Abstract
BACKGROUND AND PURPOSE: Endovascular treatment of aneurysms may result in complete or incomplete occlusions or may be followed by recurrences. The goal of the present study was to better define pathologic features associated with so-called healing or recurrences after coiling and to propose an alternative concept to the currently accepted view.
MATERIALS AND METHODS: Experimental canine venous pouch aneurysms were created by using a T-type (group A, N = 29) or a Y-type constructed bifurcation (group B, N = 37) between the carotid arteries. Coil embolization was performed 2 weeks later; and angiography, immediately after and at 12 weeks. Angiographic results, neointima formation at the neck, endothelialization, and organization of thrombus were compared between groups by using qualitative scores and immunohistochemistry.
RESULTS: Angiographic results at 3 months were significantly better in group A than in group B (P = .001). Macroscopic neointimal scores were also better (P = .012). Only 10/32 aneurysms with satisfactory results at angiography were completely sealed by neointima formation. Animals with residual or recurrent aneurysms had significantly worse neointimal scores than those with completely occluded ones (P = .0003). On histologic sections, the neointima was constantly present in “healed” and in recurrent aneurysms. This neointima was a multicellular layer of α-actin+ cells in a collagenous matrix, covered with a single layer of nitric oxide synthetase (NOS+) endothelial cells, whether it completely occluded the neck of the aneurysm or dived into the recurring or residual space between the aneurysm wall and the coil mass embedded in organizing thrombus.
CONCLUSION: Complete angiographic occlusions at 3 months can be associated with incomplete neointimal closure of the neck at pathology. Thrombus organization, endothelialization, and neointima formation can occur concurrently with recurrences.
Endovascular treatment can significantly improve the outcomes of patients with ruptured intracranial aneurysms, but angiographic exclusion of lesions remains incomplete in a significant proportion of patients at long-term follow-up.1 Although these suboptimal morphologic results do not seem to undermine significantly the clinical benefits of coil embolization, their presence implies continuing concerns for possible future hemorrhages.2 They remain a frustrating worrisome problem, necessitating follow-ups and sometimes retreatments.3
A research program relying on animal models4 rests implicitly on the premise that certain biologic events or factors may explain stable angiographic results or, conversely, recurrence with time after endovascular treatment. It is important to emphasize that such a program is not designed to inquire into the nature of aneurysms themselves or their biology. We cannot pretend that our artificial constructions, by using surgical anastomoses of venous pouches or enzymatic digestion and endovascular occlusions of normal arteries in healthy individuals of different species, can explain a specifically human pathology that occurs sporadically. The sole interest of animal models is to identify some normal repeatable mechanism, shared by healthy individuals of 1 or many species, that can be linked to satisfactory or unsatisfactory results after coil embolization.
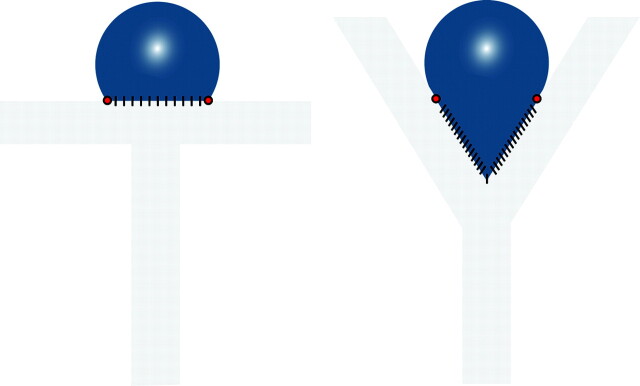
Multiple concepts have been evoked to explain recurrences after coil embolization. Many researchers focused on “deficient healing,” alternatively considered as deficient fibrosis,5,6 lack of endothelialization,7 or insufficient neointima formation.8 Recurrences can be explained, we will argue, without resorting to such concepts. We have extensively studied a canine venous pouch carotid bifurcation aneurysm model for more than a decade. The model comes in 2 varieties, one with a well-defined neck that can be coiled completely (T-type),9 and one with a wide neck that incorporates the parent vessel bifurcation, cannot be completely excluded, and is more prone to residual necks and recurrences after coiling (Y-type, Fig 1).10 By analyzing angiographic and pathologic results following coil embolization, we may be in a position to better define features associated with so-called healing (more frequent with T-type)9 or recurrences (more frequent with Y-type)10; to clarify the concepts of endothelialization, organization of thrombus, and neointima formation; and to propose in which direction research could be more fruitful.
Fig 1.
Schematic illustration of T- and Y-type carotid bifurcation aneurysm models.
We propose that as long as coiling is sufficient to provoke thrombosis, the organization and recanalization of thrombus, neointima formation, and endothelialization are all normal features of the biologic evolution after treatment.
Materials and Methods
Aneurysm Construction
Protocols for animal experimentation were approved by the institutional Animal Committee in accordance with guidelines of the Canadian Council on Animal Care.
Surgical and endovascular procedures were performed with the animals under general anesthesia. Sixty-six Beagles weighing 10–15 kg were sedated with an intramuscular injection of acepromazine (0.1 mg/kg), glycopyrrolate (0.01 mg/kg), and butorphanol (0.1 mg/kg) and were anesthetized with intravenously administered thiopental (15 mg/kg). Animals were ventilated artificially and maintained under surgical anesthesia with 2% isoflurane. Postoperative anesthesia was provided for 3 days by a 50-μg fentanyl skin patch.
Two segments of the same external jugular vein were harvested for construction of the venous pouches. Wide-necked aneurysms were constructed after a T-type bifurcation9 (group A, N = 29) or a Y-type bifurcation10 (group B, N = 37) was created between the 2 common carotid arteries. A number of animals were controls of studies performed for other purposes9,10 but were included in the current study. T- type–shaped aneurysms had approximately a 12-mm fundus and a 5-mm neck, and the Y-type had 10- to 12-mm sacs and 6- to 8-mm necks, as measured by the software provided by the manufacturer (Acom Pc lite; Siemens, Erlangen, Germany), by using the 5F angiographic catheter as the calibrating object.10
Endovascular Treatment and Coil Embolization
Endovascular treatment was instituted by means of coaxial Tracker-18 (Boston Scientific, Natick, Mass) microcatheters introduced by a transfemoral approach. Coil embolization was performed at least 2 weeks after aneurysm construction, according to the technique routinely used in clinical practice. All coils were platinum coils from Boston Scientific Target (Fremont, Calif), Micrus Endovascular (San Jose, Calif), or Microvention Terumo (Aliso Viejo, Calif). A first coil of 0.015-inch caliber, commonly called “18,” was introduced, and coils of decreasing diameters were used for additional packing. The procedure was completed when coils could no longer be introduced inside the aneurysm.
Data Analysis, Angiographic Evaluation, and Scoring
Carotid angiography was performed in anesthetized animals immediately after embolization, at 3 and 12 weeks before euthanasia by barbiturate overdose, to document the degree of aneurysmal obliteration. Multiple projections were evaluated, and the results were scored according to a previously described classification scheme.10,11 A score of 0 indicated complete obliteration, 1 indicated minimal residual or recurring neck (“dog ears”), 2 indicated a more sizable recurrent neck, and 3 indicated residual or recurrent aneurysm.
Dichotomized qualitative angiographic scores, complete or near-complete occlusion (score, 0–1) versus incomplete occlusion (score, 2–3), were compared by using the Fisher exact test, with P = .05 chosen as the level of significance.
Macroscopic Photography and Neointimal Scores
After a 10% formalin fixation, the carotid artery wall was longitudinally opened to expose the luminal surface of the neck of the aneurysms, which was photographed en face by using a computerized imaging system (Vision PE; Clemex Technologies, Montreal, Quebec, Canada). Morphologic results at the neck of treated aneurysms were graded according to a previously published classification system.9 Neointima formation was assigned a score of 0 when a thick neointima completely sealed the orifice of the aneurysmal neck, a score of 1 when the neointima was similarly sealing the neck but small areas of recanalization were seen adjacent to the transition between the neck and the wall of the aneurysm, a score of 2 when a crescent of recanalization was present around the neointima covering the coil mass or the embolic agent, a score of 3 when recanalization affected the coil mass with the neointimal covering being only partial, and a score of 4 when no neointima, but only thrombus, was covering most of the embolic material or coil mass. This grading system and the presence of neointimal tissue were validated by histopathologic studies that were conducted after careful dissection of the coils under microscopy. Again scores were dichotomized as complete or near-complete occlusion (scores, 0–1) and incomplete occlusion (scores, ≥2) and compared by using the Fisher exact test.
Histologic/Immunohistochemical Analysis
Five-micrometer-thick deparaffinized and rehydrated axial sections were stained with Movat pentachrome and, for immunohistochemistry, were incubated with 0.3% hydrogen peroxide (H2O2) in methanol to quench endogenous peroxidase activity, washed in trishydroxymethylaminomethane buffer saline (Tween-20 [TBT, polysorbate 20]; Sigma Aldrich, St. Louis, Mo.), and incubated with 10% serum in TBT (TBB) to block nonspecific binding. Smooth muscle cells and myofibroblasts were detected by overnight incubation of mouse anti-human smooth muscle α-actin (Clone 1A4; Dako, Carpinteria, Calif) antibody, and endothelial cells, by a rabbit polyclonal anti-NOS3 (Santa Cruz Biotechnology, Santa Cruz, Calif) in TBB and were revealed by a biotin-avidin-peroxidase complex (Vectastain ABC KIT; Vector Laboratories, Burlingame, Calif). Peroxidase activity was detected with 1-mg/mL 33-diaminobenzidine hydrochloric and 0.1% H2O2. Sections counterstained with hematoxylin were visualized with a computerized imaging system (Vision PE). Omission of primary antibodies and staining with an irrelevant mouse immunoglobulin of the same isotype served as negative controls.
Results
Angiograhic Results
Angiographic results are summarized in Table 1 and illustrated in Fig 2.
Table 1:
Angiographic and macroscopic scores immediately and 3 months after embolization
| Group | Occlusion | Angiographic Scores |
Macroscopic Scores | |
|---|---|---|---|---|
| Initial | Final | |||
| A, N = 27 | Complete, incomplete | 24 | 20* | 8† |
| 3 | 7 | 19 | ||
| B, N = 39 | Complete, incomplete | 22 | 12* | 2† |
| 17 | 27 | 37 | ||
P = .001.
P = .012 (Fisher exact test).
Fig 2.
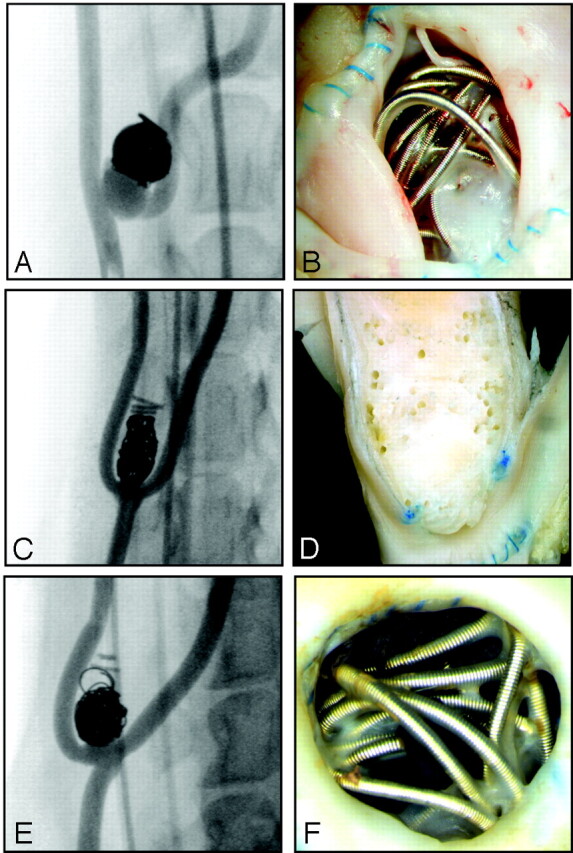
Angiographic and macroscopic results. Angiography (A, C, E) and macroscopic photographs (B, D, F) of prototypical cases of recurrence at 3 months and poor neointimal closure (A and B), complete occlusions at 3 months with good neointimal closure of the neck (C and D), and angiographic occlusion but with poor neointimal closure of the neck (E and F). Note that the coils have been removed in D.
Angiographic results initially and at 3 months were significantly better in group A than in group B, with 20/27 complete or near-complete occlusions in group A as compared with 12/39 for group B (P = .001, Fisher exact test).
Recurrences, defined as an increase in angiographic scores from 0–1 to 2–3, occurred in 4/24 initially occluded aneurysms of group A as compared with 10/22 occluded aneurysms of group B (P = .05).
Macroscopic Scores
Neointimal scores were better at 3 months in aneurysms of group A (8/27 complete) as compared with group B (2/39 complete), with a median neointimal score of 2 as compared with 3 (P = .012, Table 1). Macroscopic scores according to final angiographic results are summarized in Table 2. Specimens with complete or near-complete occlusions on angiography showed complete neointimal closure of the neck in only 10/32 of cases (scores, 0–1), with 22/32 having an incomplete neointima (scores, 2–3). Animals with angiographic residual or recurring aneurysms at 3 months had significantly worse neointimal scores as compared with those with complete or near-complete angiographic occlusions (P = .0003, Table 2).
Table 2:
Neointimal neck closure and angiographic results at 3 months
| Final Angiographic Scores | Macroscopic Scores |
|---|---|
| Complete: 32 | Complete: 10; incomplete: 22* |
| Incomplete: 34 | Complete: 0; incomplete: 34* |
P = .0003; complete occlusion, score 0–1; incomplete occlusion, score 2–3.
Macroscopic and Histopathologic Findings
Aneurysms with Complete and Stable Angiographic Occlusions.
Aneurysms that showed stable and complete occlusions at 3-month angiography and complete closure at macroscopic photography of the neck (Fig 3A) will serve as prototypical cases of aneurysms with satisfactory healing following coil occlusion.
Fig 3.
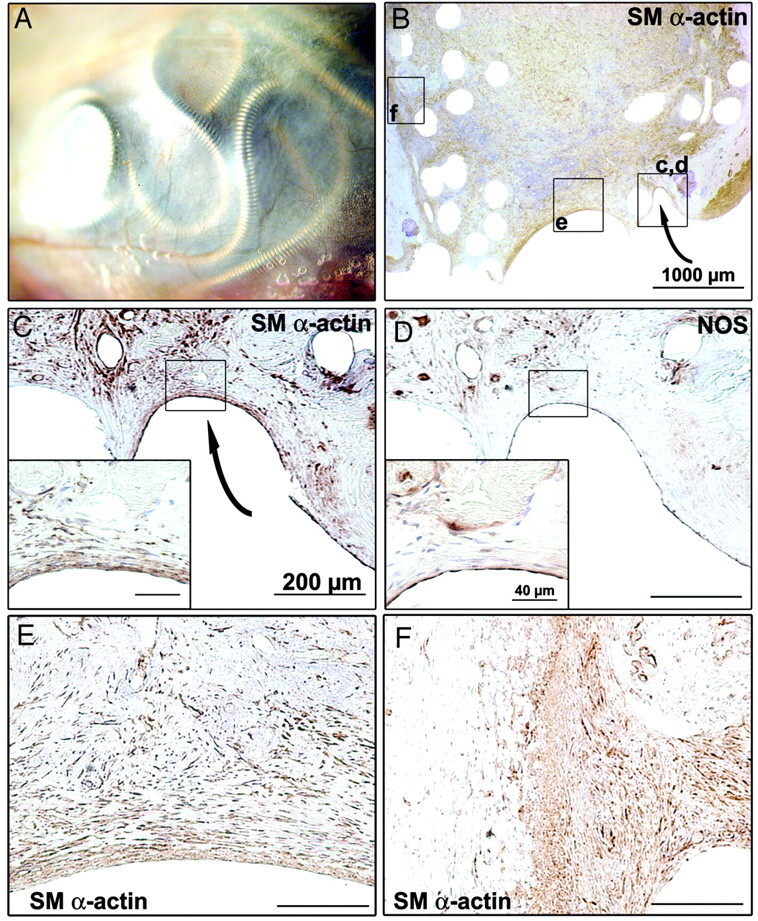
Macroscopic and pathologic findings of aneurysm with complete angiographic occlusion at 3 months after coil embolization. A, Photograph of aneurysmal neck en face. Note complete closure of the neck by a translucent layer of membrane tissue. B, Low-magnification overview of an axial aneurysm section showing a continuous layer of smooth muscle (SM) α-actin+ cells completely sealing the neck of the aneurysm. At higher magnification (C and D are magnifications of the inset c,d in B), closed corner (arrows in B and C) shows aligned SM α-actin+ cells covered by a single layer of NOS+ endothelial cells. E, The same neointimal layer is continuous, isolating the aneurysm from the circulation. F, SM α-actin immunostaining of atrophied aneurysmal wall. Sections are hematoxylin-counterstained (scale bar: 1000 μm for B; 200 μm for C–F; 40 μm for insets in C and D).
Histopathologic sections showed complete closure of the neck by a continuous multicellular layer of α-actin+ cells organized in parallel rows (Fig 3B, -C) in a collagenous matrix and covered with a single layer of NOS+ endothelial cells (Fig 3D). There was no crescentic endothelialized space between the organizing tissue inside the aneurysm and the aneurysm wall (arrow in Fig 3B and Fig 3C, -D: higher magnification of the inset c,d in Fig 3B).
This typical neointima blended imperceptibly with connective tissues deeper inside the neck and body of aneurysms between coils, tissues that always consisted of α-actin+ cells in a collagenous matrix but in a multidirectional disorganized manner (Fig 3E: higher magnification of the inset in Fig 3B). Multiple NOS+ capillaries, surrounded by α-actin+ cells, were present throughout the aneurysm body in varying attenuation. In some areas, old clot was still visible, with intermediate zones showing progressive organization.
The aneurysm wall was in areas difficult to recognize because of the discontinuity of the elastic laminae, being atrophic and infiltrated with connective tissue continuous with that filling the aneurysm lumen (Fig 3F). Coils were sometimes surrounded with giant cells and inflammatory cells, typical of a foreign body reaction.
Animals with Recurring Aneurysms.
The specimens recurring at 3 months consistently showed incomplete neck closure on macroscopic photography (Fig 4A). Nevertheless, areas of the neck that were occluded showed the same neointima, with α-actin+ cells (Fig 4B) in a collagenous matrix covered with NOS+ endothelial cells, as specimens that were completely occluded (compare Figs 3E and 4E). This neointima, instead of closing the neck-parent vessel interface, dived into the lesion, particularly between the mass of coils embedded within connective tissues and the aneurysmal wall (arrow in Fig 4A, -B), with full continuity of the NOS+ endothelial lining between the surface of the neointimal closure of the neck at the level of the parent artery and the luminal surface of recurring lesions (Fig 4B). The hallmark of recurring lesions was the presence of NOS+ crescentic spaces between the aneurysm wall and the coil mass, embedded in connective tissues and covered with neointima (Fig 4C, -D). There was no perceptible difference in the nature of the neointima closing the aneurysm neck and the one bounding the recurring lesions (Fig 4E).
Fig 4.
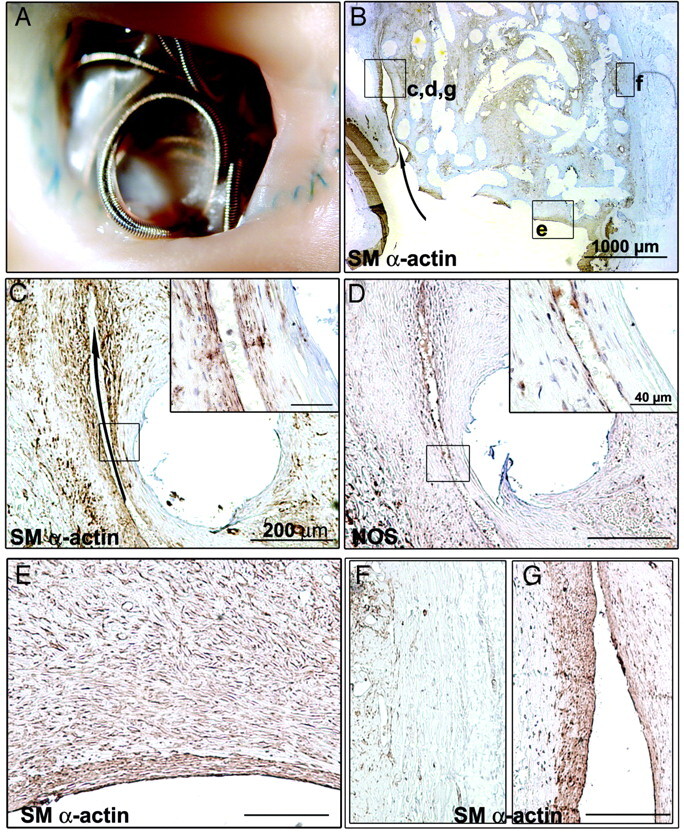
Macroscopic and pathologic findings of recurring aneurysm 3 months after coil embolization. A, Macroscopic photograph showing incomplete neck closure. B, Low magnification of overview of axial aneurysm section revealing a recurring lesion extending from the corner of the neck to the body of the aneurysm (arrow). These crescentic spaces are lined by smooth muscle (SM) α–actin+ cells (C) and covered with NOS+ endothelial cells (D). E, Higher magnification showing the same layer of SM α-actin+ cells covered with NOS+ endothelial cells. F, Atrophy of the aneurysmal wall in the occluded zone. G, No atrophy of the aneurysmal wall in the recurring zone. Sections are hematoxylin-counterstained (scale bar: 1000 μm for B; 200 μm for C–G; 40 μm for insets in C and D).
The aneurysmal sac was filled with coils and connective tissues in all respects similar to the one found in healed aneurysms. The aneurysm wall was atrophic and lost its architectural and organized layer of α-actin+ cells in areas that are not subjected to recurrences (Fig 4F), but not in areas subjected to recurring flow (Fig 4G).
Features Common to Occluded and Recurrent Aneurysms.
Coils and coil loops at the surface of the neck of aneurysms, as well as those protruding into the lumen of the parent vessel, were always covered with neointima (Fig 5A, -B), though it was sometimes so thin as to be translucid, falsely suggesting that the coils and coil loops were bare. Clearly, the neointima covering protruding coils tended to be thinner on the luminal side of the neck, presumably where flow was maximal, as compared with the aneurysm side. Again this neointima always consisted of α-actin+ cells (Fig 5C) in a collagenous matrix and covered with NOS+ endothelial cells (Fig 5D). The thickness of neontima covering the coil mass and each loop of coils varied greatly from 1 area of the neck to the other; similarly, the thickness of the neointimal layer at the site of a recurrence was not different from that found where the coil mass nearly closed the neck at the level of the parent artery.
Fig 5.
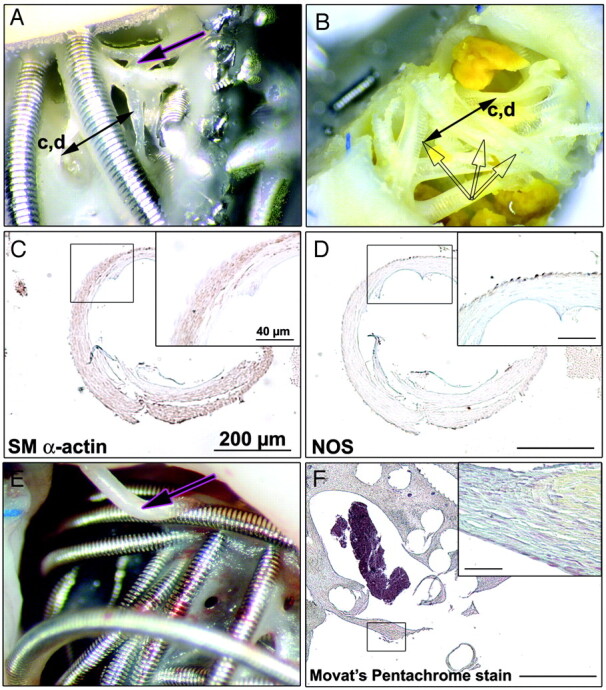
Common findings of occluded and recurrent aneurysms. Macroscopic views with coils protruding into the lumen vessel before (A) and after coil retrieval (B). Note that all coils are re-covered with neointima, which consists of smooth muscle (SM) cells (C) embedded in a collagenous matrix surrounded by a unique layer of NOS+ cells (D). Note polypoid filaments (pink arrows in A and E) observed at the neck of aneurysms, with a similar neointima organization (F). Sections are hematoxylin-counterstained (scale bar: 200 μm for C, D, and F; 40 μm for insets in C, D, and F).
Other polypoid structures, without a “skeleton” of platinum coils, were frequently found at the neck of aneurysms (Fig 5A, -E; pink arrows). They had a similar structure, with a core of connective tissue and a continuous outer layer of NOS+ endothelial cells isolating the core tissues from the intravascular space (Fig 5F). We believe that these structures cannot be explained without resorting to the concept of recanalization (see “Discussion”).
The aneurysmal cavity was always, though incompletely in recurring lesions, filled with vascularized connective tissue embedding coils that were never in direct contact with the lumen of the parent artery or the lumen of the recurring neck or sac (Fig 6).
Fig 6.
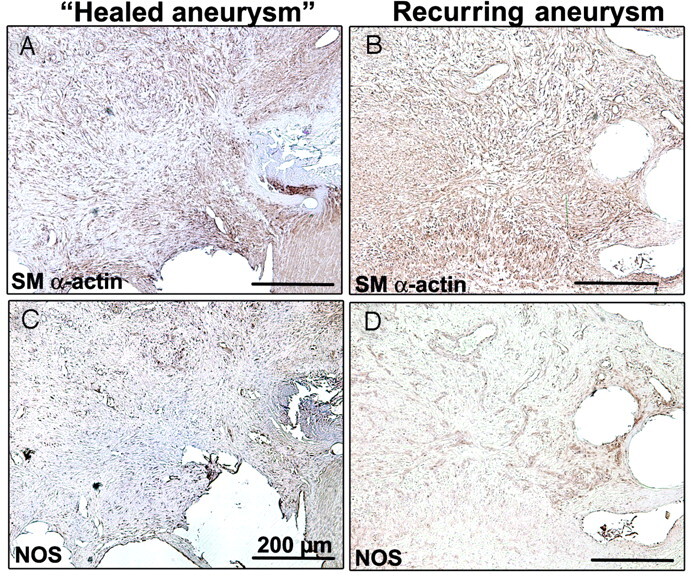
Vascularized connective tissues are similarly organized in occluded (A and C) and recurrent (B and D) aneurysm with smooth muscle (SM) α–actin+ cells, presumably myofibroblast (A and B) and NOS+ cells for neovascularization (C and D) (scale bar: 200 μm).
Within the body of coiled aneurysms, we could not perceive any difference in the amount of residual thrombus, always minimal at 3 months, between recurring and healed aneurysms, nor could we perceive any difference in the quality of the vascularized connective tissues: α-actin myofibroblast-like cells (Fig 6A, -B) and NOS+ cells for neovascularization (Fig 6C, -D).
When the tissues infiltrating the core of the coils were available for inspection, they had a structure similar to that of the connective tissues surrounding the coils (Fig 7).
Fig 7.
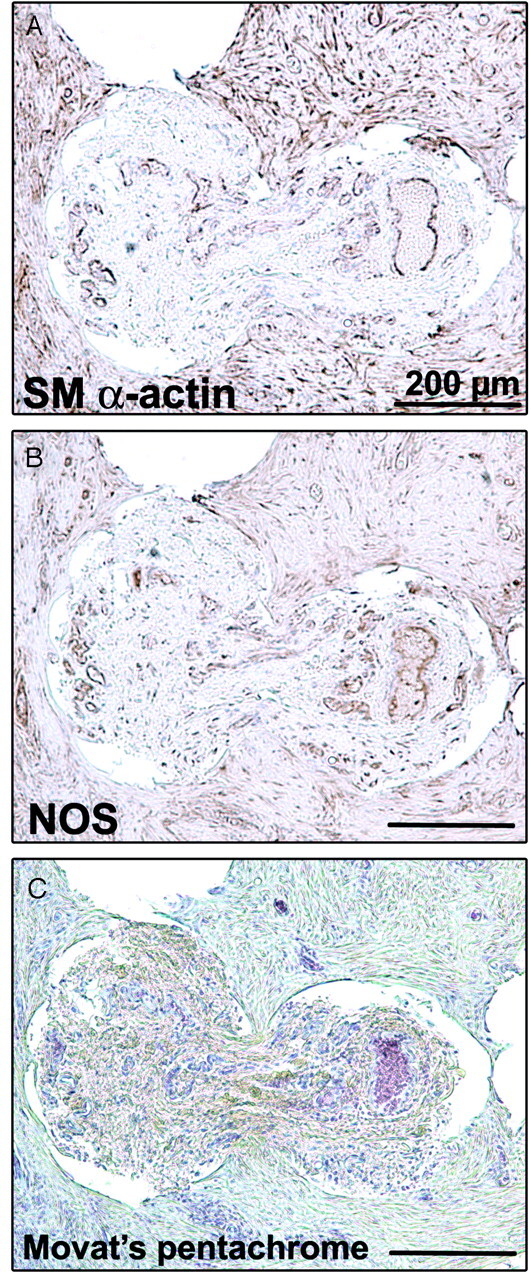
Tissues infiltrating the core of the coils. Immunostaining of smooth muscle (SM) α–actin (A), of NOS (B), and Movat pentachrome stain (C) shows the same structure as the vascularized tissue inside the aneurysm (scale bar: 200 μm).
Discussion
Main observations of this study include the ubiquity of a NOS+ endothelial lining always bounding the intravascular space, present in recurring as constantly as in stably occluded aneurysms, covering all newly formed tissues wherever they are in contact with flowing blood. A residual or recanalized portion of the aneurysm is bounded and covered by this lining; and because endothelial cells are known to possess antithrombotic and thrombolytic properties, this may perhaps explain why the aneurysm remains patent or progresses or why it does not thrombose.
Early endothelialization of the thrombus that forms after coil deposition has been documented in a few human aneurysm specimens, including one as early as 13 days, suggesting that it is not peculiar to canine models.12 Similarly, a neointima, identical in appearance to the one found in this model, has also been well documented in human aneurysms.13
The endothelial lining never occurs in isolation, or without a “subendothelial” supporting structure, sometimes very thin and composed of α-actin+ myofibroblast-like cells in a collagen matrix, forming, with the endothelial lining, a classic “neointima.” This neointima is found as constantly in recurring as in stable lesions or over loops of coils that protrude into the parent vessel and falsely appear “bare.” Thus, at least in this canine model, absent or “deficient” endothelialization or deficient neointima formation cannot explain recurrences.
The hallmark of a stable aneurysm after treatment is the fact that the neointima fully occludes the aneurysm neck.
The hallmark of a recurring lesion is not the absence of a neointima, but the fact that the neointima does not completely occlude the neck at the junction with the parent artery but dives outside the boundary of the parent vessel, inside the neck toward the fundus of the aneurysms, most often near the wall. The recurrence can thus be defined as an area of flowing blood, outside the boundary of the parent vessel, forming a crescentic space on axial sections, fully lined by a neointima (always endothelialized). This neointima is continuous from the wall of the aneurysm to the neointima, covering the coil mass embedded in newly formed connective tissues, now separated from the aneurysm wall by the recurrence and joining at the apex of the recurrence or the deepest extension of the crescent within the body of the aneurysms, presumably where it can extend more deeply toward the fundus (Fig 4B).
The tissues inside the aneurysms, whether in fully occluded aneurysms or in specimens with recurrences, can only form where thrombus provided a provisional matrix for the infiltration of α-actin myofibroblast-like cells, presumably responsible for the secretion of collagen, analogous to any other wound-healing process. These tissues, away from the lumen of the parent vessel, need oxygenation and nutrition, provided as everywhere else through capillary neovascularization. There is no perceptible difference inside the coil mass where it succeeded in thrombosing most of the aneurysm, at the fundus or between occluded and recurring lesions. In this model at least, recurrences do not seem to be caused by a deficient healing mechanism but by the fact that though healing mechanisms succeed in replacing the thrombus and forming an organized connective tissue filling the fundus, they seem to occur concurrently with the formation of a recurring space near the aneurysm wall at the neck.
It would seem that because the battle is won at the fundus, it is lost at the same time at the neck, with a progressive biologic reaction leading to growth of the residuum and displacement of the metal mass toward the fundus. Contraction of the connective tissues, composed of an interconnecting network of α-actin+ cells spreading their cell extensions in a collagenous matrix, in the presence of a completely sealed neck, leads to shrinking of the occluded fibrosed cavity. Therefore, the same phenomenon, in the presence of a crescentic endothelialized space flowing with blood separating the wall from the mass of coils embedded in organizing connective tissues, can only lead to shrinking of the coil mass and its displacement toward the fundus, where it is attached, and to opening of the recurring space, with progressive enlargement of the recurrence and apparent coil compaction (Fig 2B).
We believe there are no failures and deficiencies here. We may judge recanalization as a failure of treatment, but this is no failure from the standpoint of nature, whose aims may differ from ours. As shown in a single-coil arterial occlusion model that we have extensively studied, mechanisms of nature are very successful indeed, because they seem to be a most efficient way to re-establish flow in an occluded artery, a phenomenon that can be observed in 100% of cases when coils are not radioactive14 or when the artery is not denuded of its endothelial lining.15 Of course, recanalization of arteries and of aneurysms after coil occlusion may differ, even though we believe some common mechanism is involved.
Another important finding of this study is the association between, on the one hand, a well-defined neck, better initial and follow-up angiographic results, and better neointimal closure of the neck and, on the other, a wide neck incorporating a segment of the parent bifurcation, inferior initial results, increased recurrences, and incomplete neointimal closure of the neck at pathology. Such associations make intuitive sense and agree with clinical series.3 The difference in neck size between the 2 groups may also be an important factor, though it was not statistically significant.
A less intuitive finding is the occurrence of poorly occluded necks and incomplete neointimal coverage in many specimens that were stable at follow-up angiography. This finding may suggest that if animals had been followed for longer periods, a larger number of recurrences could have been found. This finding may also suggest some clue as to the puzzle of delayed recurrences. By postulating a time lag between an invisible incomplete neck closure and visible recurrences with coil compaction, we could tentatively propose a reconciliation between the pertinence of wound-healing phenomena, always active within the first few days and weeks after any injury, and clinical recurrences, known to occasionally occur much later.16 This finding, which reminds us of the potential limits of our radiographic evaluation, in which the high density of platinum may hide a considerable portion of residual necks after coiling, has also been documented in human aneurysms.17
There are many limitations to this work. The aneurysm is a surgical construction, venous pouches are certainly not identical to human aneurysms, and the aneurysmal environment in the soft tissues of the neck does not reproduce the subarachnoid spaces surrounding human aneurysms. We studied specimens at 3 months only; earlier and later time points could also be very informative. Canine aneurysms do not provide the “truth” about aneurysms; species may react differently, because it was shown that porcine aneurysms have a propensity for permanent cures, whereas rabbit models may provide examples of poor organization of thrombus and a higher incidence of recurrences.8 Finally, another weakness was the use of a variety of platinum coils from different manufacturers, though we doubt this had a significant effect on results.
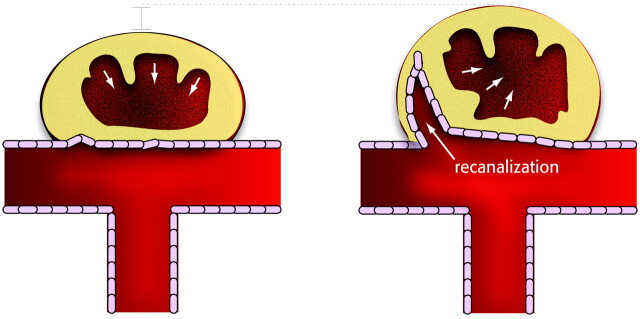
Nevertheless, we believe this experience demonstrates that both stable and complete closure of the neck, as well as incomplete closure with residual and recurrent aneurysms, can be reproduced in animal models. Both outcomes can be associated with thrombus organization, neointima formation, and full endothelialization, which can hardly be qualified as deficient; the main difference between satisfactory and unsatisfactory results is not what type of tissue is formed after coil embolization, but where it is formed, whether it completely closes the neck of the aneurysm from the start, or whether it leaves a crescentic endothelialized space at the neck, which will be followed by the development of a recurrence. This concept is illustrated in Fig 8. Because the infiltration of the thrombus by both endothelial and myofibroblastic cells is so efficient in re-establishing blood flow, at least in arterial occlusion models, we have chosen to consider the problem as one involving an active process (and not a deficiency), recanalization, increasingly recognized in vascular research.18 This concept of an active recanalization seems to depend on the presence of a healthy endothelial lining.15,19–21 Hence, future research on improving results of endovascular treatment of aneurysms could focus on the inhibition of this normal process, reproducible in animal aneurysm models.
Fig 8.
Schematic illustration summarizing the differences between occlusion and recanalization.
Conclusion
Wide-necked canine bifurcation aneurysms (Y-type) are less completely coiled and recur more frequently than narrow-necked aneurysms (T-type). Stable angiographic results at 3 months can occur with incomplete neointimal closure of the neck at pathology. Thrombus organization, endothelialization, and neointima formation can occur concurrently with recurrences.
Although research has often attempted to compensate for deficient healing mechanisms, we believe a more promising strategy is inhibition of an active process, recanalization.
Footnotes
This work was supported by grants from the Canadian Institutes of Health Research (MOP-44062) and the Hearth and Stroke Foundation of Canada.
References
- 1.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–74 [DOI] [PubMed] [Google Scholar]
- 2.Campi A, Ramzi N, Molyneux AJ, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke 2007;5:1538–44 [DOI] [PubMed] [Google Scholar]
- 3.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–403 [DOI] [PubMed] [Google Scholar]
- 4.Raymond J, Salazkin I, Gevry G, et al. Interventional neuroradiology: the role of experimental models in scientific progress. AJNR Am J Neuroradiol 2007;28:401–05 [PMC free article] [PubMed] [Google Scholar]
- 5.Dai D, Ding YH, Kadirvel R, et al. A longitudinal immunohistochemical study of the healing of experimental aneurysms after embolization with platinum coil. AJNR Am J Neuroradiol 2006;27:736–41 [PMC free article] [PubMed] [Google Scholar]
- 6.Dai D, Ding YH, Danielson MA, et al. Histopathologic and immunohistochemical comparison of human, rabbit, and swine aneurysms embolized with platinum coils. AJNR Am J Neuroradiol 2005;26:2560–68 [PMC free article] [PubMed] [Google Scholar]
- 7.Krings T, Busch C, Sellhaus B, et al. Long-term histological and scanning electron microscopy results of endovascular and operative treatments of experimentally induced aneurysms in the rabbit. Neurosurgery 2006;59:911–23 [DOI] [PubMed] [Google Scholar]
- 8.Desfaits AC, Raymond J, Muizelaar JP. Growth factors stimulate neointimal cells in vitro and increase the thickness of the neointima formed at the neck of porcine aneurysms treated by embolization. Stroke 2000;31:498–507 [DOI] [PubMed] [Google Scholar]
- 9.Raymond J, Berthelet F, Desfaits AC, et al. Cyanoacrylate embolization of experimental aneurysms. AJNR Am J Neuroradiol 2002;23:129–38 [PMC free article] [PubMed] [Google Scholar]
- 10.Raymond J, Salazkin I, Georganos S, et al. Endovascular treatment of experimental wide neck aneurysms: comparison of results using coils or cyanoacrylate with the assistance of an aneurysm neck bridge device. AJNR Am J Neuroradiol 2002;23:1710–06 [PMC free article] [PubMed] [Google Scholar]
- 11.Venne D, Raymond J, Allas S, et al. Healing of experimental aneurysms. II. Platelet extracts can increase the thickness of the neointima at the neck of treated aneurysms. J Neuroradiol 1999;26:92–100 [PubMed] [Google Scholar]
- 12.Groden C, Hagel C, Delling G, et al. Histological findings in ruptured aneurysms treated with GDCs: six examples at varying times after treatment. AJNR Am J Neuroradiol 2003;24:579–84 [PMC free article] [PubMed] [Google Scholar]
- 13.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg 1999;91:284–93 [DOI] [PubMed] [Google Scholar]
- 14.Raymond J, Lebel V, Ogoudikpe C, et al. Recanalization of arterial thrombus, and inhibition with beta-radiation in a new murine carotid occlusion model: MRNA expression of angiopoietins, metalloproteinases, and their inhibitors. J Vasc Surg 2004;40:1190–98 [DOI] [PubMed] [Google Scholar]
- 15.Raymond J, Guilbert F, Metcalfe A, et al. Role of the endothelial lining in recurrences after coil embolization: prevention of recanalization by endothelial denudation. Stroke 2004;35:1471–75 [DOI] [PubMed] [Google Scholar]
- 16.Raymond J, Guilbert F, Weill A, et al. Follow-up of treated aneurysms: the challenge of recurrences and potential solutions. Neuroimaging Clin N Am 2006;16:513–23 [DOI] [PubMed] [Google Scholar]
- 17.Bocher-Schwarz HG, Ringel K, Bohl J, et al. Histological findings in coil-packed experimental aneurysms 3 months after embolization. Neurosurgery 2002;50:379–84 [DOI] [PubMed] [Google Scholar]
- 18.Moldovan NI, Asahara T. Role of blood mononuclear cells in recanalization and vascularization of thrombi: past, present, and future. Trends Cardiovasc Med 2003;13:265–69 [DOI] [PubMed] [Google Scholar]
- 19.Raymond J, Metcalfe A, Salazkin I, et al. Endoluminal cryotherapy to prevent recanalization after endovascular occlusion with platinum coils. J Vasc Interv Radiol 2006;17:1499–504 [DOI] [PubMed] [Google Scholar]
- 20.Raymond J, Ogoudikpe C, Metcalfe A, et al. Endovascular treatment with platinum coils: recanalization is associated with early increased von Willebrand factor mRNA. Interv Neuroradiol 2006;12:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouzeghrane F, Darsaut T, Salazkin I, et al. MMP-9 may play a role in recanalization and recurrence after therapeutic embolization of aneurysms or arteries. J Vasc Interv Radiol 2007;18:1271–79 [DOI] [PubMed] [Google Scholar]