PubMed] [Google Scholar]
- 5.Korchounov A, Schipper HI, Preobrazhenskaya IS, et al. Differences in age at onset and familial aggregation between clinical types of idiopathic Parkinson's disease. Mov Disord 2004;19:1059–64 [DOI] [PubMed] [Google Scholar]
- 6.Kang GA, Bronstein JM, Masterman DL, et al. Clinical characteristics in early Parkinson's disease in a central California population-based study. Mov Disord 2005;20:1133–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiess MC, Zheng H, Soukup VM, et al. Parkinson's disease subtypes: clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism Relat Disord 2000;6:69–76 [DOI] [PubMed] [Google Scholar]
- 8.Burton EJ, McKeith IG, Burn DJ, et al.. Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain 2004;127:791–800 [DOI] [PubMed] [Google Scholar]
- 9.Ramirez-Ruiz B, Marti MJ, Tolosa E, et al. Longitudinal evaluation of cerebral morphological changes in Parkinson's disease with and without dementia. J Neurol 2005;252:1345–52. Epub 2005 Jul 5 [DOI] [PubMed] [Google Scholar]
- 10.Nagano-Saito A, Washimi Y, Arahata Y, et al. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 2005;64:224–29 [DOI] [PubMed] [Google Scholar]
- 11.Summerfield C, Junqué C, Tolosa E, et al. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol 2005;62:281–85 [DOI] [PubMed] [Google Scholar]
- 12.Beyer MK, Janvin CC, Larses JP, et al. A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry 2007;78:254–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen BC, Block RE, Sanchez-Ramos J, et al. Proton MR spectroscopy of the brain in 14 patients with Parkinson disease. AJNR Am J Neuroradiol 1995;16:61–68 [PMC free article] [PubMed] [Google Scholar]
- 14.Hu MT, Taylor-Robinson SD, Chaudhuri KR, et al. Evidence for cortical dysfunction in clinically non-demented patients with Parkinson's disease: a proton MR spectroscopy study. J Neurol Neurosurg Psychiatry 1999;67:20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eidelberg D, Moeller JR, Dhawan V, et al. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab 1994;14:783–801 [DOI] [PubMed] [Google Scholar]
- 16.Piert M, Koeppe RA, Giordani B, et al. Determination of regional rate constants from dynamic FDG-PET studies in Parkinson's disease. J Nucl Med 1996;37:1115–22 [PubMed] [Google Scholar]
- 17.Bohnen NI, Minoshima S, Giordani B, et al. Motor correlates of occipital glucose hypometabolism in Parkinson's disease without dementia. Neurology 1999;52:541–46 [DOI] [PubMed] [Google Scholar]
- 18.Hu MT, Taylor-Robinson SD, Chaudhuri KR, et al. Cortical dysfunction in non-demented Parkinson's disease patients: a combined 31P-MRS and 18 FDG-PET study. Brain 2000;123:340–52 [DOI] [PubMed] [Google Scholar]
- 19.Abe Y, Kachi T, Kato T, et al. Occipital hypoperfusion in Parkinson's disease without dementia: correlation to impaired cortical visual processing. J Neurol Neurosurg Psychiatry 2003;74:419–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oishi N, Udaka F, Kameyama M, et al. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology 2005;65:1708–15 [DOI] [PubMed] [Google Scholar]
- 21.Bozzali M, Franceschi M, Falini A, et al. Quantification of tissue damage in AD using diffusion tensor and magnetization transfer MRI. Neurology 2001;57:1135–37 [DOI] [PubMed] [Google Scholar]
- 22.Mascalchi M, Lolli F, Della Nave R, et al. Volumetric, diffusion and magnetization transfer MR imaging of the brain in individuals with Huntington's disease. Radiology 2004;232:867–73 [DOI] [PubMed] [Google Scholar]
- 23.Della Nave R, Foresti S, Tessa C, et al. ADC mapping of neurodegeneration in the brainstem and cerebellum in patients with progressive ataxias. Neuroimage 2004;22:698–705 [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust and automated longitudinal and cross-sectional brain changes analysis. Neuroimage 2002;17:479–89 [DOI] [PubMed] [Google Scholar]
- 25.Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 1988;51:745–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res 1975;12:189–98 [DOI] [PubMed] [Google Scholar]
- 27.Fahn S, Elton RL, and members of the UPDRS development committee. The Unified Idiopathic Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, eds. Recent Developments in Parkinson's Disease. Vol 2. Florham Park, NJ: Macmillan Healthcare Information;1987. :153–64
- 28.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–42 [DOI] [PubMed] [Google Scholar]
- 29.Behrens TEJ, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003;50:1077–88 [DOI] [PubMed] [Google Scholar]
- 30.Smith S, Jenkinson M, Woolrich M, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:208–19 [DOI] [PubMed] [Google Scholar]
- 31.Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5T in Alzheimer dementia and normal aging. AJNR Am J Neuroradiol 1987;8:421–26 [DOI] [PubMed] [Google Scholar]
- 32.Della Nave R, Magaudda A, Michelucci R, et al. Whole-brain histogram and voxel-based analyses of apparent diffusion coefficient and magnetization transfer ratio in celiac disease, epilepsy, and cerebral calcifications syndrome. AJNR Am J Neuroradiol 2007;28:479–85 [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging 2001;20:45–57 [DOI] [PubMed] [Google Scholar]
- 34.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol 2000;12:191–200 [DOI] [PubMed] [Google Scholar]
- 35.Huber JB, Shuttleworth E, Christy JA, et al. Magnetic resonance imaging in dementia of Parkinson's disease. J Neurol Neurosurg Psychiatry 1989;52:1221–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laasko MP, Partanen K, Riekkinen P, et al. Hippocampal volumes in Alzheimer disease, Parkinson's disease with and without dementia, and in vascular dementia: an MRI study. Neurology 1996;46:678–81 [DOI] [PubMed] [Google Scholar]
- 37.Camicioli R, Moore MM, Kinney A, et al. Parkinson's disease is associated with hippocampal atrophy. Mov Disord 2003;18:784–90 [DOI] [PubMed] [Google Scholar]
- 38.Tam CW, Burton EJ, McKeith IG, et al. Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology 2005;8:861–65 [DOI] [PubMed] [Google Scholar]
- 39.Brück A, Kurki T, Kaasinen V, et al. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson's disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry 2004;75:1467–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu MT, White SJ, Chaudhuri KR, et al. Correlating rates of cerebral atrophy in Parkinson's disease with measures of cognitive decline. J Neural Transm 2001;108:571–78 [DOI] [PubMed] [Google Scholar]
- 41.Burton EJ, McKeith IG, Burn DJ, et al. Brain atrophy rates in Parkinson's disease with and without dementia using serial magnetic resonance imaging. Mov Disord 2005;20:1571–76 [DOI] [PubMed] [Google Scholar]
- 42.Seppi K, Schocke MF, Esterhammer R, et al. Diffusion-weighted imaging discriminates progressive supranuclear palsy from PD, but not from the Parkinson variant of multiple system atrophy. Neurology 2003;60:922–27 [DOI] [PubMed] [Google Scholar]
- 43.Schocke MF, Seppi K, Esterhammer R, et al. Trace of diffusion tensor differentiates the Parkinson variant of multiple system atrophy and Parkinson's disease. Neuroimage 2004;21:1443–51 [DOI] [PubMed] [Google Scholar]
- 44.Nicoletti G, Lodi R, Condino F, et al. Apparent diffusion coefficient measurements of the middle cerebellar peduncle differentiate the Parkinson's variant of MSA from Parkinson's disease and progressive supranuclear palsy. Brain 2006;129:2679–87 [DOI] [PubMed] [Google Scholar]
- 45.Paviour DC, Thornton JS, Lees AJ, et al. Diffusion-weighted magnetic resonance imaging differentiates Parkinsonian variant of multiple-system atrophy from progressive supranuclear palsy. Mov Disord 2007;22:68–74 [DOI] [PubMed] [Google Scholar]
- 46.Köllensperger M, Seppi K, Liener C, et al. Diffusion weighted imaging best discriminates PD from MSA-P: a comparison with tilt table testing and heart MIBG scintigraphy. Mov Disord 2007;22:1771–76 [DOI] [PubMed] [Google Scholar]
- 47.Scherfler C, Schocke MF, Seppi K, et al. Voxel-wise analysis of diffusion-weighted imaging reveals disruption of the olfactory tract in Parkinson's disease. Brain 2006;129:538–42 [DOI] [PubMed] [Google Scholar]
- 48.Rovaris M, Iannucci G, Sormani MP, et al. Age-related changes in conventional, magnetization transfer and diffusion-tensor MR imaging findings: study with whole-brain tissue histogram analysis. Radiology 2003;227:731–38 [DOI] [PubMed] [Google Scholar]
- 49.Zivadinov R, Grop A, Sharma J, et al. Reproducibility and accuracy of quantitative magnetic resonance imaging techniques of whole brain atrophy measurements in multiple sclerosis. J Neuroimaging 2005;15:27–36 [DOI] [PubMed] [Google Scholar]
- 50.Smith SM, Rao A, De Stefano N, et al. Longitudinal and cross-sectional analysis of atrophy in Alzheimer's disease: cross-validation of BSI, SIENA and SIENAX. Neuroimage 2007;36:1200–06. Epub 2007 Apr 27 [DOI] [PubMed] [Google Scholar]
- 51.Peters N, Holtmannspotter M, Opherk C, et al. Brain volume changes in CADASIL: a serial MRI study in pure subcortical ischemic vascular disease. Neurology 2006;66:1517–22 [DOI] [PubMed] [Google Scholar]
- 52.Cercignani M, Inglese M, Pagani E, et al. Mean diffusivity and fractional anisotropy histograms of patients with multiple sclerosis. AJNR Am J Neuroradiol 2001;22:952–58 [PMC free article] [PubMed] [Google Scholar]
- 53.Della Nave R, Foresti S, Pratesi A, et al. Whole-brain histogram and voxel-based analyses of diffusion tensor imaging in patients with leukoaraiois: correlation with motor and cognitive impairment. AJNR Am J Neuroradiol 2007;28:1313–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jellinger KA. Post-mortem studies in Parkinson's disease: is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl 1999;56:1–26 [DOI] [PubMed] [Google Scholar]
- 55.Jellinger KA. Recent developments in the pathology of Parkinson's disease. J Neural Trans Suppl 2002;62:347–46 [DOI] [PubMed] [Google Scholar]
- 56.Alves G, Larsen JP, Emre M, et al. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord 2006;21:1123–30 [DOI] [PubMed] [Google Scholar]
- 57.Burn DJ, Rowan EN, Allan LM, et al. Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006;77:585–89 [DOI] [PMC free article] [PubMed] [Google Scholar]