Abstract
BACKGROUND AND PURPOSE: Inferior petrosal sinus sampling (IPSS) is a useful diagnostic technique in adrenocorticotropic hormone (ACTH)-dependent hypercortisolism with normal or equivocal MR imaging. The procedure is believed to be safe, with mostly minor complications. However, there are rare, but severe, neurologic complications that need to be considered.
MATERIALS AND METHODS: We performed an institutional review board–approved retrospective review of our institutional IPSS experience from July 2001 to January 2007. IPSS was performed for the evaluation of Cushing disease. The end points of particular interest were the indications for IPSS and the incidence of associated complications.
RESULTS: During the study period of 5½ years, 44 patients underwent IPSS for evaluation of Cushing disease. There were 33 women and 11 men with a mean age of 43.1 years. Because of equivocal imaging and endocrine testing, 36 of 44 patients underwent IPSS, and 8 of 44 underwent IPSS after failed transsphenoidal exploration. The only complication was injury to the brain stem that occurred after an unremarkable procedure in a 42-year-old woman. She developed clinical evidence of pontomedullary dysfunction with MR imaging consistent with brain stem infarction. The cause of this injury is unclear, but a venous variant leading to transient venous hypertension or thrombosis is suspected.
CONCLUSION: Neurologic injury is a rare but serious complication associated with IPSS. Despite this, if performed under a strict paradigm, IPSS is both accurate and safe and can be very useful in the management of Cushing disease.
Although transsphenoidal microsurgery can be curative in 84% of patients with Cushing disease (personal data), the precise localization of the tumor can be challenging. Corticotrophic microadenomas are not detectable on imaging studies in 40% to 50% of patients.1 Bilateral inferior petrosal sinus sampling (IPSS) with corticotropin-releasing hormone (CRH) stimulation is a diagnostic technique that can aid with both the localization of the tumor to the pituitary gland as well as suggest lateralization within the gland. This procedure can also be used in cases of equivocal endocrine testing but established hypercortisolism or before reoperation. Although it is thought to be a relatively innocuous diagnostic test, some significant complications have been reported in the literature that include deep venous thrombosis,2 pulmonary embolism,2 venous subarachnoid hemorrhage (SAH),3 and brain stem injury.4 These reported morbidities are rare but have fueled the controversy surrounding the exact role of IPSS in the evaluation of Cushing disease.
During the past 5½ years at our institution, we have performed 44 IPSSs for the evaluation of Cushing disease. In this report, we review our institution's experience with IPSS and detail a rare case of brain stem injury. The potential causes, strategies to avoid complications, and indications of IPSS are discussed.
Methods
Patient Population
Hospital records and available imaging findings for patients presenting to Mount Sinai Medical Center from July 2001 to January 2007 with presumed Cushing disease who underwent IPSS were reviewed. The hospital's institutional review board approved the record review.
For each patient, various demographic and procedural characteristics were reviewed. The age and sex of each patient were recorded. In addition, the indications for IPSS, endocrine profile, and preprocedure imaging of the sella were reviewed. Finally, any reported difficulties during the procedure or complications as a result of the procedure were also recorded.
At our institution, there are 2 common indications for IPSS. It is commonly performed in patients with the clinical stigmata of Cushing disease but equivocal imaging and endocrine testing in the presence of documented hypercortisolism. Endocrine work-up is commonly performed by the patient's physician before referral and usually consists of 24-hour urinary free cortisol, serum or salivary cortisol, serum adrenocorticotropic hormone (ACTH), and a dexamethasone-suppression test at a low or high dose. Occasionally, a CRH stimulation test is also performed. IPSS is also performed in patients with presumed Cushing disease undergoing reoperation for continued elevation of serum ACTH levels when initial exploration did not demonstrate ACTH secreting adenoma.
The samples collected during the procedure assess ACTH levels within the IPS and the peripheral venous system before and after CRH injection. A gradient of 2:1 is diagnostic without CRH, and 3:1 is diagnostic with CRH for an ACTH-secreting pituitary microadenoma and helps in excluding adrenal and ectopic sources.
IPSS Technique
Either our endovascular neurosurgeon (A.B.P.) or our interventional neuroradiologist (D.M.J.) performed all of the sampling procedures.
The patients were brought to the neurointerventional angiography suite, where light intravenous sedation with midazolam (Versed) and fentanyl was administered. Both groins were then shaved and then sterilely cleaned and draped. A 19-gauge single-wall puncture needle was used to access both the right and left common femoral vein, and the Seldinger technique was used to place a 5F sheath (Terumo Medical, Somerset, NJ) into the right femoral vein and a 4F sheath into the left femoral vein. Both sheaths were attached to continuous flush drips of heparinized saline. The patient was then given 3000 U of heparin intravenously as a bolus before catheterization. A 4F angled glide catheter (Terumo Medical) was advanced over a Bentson wire (Cook Medical, Bloomington, Ind) into the right inferior petrosal sinus. The guidewire was removed, and an inferior petrosal sinus venogram was performed to evaluate contrast filling within the ipsilateral inferior petrosal (IPS) and cavernous sinuses as well as across the intracavernous sinus into the contralateral cavernous and inferior petrosal sinuses. A catheter was then placed into the left IPS, commonly under roadmap guidance, in a similar manner, and a confirmatory venogram was again performed to verify placement. Both catheters needed to be positioned symmetrically, and intermittent fluoroscopy was used during the procedure to ensure that there was no migration of either catheter. Baseline samples were then obtained from both the catheters as well as from the right femoral sheath. CRH (Acthrel; Ferring Pharmaceuticals, Parsippany, NJ) was then administered through the right femoral sheath at a dose of 1 μg/kg to a maximum dose of 100 μg, and samples were obtained at 2, 5, 10, and 15 minutes after the injection from both sinuses and the right femoral sheath. The samples were carefully labeled and were placed in sterile tubes in an ice bath. After the final sample, the catheters and sheath were removed, and manual pressure was used to achieve hemostasis. Patients were most commonly observed in the recovery room for 1 to 2 hours and sent home on the day of the procedure.
Results
During the study period of 5½-years, 44 patients underwent IPSS. The female-to-male ratio was 3:1 (33 women and 11 men) with a mean age of 43.1 years (range, 15–70 years). Of these 44 patients, 36 had a high clinical suspicion for Cushing disease with an equivocal endocrine profile and imaging of the sella. The remaining 8 patients were undergoing reoperation after a previous unsuccessful transsphenoidal exploration, either at our institution or elsewhere.
Our method for IPSS has been the same for all 44 patients. In the first 35 consecutive patients, no complications were encountered. All of these patients were sent home on the day of the procedure without any complaints at discharge. Patient 36 underwent an uncomplicated IPSS but sustained a brain stem injury. The 8 most recent patients who had this complication all underwent uncomplicated IPSS. Because of the rarity of brain stem injury from IPSS, the case is further detailed.
Patient History
This is a 42-year-old woman who presented with recent weight gain and new-onset diabetes mellitus and hypertension. On physical examination she was overweight, with upper body obesity and a rounded face. She reported easy bruising and had multiple purple abdominal striae. Endocrinologic testing found elevated levels of cortisol in both urine and serum. Although ACTH was elevated, provocative testing that included a dexamethasone-suppression test was equivocal. In addition, MR imaging of the sella was also normal. To confirm the diagnosis of Cushing disease and to aid with lateralization within the sella, an IPSS was requested.
Procedure
The patient underwent an IPSS as detailed earlier, without any difficulties during the procedure. After the intravenous bolus of heparin, the first catheter was placed into the right IPS, and a routine venogram was performed (Fig 1A,B). Normal contrast filling was seen in the IPS and cavernous sinuses as well as across the intracavernous sinus into the contralateral cavernous and inferior petrosal sinuses. In addition, prominent venous drainage was observed caudally into the anterior pontomesencephalic venous plexus (Fig 1A). The lateral angiogram demonstrates cranially directed contrast reflux into the posterior mesencephalic vein that drains superiorly into the vein of Galen and straight sinus (Fig 1B). This may represent some degree of outflow obstruction causing the excess contrast to reflux superiorly rather than to drain inferiorly. A catheter was then placed into the left IPS in a similar manner, and a confirmatory venogram demonstrated normal filling into the smaller left IPS, with normal outflow through the internal jugular vein (IJV) and intracavernous sinus. The anterior pontomesencephalic plexus was visualized, but it was not as prominent. In addition, there was no evidence of superior contrast reflux. Both catheters were positioned symmetrically. Normal washout of contrast was seen during both venograms, without any regions of contrast stagnation or extravasation. Baseline samples were then obtained, after which 100 μg of CRH was administered intravenously. Appropriate samples were sent, after which both catheters and groin sheaths were removed and hemostasis was achieved. The patient reported no complaints during the procedure, and she was neurologically intact at its completion.
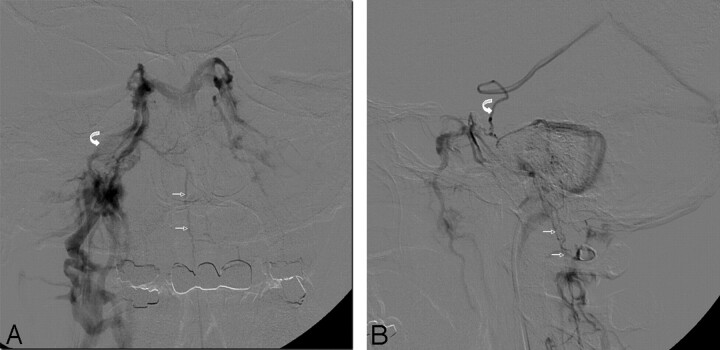
Fig 1.
A,B. Venograms performed after catheterization of IPS. A, AP venogram of right IPS (curved arrow) demonstrating normal opacification of the right sinus as well as cross-filling into the left IPS. The anterior pontomesencephalic vein (small arrows) is seen draining inferiorly into the vertebral plexus. B, Lateral venogram of right IPS demonstrating the anterior pontomesencephalic vein (small arrows) and reflux into the cranial aspect of the anterior mesencephalic vein (curved arrow).
Postoperative Course
One hour after the procedure, the patient began to complain of “jumping” vision and unsteadiness associated with nausea and vomiting. On examination, she had a right internuclear ophthalmoplegia, mild right-sided facial weakness involving the lower motor neuron, drift of the left upper extremity, and diminished sensation in the left distal arm. An urgent MR imaging demonstrated T2-weighted abnormality in the pons and upper medulla and a diffusion-weighted imaging (DWI) abnormality within the dorsal pons (Fig 2) that was much smaller than the region of T2 change. The MR angiogram and MR venogram were notable for a dominant right transverse sinus and a patent basilar artery. These imaging findings were concerning for a brain stem infarction. She was admitted to the neurosurgical intensive care unit for aggressive fluid hydration and was given 10 mg of dexamethasone (Decadron) every 6 hours and 325 mg of aspirin daily.
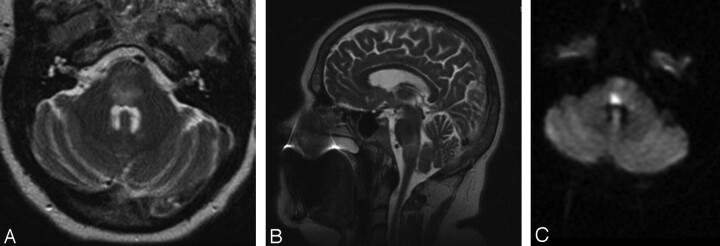
Fig 2.
A–C. Initial MR imaging after a change in the patient's neurologic examination. A, Axial T2-weighted MR imaging demonstrating diffuse edema within the pons. B, Sagittal T2-weighted MR imaging demonstrating the cranial-caudal extent of edema at the pontomedullary junction. C, Axial DWI MR imaging showing a region of restricted diffusion, highly suggestive of infarct, within the dorsal pons.
Overnight, she deteriorated further with complaints of severe diplopia bilaterally on lateral gaze. On examination, she had complete paralysis of horizontal gaze as well as increased right facial and left upper extremity weakness. She also reported some difficulty swallowing, and a formal swallowing evaluation confirmed moderate dysphagia. For the next 48 hours, she developed new weakness involving the left leg, and she had decreasing oxygen saturations and increasing stridor, requiring intubation and an eventual tracheostomy. Repeat MR imaging demonstrated diminished T2-weighted abnormality within the pons and evidence of hemorrhagic conversion on gradient-echo sequences. Another MR imaging examination performed 3 weeks later revealed diminished T2-weighted abnormality within the pons and medulla but continued DWI abnormality within the dorsal pons, extending in a linear wedge to the right ventral pontine surface. She was transferred to acute rehabilitation after 3 weeks on the neurosurgical service, at which time her hemiparesis and facial weakness were improving and the sensory deficits had resolved.
Follow-Up
Repeat MR imaging 3 months after IPSS demonstrated very scant T2-weighted abnormality, except in the region coinciding with the fixed DWI abnormality in the dorsal pons (Fig 3). At 6-month follow-up, she reported diminished diplopia but had persistent bilateral lateral gaze palsy. She did, however, have complete resolution of her right facial weakness and left-sided hemiparesis. In addition, her tracheostomy had been reversed and her swallowing was substantially improved.
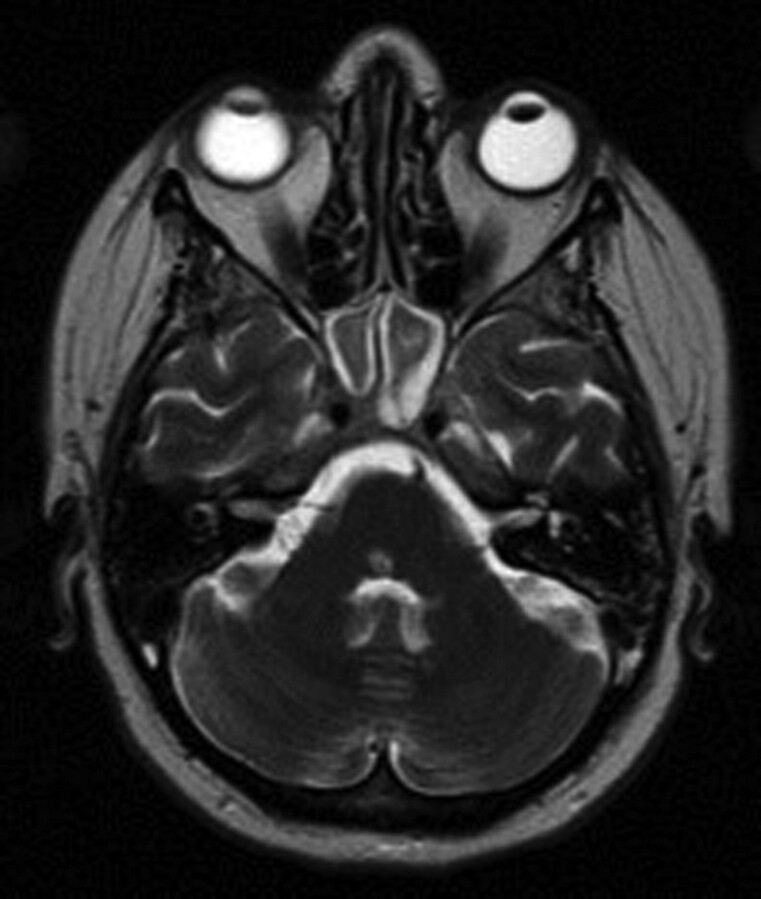
Fig 3.
MR imaging at 3 months after IPSS. Axial T2-weighted MR imaging demonstrating near-complete resolution of edema within the brain stem but a region of hyperintensity within the dorsal pons corresponding to the region of infarction.
Discussion
The literature documents a few cases of both reversible and irreversible brain stem injury related to IPSS. In a large series from the National Institutes of Health, there was 1 (0.2%) major neurologic complication of 508 patients undergoing IPSS.5 A second, more recent report from the National Institutes of Health describes 2 patients in whom potential brain stem injury was avoided and 2 other patients who sustained brain stem injury.4 Both patients in the first group reported feeling “woozy” or “funny” during the procedure, and the catheterization was immediately terminated. These patients both had changes in their neurologic examination such as slurred speech, vertigo, or paresthesias that resolved within 4 hours. MR imaging results were normal in both patients. In contrast, the 2 patients in the second group both sustained brain stem injury. They both had more prolonged intraprocedural complaints of facial numbness, speech difficulty, or extremity paresthesias. One of the patients also had a prolonged period of elevation of blood pressure during the case and eventually developed paresis of contralateral gaze and hemiplegia. MR imaging examination demonstrated a dorsal pontine hemorrhagic infarction that seemed to be remarkably similar to the MR imaging in our case. On follow-up, both the patients had significant neurologic deficits. This report emphasizes the importance of immediately terminating the procedure if the patient has complaints or changes in vital signs during IPSS. Some of the changes may be subtle, requiring elevated vigilance during the procedure.
Another recent report by Sturrock and Jeffcoate6 describes a case of a 45-year-old woman who underwent bilateral IPSS and developed otalgia, nausea, and hypotension during contrast injection into the right IPS. Two hours after the procedure, she developed difficulty swallowing, reduced right-sided palatal movement, right lower motor neuron sixth and seventh cranial nerve palsies, horizontal nystagmus, and left-sided sensory deficits. In addition, the patient did have right upper extremity ataxia, though no motor weakness was present. MR imaging demonstrated an infarct at the pontocerebellar junction. The cause of the injury in this patient remained unclear.
Seyer et al7 report on a 14-year-old boy who had persistent Cushing disease despite resection of a right-sided microadenoma. The patient did not have any intraoperative symptoms but complained of diplopia immediately after IPSS. He developed a complete palsy of the sixth cranial nerve and contralateral hemiparesis within 1 hour and also complained of hyperacusis for the next 2 days. He eventually recovered except for a persistent palsy of the left sixth cranial nerve.
Lefournier et al8 present a series of 166 consecutive IPSSs with 1 transient neurologic complication. Of particular interest was that this particular patient developed right-sided sixth nerve palsy during a right IPSS with an occluded left IJV. The authors suggest that perhaps the occlusion of the left IJV resulted in increased venous pressure within the right IJV and possibly resulted in thrombosis of the right IPS. However, 3 other patients in the same series also had 1 occluded IJV and had no complications resulting from IPSS.
The exact mechanism for brain stem injury remains unclear, but various theories exist. Kitaguchi et al9 describe 2 cases of a brain stem variant of posterior reversible encephalopathy syndrome (PRES) that is most commonly seen in the occipital lobes. PRES is a condition often associated with hypertension, renal failure, cytotoxic agents, and eclampsia.10 These underlying disorders can cause disruption of autoregulation in the cerebral vasculature. Because the vertebrobasilar system has poor sympathetic innervation,11 abnormalities are usually seen in the posterior cerebral hemispheres with resulting, characteristic, T2-weighted changes in signal intensity on MR imaging. Brain stem involvement is rare, and only 1 of the 2 patients in the report by Kitaguchi et al9 had hypertension. In both cases, the patients completely recovered but retained residual pontine lacunar abnormalities on follow-up imaging. The MR imaging abnormalities in both these cases seem very similar to that seen in our case. In addition, transient occipital blindness, with radiographic findings very similar to PRES, has been reported as a rare complication of radiologic contrast dye injection with a cause that remains unclear.12 Because of the radiographic similarities, our patient's history of hypertension, and the rare report of contrast dye neurotoxicity, we had originally believed brain stem PRES to be the probable explanation in our case. In most of the reported cases, the symptoms of PRES are reversible. However, irreversible brain injury has also been described as a very rare outcome.13 Despite these infrequent reports, we now believe that though PRES may still be a possible explanation for the injury in our case, it is unlikely. Contrast neurotoxicity has only been described in the occipital lobes but has not been described in the brain stem. The only other agents received during the procedure were Versed, fentanyl, and CRH, and none of these have been associated with PRES. In addition, early hemorrhage, as seen on the first follow-up MR imaging, has not been described with PRES, again making this cause less likely.
Another proposed explanation is that venous hypertension or thrombosis results in brain stem injury. Doppman14 describes how a temporary occlusion of any number of the small veins draining the posterior cerebellopontomedullary angle could lead to venous hypertension that causes brain stem injury. The types of catheter used for selective catheterization may also have an impact.4 The use of smaller catheters that are capable of canalizing more distally can potentially occlude a smaller bridging vein and can also cause more distal, focal, venous hypertension during contrast injection. Either of these mechanisms can result in brain stem injury. Because catheter-related occlusion is temporary, prompt termination of the procedure may help spare irreversible injury if the patient manifests any symptoms during the procedure. In our series, the type of catheter used is larger than that described by Miller et al,4 reducing the risk for distal catheterization. Another possible cause that may also be related to catheterization is venous emboli, but no literature describing this entity currently exists. Our patient received heparin before catheterization, minimizing the possibility of emboli.
Venous SAH after IPSS has also been reported to occur and to lead to brain stem injury. Bonelli et al3 reported a case of a patient with venous subarachnoid hemorrhage and subsequent hydrocephalus as a severe complication of IPSS. In one of the cases described by Miller et al,4 extravasated contrast along with blood was seen in the pons and fourth ventricle on a postprocedure CT, after a deficit was noted. In both of these patients, venous perforation causing the SAH resulted in brain stem injury. Both authors suggest that increased luminal pressure within the affected veins was a result of contrast injection. There was no evidence of SAH in our patient, and the signal intensity abnormality seen on the second MR imaging on gradient echo was most probably from hemorrhagic conversion of the original ischemic infarct.
The venous anatomy surrounding the brain stem has been found to be highly variable, especially in the region of the petrosal veins.15 Small venous channels originating in the cerebellopontomedullary angle can infrequently drain into the IPS. Additional, small bridging veins can also be found connecting the IPS with the transverse pontine vein, the vein of the pontomedullary sulcus, or the lateral medullary vein near the jugular bulb.16 As suggested by Miller et al,4 some of the venous variations may allow for focal venous hypertension in the small bridging veins near the brain stem and cause certain patients to have a higher risk for injury. Unfortunately, there is no way to identify these patients before IPSS.
In our 5½-year experience, 44 patients underwent IPSS after either an equivocal work-up or after previous failed surgery. The initial 35 consecutive patients sustained no complications as a result of the procedure, and the 8 most recent procedures have also been free of complications. However, 1 of 44 patients did sustain a partially reversible brain stem injury. After reviewing the literature, we believe that the prominent anterior pontomesencephalic veins seen in our patient during the right IPS venogram was an example of a variant pattern of venous drainage surrounding the brain stem and contributed to her injury. Whether this was from the catheter occluding venous outflow or from focal venous hypertension as a result of contrast injection remains unclear. There is some evidence of contrast reflux superiorly into the cranial aspect of the anterior mesencephalic vein instead of only inferior venous drainage (Fig 1B). This raises the possibility of some degree of outflow obstruction or slowing. During the procedure, the patient reported no symptoms, and there were no changes in the patient's examination or vital signs that would have caused us to terminate the test. The resulting injury on the first MR showed diffuse brain stem edema with a smaller region of actual infarction. The region of injury seen on T2-weighted sequences is consistent with our patient's neurologic examination with involvement of the fibers of pontomedullary cranial nerves (VI, VII, IX, and X), the paramedian pontine reticular formation, medial longitudinal fasciculus, portions of the corticospinal tract, medial lemniscus, and spinothalamic tracts. As the edema in the brain stem resolved, best seen on the evolution of T2-weighted sequences on serial MR imaging, the patient's sensory, motor, and lower cranial nerve deficits also resolved. The resulting infarct on the 3-month follow-up MR imaging confirmed a persistent abnormality on T2-weighted sequences within the dorsal pons that extends ventrally (Fig 3). The patient's neurologic examination after 6 months suggests that with the resolution of brain stem edema, only the fibers of the sixth cranial nerve, medial longitudinal fasciculus, and paramedian pontine reticular formation remain damaged. On the basis of the MR imaging findings, this is not surprising.
Although initial reports on IPSS claimed that the sensitivity and specificity of the test were near 100%,17 later reports have shown the sensitivity and specificity to be approximately 94% for the detection of a pituitary source of ACTH after CRH stimulation.18,19 In the report by Bonelli et al,18 this corresponds to 6 false-negative results and 1 false-positive result of 94 IPSS procedures. In the same study, there was only a 70% correlation between the lateralization results from IPSS and the surgical location. This suggests that though IPSS can be of significant benefit in the diagnosis of a pituitary source for the elevated ACTH secretion, it is not reliable for lateralization within the gland. By comparison, jugular venous sampling (JVS), which some advocate because of the decreased technical difficulty of the procedure and the potential for less complications, has a sensitivity of 83% and a specificity of 100%.20 On the basis of both our experience and the current literature, we believe that IPSS remains superior to JVS in accuracy, and the theoretic difference in the safety profile is not well characterized. JVS may be a good alternative for smaller institutions with limited technical expertise to perform IPSS. In the limited number of JVS cases we have performed, the ACTH response to CRH stimulation is often blunted, making the results far less useful than a comparable IPSS evaluation.
This report is one of the largest series detailing IPSS in the literature, and our experience with 44 consecutive patients highlights a few key points. It further confirms that IPSS is not benign, even at the most experienced institutions. Only by slowly adding to the current body of literature on IPSS complications will we eventually be able to formulate treatment decisions based on accurate risk/benefit analyses. Currently, there are very limited data. In addition, the anatomic venous variant described in this report may be particularly high-risk for brain stem injury. At the time of the procedure, the venous variant was not been established as a higher-risk pattern, so it was not possible for us to have modified the case when we saw the venous pattern. We strongly believe that the injury was sustained during the initial catheterization and injections of contrast and not from the subsequent IPS sampling. However, without additional evidence, it is impossible to be absolutely certain. If faced with the same venous variant in a patient manifesting no symptoms at that time, we would promptly remove the catheter from the IPS and proceed with sampling from the IJV. Injury may result even with this strategy because there is no clear way to predict this venous variant in a patient until the IPS is at least briefly catheterized but minimized.
IPSS can be of significant value in the evaluation of Cushing disease. However, as illustrated by previous rare reports and our recent case, the procedure is not benign and the indiscriminate use of the study can be very dangerous. The indications for the procedure are not clearly established and can vary between practitioners. As we described earlier, the 2 common indications for IPSS at our institution are either in patients with the clinical stigmata of Cushing disease with equivocal imaging and endocrinologic testing in the presence of hypercortisolism or in patients with presumed Cushing disease undergoing reoperation. With strict adherence to these indications, we believe that IPSS remains a safe and valuable procedure, and we continue to perform it despite our single, severe complication.
Conclusion
IPSS is a test commonly used in the evaluation of Cushing disease, but it is not benign. There are reports of rare but severe complications, the most dangerous of which is irreversible brain stem injury. Reports suggest that irreversible brain stem injury can be avoided if the procedure is promptly terminated if there are any patient complaints, changes in blood pressure, or changes in neurologic examination. In the1 complication in our series, there was radiographic evidence of venous outflow variance that may have contributed to the injury. Venous hypertension as a result of catheter-related occlusion of a venous channel, or from the pressure or toxicity of contrast injection, probably resulted in the pontine infarction and resulting gaze palsy. This complication suggests that recognizing a venous variant, especially one with contrast reflux suspicious for outflow obstruction, early in the procedure may reduce the risk for brain stem injury in the asymptomatic patient. However, despite this single complication, we believe that the procedure is both safe and useful if used in the appropriate circumstance and a universal paradigm would be beneficial.
References
- 1.Tabarin A, Laurent F, Catargi B, et al. Comparative evaluation of conventional and dynamic magnetic resonance imaging of the pituitary gland for the diagnosis of Cushing's disease. Clin Endocrinol (Oxf) 1998;49:293–300 [DOI] [PubMed] [Google Scholar]
- 2.Oboubie K, Davies JS, Ogunko A, et al. Venous thrombo-embolism following inferior petrosal sinus sampling in Cushing's disease. J Endocrinol Invest 2000;23:542–44 [DOI] [PubMed] [Google Scholar]
- 3.Bonelli FS, Huston J, Meyer FB, et al. Venous subarachnoid hemorrhage after inferior petrosal sinus sampling for adrenocorticotropic hormone. AJNR Am J Neuroradiol 1999;20:306–07 [PMC free article] [PubMed] [Google Scholar]
- 4.Miller DL, Doppman JL, Peterman SB, et al. Neurologic complications of petrosal sinus sampling. Radiology 1992;185:143–47 [DOI] [PubMed] [Google Scholar]
- 5.Brismar G, Brismar J, Cronqvist S. Complications of orbital and skull base phlebography. Acta Radiol Diagn (Stockh) 1976;17:274–80 [DOI] [PubMed] [Google Scholar]
- 6.Sturrock ND, Jeffcoate WJ. A neurological complication of inferior petrosal sinus sampling during investigation for Cushing's disease: a case report. J Neurol Neurosurg Psychiatry 1997;62:527–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seyer H, Honegger J, Schott W, et al. Raymond's syndrome following petrosal sinus sampling. Acta Neurochir (Wien) 1994;131:157–59 [DOI] [PubMed] [Google Scholar]
- 8.Lefournier V, Gatta B, Martinie M, et al. One transient neurological complication (sixth nerve palsy) in 166 consecutive inferior petrosal sinus samplings for the etiological diagnosis of Cushing's syndrome. J Clin Endocrinol Metab 1999;84:3401–02 [DOI] [PubMed] [Google Scholar]
- 9.Kitaguchi H, Tomimoto H, Miki Y, et al. A brainstem variant of reversible posterior leukoencephalopathy syndrome. Neuroradiology 2005;47:652–56 [DOI] [PubMed] [Google Scholar]
- 10.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494–500 [DOI] [PubMed] [Google Scholar]
- 11.Edvinsson L, Owman C, Sjöberg NO. Autonomic nerves, mast cells, and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res 1976;115:377–93 [DOI] [PubMed] [Google Scholar]
- 12.Schulte-Altedorneburg G, Rüb K, Scheglmann K. Simultaneous ischemic and neurotoxic brain damage after coronary angiography. Neurol Res 2004;26:79–82 [DOI] [PubMed] [Google Scholar]
- 13.Antunes NL, Small TN, George D, et al. Posterior leukoencephalopathy syndrome may not be reversible. Pediatr Neurol 1999;20:241–43 [DOI] [PubMed] [Google Scholar]
- 14.Doppman JL. There is no simple answer to a rare complication of inferior petrosal sinus sampling. AJNR Am J Neuroradiol 1999;20:191–92 [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YP, Wolf BS, Antin SP, et al. The veins of the posterior fossa–anterior or petrosal draining group. Am J Roentgenol Radium Ther Nucl Med 1968;104:36–56 [DOI] [PubMed] [Google Scholar]
- 16.Matsushima T, Rhoton AL Jr, de Oliveira E, et al. Microsurgical anatomy of the veins of the posterior fossa. J Neurosurg 1983;59:63–105 [DOI] [PubMed] [Google Scholar]
- 17.Oldfield EH, Doppman JL, Nieman LK, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing's syndrome [published erratum appears in N Engl J Med 1992;326:1172]. N Engl J Med 1991;325:897–905 [DOI] [PubMed] [Google Scholar]
- 18.Bonelli FS, Huston J 3rd, Carpenter PC, et al. Adrenocorticotropic hormone-dependent Cushing's syndrome: sensitivity and specificity of inferior petrosal sinus sampling. AJNR Am J Neuroradiol 2000;21:690–96 [PMC free article] [PubMed] [Google Scholar]
- 19.Colao A, Faggiano A, Pivonello R, et al. Inferior petrosal sinus sampling in the differential diagnosis of Cushing's syndrome: results of an Italian multicenter study. Eur J Endocrinol 2001;144:499–507 [DOI] [PubMed] [Google Scholar]
- 20.Ilias I, Chang R, Pacak K, et al. Jugular venous sampling: an alternative to petrosal sinus sampling for the diagnostic evaluation of adrenocorticotropic hormone-dependent Cushing's syndrome. J Clin Endocrinol Metab 2004;89:3795–800 [DOI] [PubMed] [Google Scholar]