Abstract
Background
The conventional type 1 dendritic cell subset (cDC1) is indispensable for tumor immune responses and the efficacy of immune checkpoint inhibitor (ICI) therapies in animal models but little is known about the role of the human CD141+ DC cDC1 equivalent in patients with melanoma.
Methods
We developed a flow cytometry assay to quantify and characterize human blood DC subsets in healthy donors and patients with stage 3 and stage 4 metastatic melanoma. To examine whether harnessing CD141+ DCs could improve responses to ICIs in human melanoma, we developed a humanized mouse model by engrafting immunodeficient NSG-SGM3 mice with human CD34+ hematopoietic stem cells (HSCs) from umbilical cord blood followed by transplantation of a human melanoma cell line and treatment with anti-programmed cell death protein-1 (anti-PD-1).
Results
Blood CD141+ DC numbers were significantly reduced in patients with stage 4 melanoma compared with healthy controls. Moreover, CD141+ DCs in patients with melanoma were selectively impaired in their ability to upregulate CD83 expression after stimulation with toll-like receptor 3 (TLR3) and TLR7/8 agonists ex vivo. Although DC numbers did not correlate with responses to anti-PD-1 and/or anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) ICIs, their numbers and capacity to upregulate CD83 declined further during treatment in non-responding patients. Treatment with anti-PD-1 was ineffective at controlling tumor growth in humanized mice but efficacy was enhanced by indirectly expanding and activating DCs in vivo with fms-like tyrosine kinase-3 ligand (Flt3L) and a TLR3 agonist. Moreover, intratumoral injections of CD141+ DCs resulted in reduced tumor growth when combined with anti-PD-1 treatment.
Conclusions
These data illustrate quantitative and qualitative impairments in circulating CD141+ DCs in patients with advanced melanoma and that increasing CD141+ DC number and function is an attractive strategy to enhance immunogenicity and response rates to ICIs.
Keywords: dendritic cells, immunogenicity, vaccine, immunotherapy, Melanoma
Background
Dendritic cells (DCs) are professional antigen-presenting cells that serve as master regulators of CD4+ and CD8+ T cell responses. They are attractive targets to harness as vaccines for enhancing tumor immune responses, particularly when combined with other treatments such as immune checkpoint inhibitors (ICIs).1 2 Circulating DCs in humans are comprised of distinct subsets with specialized functions, namely conventional (c) DC1 (CD141/BDCA-3+ DCs), cDC2 (CD1c/BDCA-1+ DCs) and plasmacytoid (p) DCs. A separate subset, monocyte-derived (Mo) DCs, arises during inflammation and infection.1 3 Conserved across species, cDC1 specialize in eliciting CD8+ T cell responses, while cDC2 primarily orchestrate CD4+ T cell responses.1 3 Plasmacytoid DCs (pDCs) are known as potent type I interferon (IFN-I) producers important for anti-viral immunity, while MoDCs appear more specialized in controlling inflammatory responses.1 3
Human MoDCs generated from monocytes with interleukin (IL)-4 and granulocyte-macrophage colony-stimulating factor in vitro have been the DC subset most commonly used as vaccines in the clinic.3 4 While CD1c+ DCs and pDCs have also shown potential utility, accumulating evidence points to a critical role for CD141+ DCs in CD8+ T cell-mediated tumor immunity.1 Mouse cDC1 are required for anti-tumor responses in inducible and spontaneously regressing tumor models including melanoma, lymphoma, breast cancer, colorectal cancer, thymoma, pancreatic cancer, and others, and are required for the efficacy of adoptive T cell therapies, anti-PD-1/anti-CTLA-4 ICI, and anti-CD137 agonistic immunotherapies.1 5–15 Enhancing mouse cDC1 numbers and activation with combined Flt3L and the TLR3 agonist polyinosinic:polycytidylic acid (PolyIC), respectively, has provided proof of principle that cDC1 can be therapeutically targeted to increase immunogenicity and responses to immunotherapy in these models.5 9 11 12 14 15 CD141+ DC/cDC1 gene signatures within human tumors are highly correlated with CD8+ T cell gene signatures,16 with survival in melanoma, lung cancer, breast cancer, and head and neck squamous cell carcinoma (HNSCC),17 18 and with responses to anti-PD-1 in melanoma,17 suggesting that human CD141+ DCs may play a similar role in tumor immune responses to cDC1 in mice.
Blood-based assays are attractive because of the non-invasive nature of sample collection and their potential to serve as predictive biomarkers of responses to therapeutics. For example, PD-L1 and CD137 expression on peripheral T cells have been reported as response biomarkers to ICIs in melanoma.19 Circulating DCs are known to be numerically and functionally impaired in patients with cancer, including melanoma, breast cancer, prostate cancer, and mesothelioma,1 but a detailed subset analysis in advanced melanoma or within the context of immunotherapy has not been performed. We examined human DC subsets in the blood of patients with advanced melanoma and found numerical and functional defects in the CD141+ DC compartment, however, these did not correlate with responses to ICI. Using a humanized mouse model comprised of a human melanoma growing alongside a reconstituted human immune system, we demonstrated that enhancing CD141+ DC number and function, via Flt3L and PolyIC, respectively,5 9 11 12 14 15 synergized with anti-PD-1 to limit tumor growth. Further, vaccination with CD141+ DCs as a cellular therapy was effective at enhancing anti-PD-1 efficacy. Taken together, our findings demonstrate that while CD141+ DCs may be decreased and impaired in advanced melanoma, they can be harnessed and targeted to restore immunogenicity to melanoma and responses to ICIs.
Materials and methods
Human samples
Blood was obtained from patients with stage 3 (n=32) and stage 4 (n=21) advanced melanoma and age-matched and sex-matched healthy volunteers (n=25) (online supplemental table 1). Samples were also collected from patients with melanoma pre-treatment and at 6, 12, and 24 weeks during treatment with ICIs including pembrolizumab (anti-PD-1, Keytruda), ipilimumab (anti-CTLA-4, Yervoy), or combined nivolumab (anti-PD-1, Opdivo) and ipilimumab (n=18) (online supplemental table 2). Clinical responses were evaluated by radiological scans according to standard response criteria20 (online supplemental table 2 and figure 1). Umbilical cord blood (UCB) was obtained from the Queensland Cord Blood Bank. CD34+ hematopoietic stem cells (HSCs) were isolated from UCB via density gradient centrifugation with Ficoll-Paque (GE Healthcare) followed by a CD34+ isolation kit (Miltenyi Biotec) and cryopreserved until use.
jitc-2020-001963supp001.pdf (3.5MB, pdf)
Whole blood assays
For quantitation of cell subsets, 400 µL whole blood collected in EDTA anticoagulant was reverse pipetted into a 5 mL round-bottom polystyrene tube (Corning), and incubated for 15 mins in the dark at room temperature with anti-human antibodies (all purchased from BioLegend unless otherwise stated) CD45-APC-Cy7 (HI30), CD14-Alexa Fluor 700 (HCD14), CD16-BV785 (3G8), CD3- (OKT3), CD19- (HIB19), CD20- (2H7), CD56-Pacific Blue (HCD56), HLA-DR-PerCP-Cy5.5 (L243), CD123-BUV395 (1A4, BD Biosciences), CD1c-PE Dazzle 594 (L161), and CD141-PECy7 (M80). Red cells were lysed using 1x FACS lysing solution (BD), then leukocytes were centrifuged at 500 g for 5 mins and resuspended in 700 µL autoMACS running buffer (MACS buffer) (Miltenyi) containing 10,050 TruCount beads (BD). Samples were acquired on a LSR Fortessa X-20 cytometer (BD) and analyzed using FlowJo software V.10.2. Absolute cell counts were reported as cells per mL blood, calculated as number of gated cells/number of beads acquired x 10,050 beads per tube/400 µL x 1000 µL.
For DC phenotyping, 1 mL EDTA whole blood was cultured in 15 mL Falcon tubes (Corning) alone or with 25 µg/mL high molecular weight PolyIC (InvivoGen) and 1 µg/mL R848 (InvivoGen) for 4 hours at 37°C, 5% CO2. Samples were stained with the antibodies listed above except for CD14-Alexa Fluor 700 and CD16-BV785, and with the addition of CD14- (HCD14) and CD16-Pacific Blue (3G8), CD40-APC (5C3) or BTLA-APC (J168-540, BD), CD83-FITC (HB15e, BD), PD-L1-PE (MIH1, BD), and Live/Dead Aqua (Thermo Fisher). Readouts for phenotyping markers were reported as geometric mean fluorescence intensity corrected for background from the fluorescence minus one controls. Chemokine and cytokine analysis was performed on plasma collected from lithium heparin whole blood cultured alone or with PolyIC and R848 for 4 hours, using a LEGENDplex human antiviral kit (BioLegend), or ELISA for Flt3L (Quantikine kit, R&D Systems) and G-CSF (Abcam), as per manufacturer’s instructions.
Generation of a humanized mouse melanoma model
Neonatal NSG-SGM3 mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3, CSF2, KITLG)1Eav/MloySzJ; stock no. 013062, The Jackson Laboratory) were irradiated with 1 Gy prior to intra-hepatic injection of human UCB CD34+ HSCs. Human CD45 chimerism was confirmed approximately 8 weeks post engraftment by staining peripheral blood with anti-human CD45-APC-Cy7, anti-mouse CD45-V500 (30-F11, BD) and Live/Dead Aqua.
The human melanoma cell line LM-MEL2821 was subcutaneously injected (5×106 cells) into the flank of 9 to 15 weeks old humanized or non-humanized NSG-SGM3 mice. Tumors were measured two to three times per week using digital calipers. Mice were injected with pembrolizumab or isotype control antibody (mouse IgG1κ, MGI-45, BioLegend) intraperitoneally (100 µg/100 µL HBSS) on days 13, 15, and 18 post tumor injection. Human recombinant Flt3L (BioXCell) was administered (50 µg/100 µL HBSS) subcutaneously at days 9 and 15 post tumor injection. PolyIC (50 µg/10 µL HBSS) was intratumorally injected on days 14 and 19. Mice were culled 35 days post tumor injection or when tumors reached 1000 mm3. Blood and bone marrow were collected, while spleens and tumors were digested in collagenase IV (Sigma) and DNase I (Sigma) followed by Percoll density gradient (spleen only) as previously described22. Five million cells from each organ were stained with anti-human-CD45-APC-Cy7, anti-CD3-BV711 (OKT3), anti-CD8-BUV395 (RPA-T8, BD), anti-CD19/CD20-Pacific Blue, anti-CD11c-PE-CF594 (B-ly6, BD), anti-CLEC9A-PE (8F9), anti-CD1c-Alexa Fluor 700 (L161), anti-CD141-PECy7, anti-CD11b-BV650 (3.9), anti-HLA-DR-PerCP-Cy5.5, anti-CD56-FITC (HCD56), anti-CD123-BUV395 (7G3, BD), anti-mouse CD45-V500, and Live/Dead Aqua in 100 µL PBS for 20 to 30 mins at 4°C before acquisition on LSR Fortessa X-20 cytometer.
Intratumoral administration of DC subsets in humanized mice
DC subsets for vaccination were isolated from spleens and bone marrow of humanized non-tumor-bearing littermates treated with Flt3L as previously described.22 Briefly, DCs were enriched by negative selection from bone marrow cells by incubation with anti-mouse Ter119 (TER-119), anti-human CD14 (RMO52, Beckman Coulter), anti-human CD19 (J3-119, Beckman Coulter), anti-human CD3 (OKT3, BioXCell), anti-human CD34 (My10, BD), and anti-mouse CD45 (30-F11, BD) followed by removal of antibody-bearing cells using sheep anti-rat IgG Dynabeads (Invitrogen). Enriched cells were stained with anti-human CD45-APC-Cy7, anti-HLA-DR-PECy7 (L243), anti-CD123-PerCP-Cy5.5 (6H6), anti-CD141-APC (M80), anti-CD1c-PE (L161), anti-CD11c-PE-CF594, and anti-CD3/CD19/CD20-Pacific Blue. DC subsets were sorted as CD3/CD14/CD19/CD20–HLA-DR+CD11c+CD141+ (cDC1) and CD3/CD14/CD19/CD20–HLA-DR+CD11c+CD141– (cDC2) using a MoFlo Astrios cell sorter (Beckman Coulter). DCs were activated for 2 hours with 10 µg/mL PolyIC prior to intratumoral injection of 20-25 x 103 DCs per mouse.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 7.0. Normality of data was assessed by Shapiro-Wilk test. Comparisons between two groups were performed using paired or unpaired t-test as indicated. For comparisons between three or more groups, one-way or two-way analysis of variance (ANOVA) with Bonferroni’s multiple comparisons test was used. Results were considered statistically significant when p value was<0.05.
Results
CD141+ DCs are numerically and functionally impaired in patients with advanced melanoma
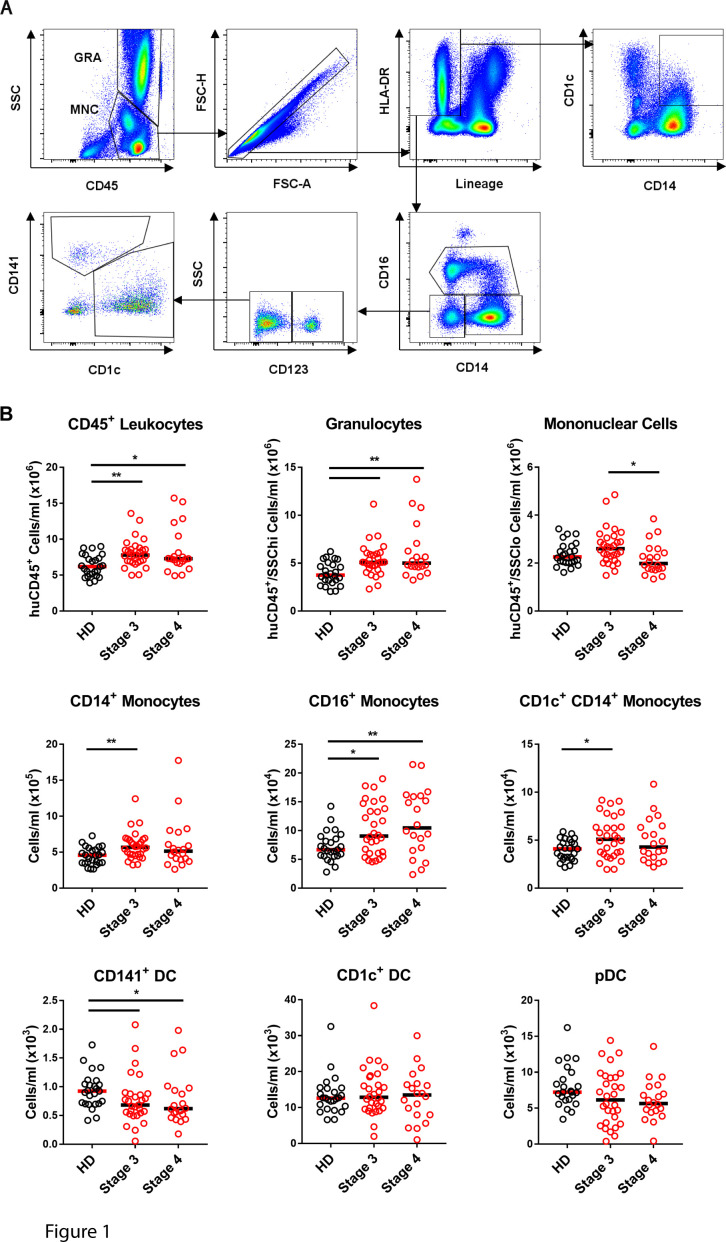
We developed a modified TruCount assay23 using 400 µL of fresh whole blood to compare DC numbers in patients with advanced melanoma (stage 3, n=29–30, stage 4, n=20) with age-matched and sex-matched healthy donor controls (n=24–25) (figure 1A). Some of the patients with melanoma were newly diagnosed while others had previous surgery (35.8%), radiation (17%) or systemic therapy (1.9%, online supplemental table 1). Total CD45+ leukocytes were elevated both in stage 3 and stage 4 patients relative to controls (figure 1B). Within the CD45+ compartment, patients with both stage 3 and stage 4 melanoma had increased granulocytes and CD16+ monocytes compared with healthy donor controls (figure 1B). CD14+ monocytes, including a CD1c+ CD14+ subpopulation previously found to be increased in patients with melanoma,24 were significantly increased in patients with stage 3 but not stage 4 (figure 1B). While CD1c+ DC and pDC numbers were unaltered in patients with melanoma, CD141+ DCs were significantly lower in patients with both stage 3 and stage 4 relative to controls (figure 1B).
Figure 1.
CD141+ DCs are selectively decreased in patients with stage 3 and stage 4 melanoma. (A) Gating strategy for immune cell profiling from 400 µL whole blood. CD45+ leukocytes were gated as granulocytes (GRA) and mononuclear cells (MNCs) combined. DCs and monocytes were gated as singlet lineage (CD3, CD19, CD20, CD56)– HLA-DR+ events and monocytes were then gated as CD14+ CD16–, CD16+ and CD1c+ CD14+ subsets. DCs were defined as lineage- HLA-DR+ CD14– CD16– CD123+ pDCs, CD123– CD141– CD1c+ DCs or CD123– CD1c– CD141+ DCs. (B) Absolute cell numbers (per mL whole blood) of each subset in healthy donors (HD, n=24–25), and patients with stage 3 (n=29–30) and stage 4 (n=20) melanoma. Median values are shown. *p<0.05, **p<0.01 by one-way analysis of variance. DCs, dendritic cells.
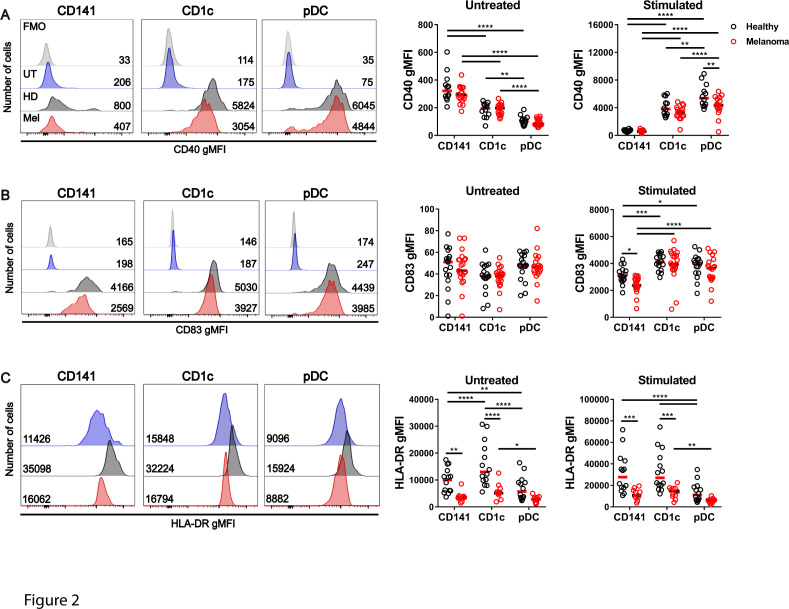
To examine DC function, whole blood was stimulated for 4 hours with PolyIC (TLR3 agonist) combined with R848 (TLR7/8 agonist) to activate all DC subsets25 26 (controls, n=12–16, patients, n=11–22). In the absence of activation, CD40 was expressed by a subpopulation of CD141+ DCs in both healthy donors and patients with advanced melanoma, and was only modestly increased after activation (figure 2A). In contrast, CD40 was not expressed by CD1c+ DCs or pDCs in the steady state but induced within 4 hours of activation. CD1c+ DCs and CD141+ DCs from patients with advanced melanoma expressed similar levels of CD40 to healthy donors after activation, however pDCs from patients with melanoma expressed significantly lower CD40 after activation when compared with healthy donors (figure 2A). CD83 expression was not detectable in the absence of activation but was induced on all DC subsets after activation (figure 2B). CD83 was upregulated to a similar degree by CD1c+ DCs and pDCs from patients with melanoma and healthy donors, but expression levels were selectively impaired on CD141+ DCs from patients with melanoma (figure 2B). HLA-DR was constitutively expressed by all DC subsets in the steady state and was further upregulated after activation, but was significantly lower in patients with melanoma compared with healthy donors, both in the steady state and after activation (figure 2C). However the fold change in expression of CD40, CD83 and HLA-DR after activation relative to the matched non-activated samples was similar for all DC subsets in healthy donors and patients with melanoma (online supplemental figure 2). This suggests basal defects in expression of these molecules in melanoma patient DCs that are maintained after activation rather than specific defects in their upregulation. BTLA was highly expressed on CD141+ DCs, moderately on pDCs, and weakly on CD1c+ DCs in the steady state, and only downregulated on pDCs after activation (online supplemental figure 3A). PD-L1 was not expressed by any DC subset in the steady state but induced on all DC subsets after activation (online supplemental figure 3B). However, no differences were observed between patients with melanoma and healthy donors in expression of BTLA and PD-L1 by DC subsets (online supplemental figure 3A, B). Taken together, these data reveal that patients with advanced melanoma have subset-specific deficits in DC quantity and quality. Notably, CD141+ DCs are decreased in number and expression of CD83 and HLA-DR after activation.
Figure 2.
Activation of CD40, CD83, and HLA-DR by DC subsets in patients with advanced melanoma and healthy donors. Expression of CD40 (A), CD83 (B), and HLA-DR (C) by DC subsets from patients with advanced (stage 3/4) melanoma and healthy donor controls after ex vivo whole blood culture alone (untreated) or after activation with PolyIC +R848. Left panels: Representative histograms showing fluorescence minus one (FMO) control (light gray), and marker expression on untreated (blue) or activated DC subsets from healthy donors (dark gray) and patients with melanoma (red). Number represents geometric mean fluorescence intensity (gMFI) values. Right panels: Compiled background-subtracted gMFI of expression of each marker (healthy controls, n=12–16, patients, n=11–22). Median values are shown. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by two-way analysis of variance. pDC, plasmacytoid dendritic cell.
Plasma IL-1β and IL-8 detected from stimulated whole blood is elevated in advanced melanoma patients
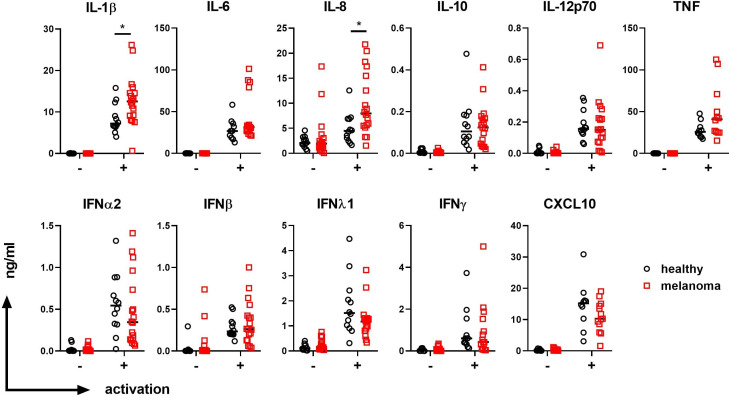
We examined DC and monocyte-associated cytokines in the plasma of healthy donors (n=10–12) and patients with advanced melanoma (n=12–18) after a 4-hour stimulation of whole blood in the presence or absence of PolyIC+R848. Under these conditions, CD141+ DCs are the major producers of IFNλ1, CD1c+ DCs the major producers of IL-12p70, and pDCs the major producers of IFNα2.22 25 IFNλ1 was present at low levels in unstimulated plasma and increased to a similar degree in the plasma of healthy donors and patients with melanoma after activation (figure 3). IL-12p70 and IFNα2 were undetectable in the absence of activation and were induced after activation, with no significant differences in their levels between healthy controls and patients with melanoma (figure 3). Inflammatory cytokines produced by DCs as well as other leukocyte subsets were also examined. In the absence of activation, melanoma patient and healthy donor plasma had similar levels of inflammatory cytokines IL-6, IL-8, and TNFα, the hematopoietic growth factor Flt3L, and the chemokine CXCL10, while IL-1β was undetectable. IL-1β, IL-6, IL-8, TNFα and CXCL10 were increased after activation with PolyIC+R848 (figure 3). Notably, significantly higher levels of IL-1β and IL-8 levels were found in melanoma patient plasma compared with healthy donor plasma after activation. No differences were observed in the levels of IL-6, IL-10, TNFα, IFNβ, IFNγ, and CXCL10 between patients with melanoma and healthy donors (figure 3).
Figure 3.
Plasma IL-1β and IL-8 after whole blood stimulation is elevated in patients with advanced melanoma. Plasma, IL-1β, IL-6, IL-8, IL-10, IL-12p70, TNF, IFNα2, IFNβ, IFNλ1, IFNγ, and CXCL10 in the absence (-) or presence (+) of activators PolyIC+R848, in healthy donor controls (n=10–12) and patients with stage 3 and stage 4 melanoma (n=12–18). Median values are shown. *p<0.05 by Welch’s t-test. IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
CD141+ DC number and function are decreased in patients who do not respond to immunotherapy
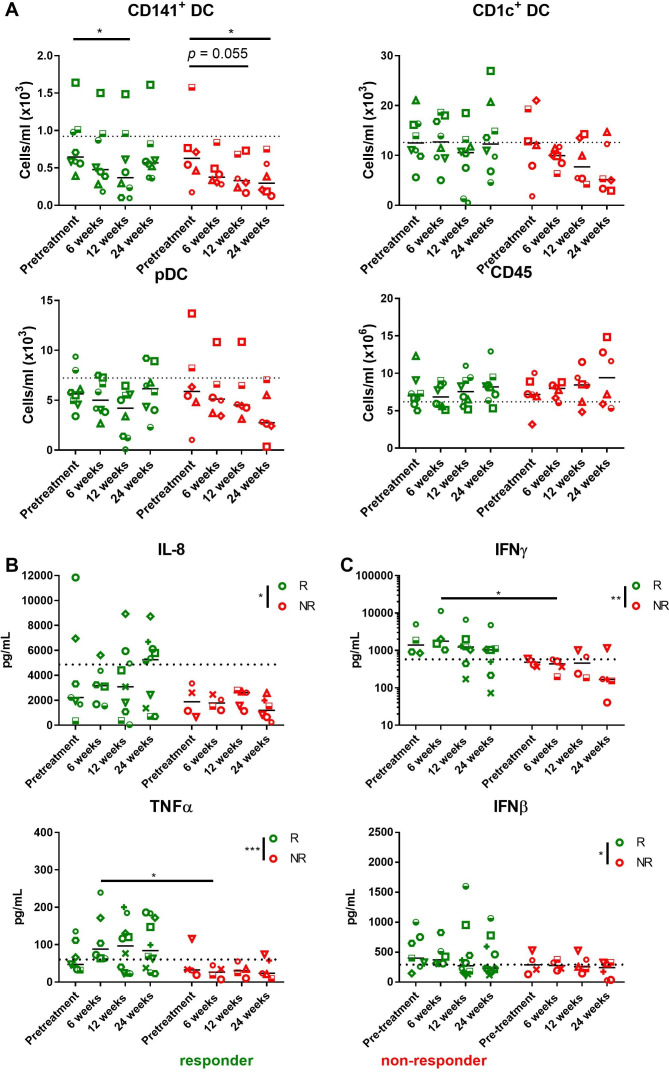
DC subset numbers and function were further investigated in patients receiving anti-PD-1 and/or anti-CTLA-4 immunotherapy (n=18) (online supplemental table 2 and figure 1). Patients were categorized as responders to immunotherapy (complete or partial responders) and non-responders. Prior to treatment, there were no significant differences in total CD45+ leukocytes, granulocytes, mononuclear cells (MNCs), or monocyte and DC subsets in responding compared with non-responding patients (online supplemental figure 4A). Total CD45+ leukocytes counts were not significantly altered in responding or non-responding patients over the 24 weeks treatment duration (figure 4A). Although CD141+ DC numbers were significantly decreased in responding patients after 12 weeks of immunotherapy, their levels were comparable to pretreatment by 24 weeks (figure 4A). This observation is consistent with the time point at which clinical responses were beginning to be observed (mean 22 weeks, online supplemental figure 1). By contrast, CD141+ DC numbers had significantly declined after 24 weeks of treatment compared with pre-treatment counts in non-responding patients (figure 4A). A similar trend was observed for CD1c+ DC and pDC counts in non-responding patients, however the results did not reach statistical significance.
Figure 4.
CD141+ DCs and plasma IL-8, TNFα, IFNγ, and IFNβ are decreased in patients with melanoma who do not respond to immunotherapy. (A) DC subset and CD45+ leukocyte numbers in patients with advanced melanoma at pre-treatment, and at 6, 12, and 24 weeks on treatment, segregated as responders (green, n=8) and non-responders (red, n=6). Dotted lines represent median numbers of each subset in healthy donors. Median values are shown. Each symbol represents individual patient. Statistical significance was determined using two-way ANOVA. *p<0.05. (B, C) Comparison between responders and non-responders in plasma cytokine and IFN production. (B) IL-8 and TNFα were measured at steady state and (C) IFNγ, and IFNβ were measured post stimulation with PolyIC+R848. Samples were collected at baseline (pre-treatment) or later time points as indicated, across n=4–10 responders and n=4–6 non-responders. Dotted lines represent median values of cytokine production based on healthy controls. Median values are shown. Statistical significance was determined using two-way ANOVA followed by Bonferroni’s multiple comparison test. *p<0.05, **p<0.01, ***p<0.001. ANOVA, analysis of variance; DCs, dendritic cells; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
We next examined DC function in responders and non-responders across time through phenotypic assessment of DC subsets post PolyIC+R848 stimulation. When pre-treatment samples were compared between responders and non-responders, no differences in CD83 expression were observed in any DC subset (online supplemental figure 4B). At 24 weeks into treatment, however, CD83 expression on stimulated CD141+ and CD1c+ DCs was significantly lower in non-responders than responders, while that on stimulated pDCs was higher in non-responders at 12 weeks into treatment (online supplemental figure 4B). Interestingly, in non-responding patients only there was significantly lower CD83 expression on all DC subsets at 24 weeks into treatment compared with 12 weeks, and in CD1c+ DCs in particular, CD83 expression was significantly lower at 24 weeks compared with all previous time points (online supplemental figure 4B). CD40 expression on stimulated CD1c+ DCs and pDCs also significantly decreased between 12 and 24 weeks into treatment in non-responders, with a similar, but not statistically significant trend observed for CD141+ DCs in non-responders and in all DC subsets in the responding patients (online supplemental figure 4C). No significant differences in CD40 expression were observed between responders and non-responders in any DC subset when comparing between samples at matched time points (online supplemental figure 4C). Taken together, these data show a further decline in DC numbers, and functional deficits in upregulation of CD83 and CD40 by DC subsets in patients who fail to respond to immunotherapy after 24 weeks of treatment.
Lower plasma TNFα, IL-8, IFNγ, and IFNβ were observed in non-responders to immunotherapy
Cytokine levels before and after whole blood TLR stimulation were examined in the plasma of patients with melanoma receiving immunotherapy (n=4–10 responders and n=4–6 non-responders). In the absence of TLR stimulation, significantly lower levels of plasma IL-8 and TNFα were observed in non-responding compared with responding patients during treatment with immunotherapy (figure 4B). Lower levels of IL-6 and CXCL10 were also observed in non-responding patients but these did not reach statistical significance (online supplemental figure 5). After whole blood stimulation with PolyIC+R848, non-responding patients produced lower levels of IFNγ and IFNβ (figure 4C). No differences between responding and non-responding patients were found in the levels of plasma cytokines IL-6, IL-10, IL-12p70, IFNα, and IFNλ prior to or during treatment with immunotherapy (online supplemental figure 5). In summary, while cytokine response was variable between responders and non-responders, and dynamic across time, lower levels of plasma TNFα and IL-8 in the absence of activation, and lower IFNγ, and IFNβ after TLR activation were characteristic of patients who failed to respond to immunotherapy.
Increased numbers of CD141+ DC enhances response to pembrolizumab in a humanized mouse model of melanoma
To examine whether improving human CD141+ DC quantity or quality can improve responses to ICIs in human melanoma, we developed a humanized mouse model where human melanoma develops in the presence of a human immune system in NSG-SGM3 mice (figure 5A). In this model, the frequency of human CD45+ cells among total leukocytes in blood was 23.3% (±11%, n=25) at 8 weeks, and 40% (±23%, n=23) at 12 weeks post reconstitution of irradiated neonatal NSG-SGM3 mice with human cord blood CD34+ HSCs (online supplemental figure 6A, B). Human CD45+ leukocytes developed in the bone marrow, liver, spleen, and blood, and comprised CD3+ T cells, CD19/20+ B cells, CD56+ NK cells, along with plasmacytoid and both subsets of conventional DCs (figure 5B, C, online supplemental figure 6C, D). The presence of a human melanoma cell line LM-MEL2821 implanted subcutaneously in the flanks of humanized NSG-SGM3 (huNSG-SGM3) mice did not significantly alter frequencies of human immune populations in spleens or blood of humanized mice in the absence of immunotherapy (figure 5C, online supplemental figure 6C). LM-MEL-28 tumors were infiltrated with human CD45+ leukocytes, the majority of which were CD11b+ myeloid cells (mean 43.72%±7.97 SEM, n=9) and CD3+ T cells (mean 25.07%±5.38 SEM, n=9; figure 5D). CD141+ DCs (0.32%), CD1c+ DCs (0.76%) and pDCs (4.59%) were also detectable in very low frequencies in some tumors (online supplemental figure 7A).
Figure 5.
Development of a melanoma model in humanized NSG-SGM3 mice. (A) Neonatal NSG-SGM3 mice were treated with 1 Gy irradiation before intrahepatic injection of human CD34+ cells. Eight weeks later, human chimerism in peripheral blood was confirmed with flow cytometry. Mice were then injected subcutaneously with the patient-derived melanoma cell line LM-MEL28 before treatment with Flt3L, PolyIC and/or pembrolizumab (immunotherapy). (B) Human immune populations in spleens of humanized (hu) NSG-SGM3 mice at 12 weeks post engraftment showing identification of DC subtypes (n=5–6 mice per group). (C) Proportions of human immune lineages in spleens of non-treated huNSG-SGM3 mice±subcutaneous LM-MEL28 tumors (n=5 tumor-bearing, 3 non-tumor-bearing mice). (D) Infiltration of human CD45+ leukocytes in LM-MEL28 tumors in vivo, a representative gating strategy is shown on the left and percentages of each subset within the total human CD45+ cells (mean±SEM, n=9 mice) shown on the right. (E) NSG-SGM3 mice injected with human CD34+ cells (‘engrafted’) were injected with melanoma cells alongside age-matched non-engrafted mice. Tumor growth curves and tumor weights (excised at day 35) are shown; no significant difference between groups was observed using Student’s t-test (n=5–6 mice per group).
Tumors grew at a similar rate following implantation into NSG-SGM3 mice in the presence or absence of a human immune system (figure 5E). Thus, we established a manipulable immunocompetent in vivo model containing integral human cell types necessary for immunotherapy.
Murine melanoma models have provided evidence to demonstrate that cDC1 (equivalent of human CD141+ DCs) are indispensable for the efficacy of anti-PD-L1, anti-PD-1, and agonistic anti-CD137 therapies.7 9 Responses to ICIs in these melanoma models can be enhanced by increasing cDC1 quantity via administration of the cytokine Flt3L, along with site-specific activation using intratumoral PolyIC.6 7 We therefore examined whether improving human CD141+ DC quantity and quality could increase response to pembrolizumab in huNSG-SGM3 mice (figure 6A).
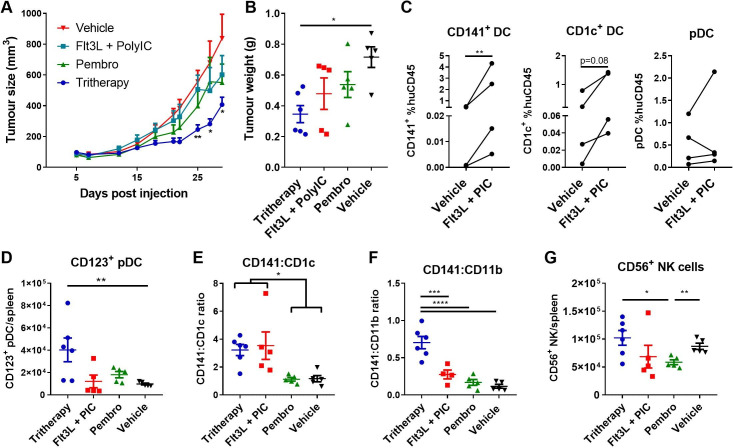
Figure 6.
In vivo DC targeting with Flt3L and PolyIC increases efficacy of pembrolizumab. (A) Tumor growth curves for tumor-bearing huNSG-SGM3 mice treated with respective regimens are shown (n=5–6 mice per group). Data are the combined replicates from two independent experiments. (B) Corresponding tumor weights from mice shown in (A). (C) Percentage of DC subsets within the human CD45+ population in spleens of Flt3L and PolyIC-treated mice 10 days after treatment commencement (day 19). Interconnecting lines represent mice engrafted with the same human donor HSCs. (D) Total number of CD123+ pDCs/spleen is shown for each treatment group. (E) Ratio of CD141+ DC:CD1c+ DC in spleens of mice in each treatment group. (F) Ratio of CD141+ DC:CD11b+ myeloid cells in spleens of mice in each treatment group. (G) Total number of CD56+ NK cells in spleens of huNSG-SGM3 mice. Statistical significance was determined using one-way analysis of variance with Bonferroni’s multiple comparisons test with the exception of ratio-paired t-test in (C). All mice were analyzed at day 35 post-treatment. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Flt3L, fms-like tyrosine kinase-3 ligand; HSCs, hematopoietic stem cells; pDC, plasmacytoid dendritic cell; PolyIC, polyinosinic:polycytidylic acid.
Treatment with pembrolizumab alone had a negligible effect on LM-MEL28 melanoma growth in huNSG-SGM3 mice (figure 6A, B). Flt3L+PolyIC had little effect on tumor growth (figure 6A, B) but increased splenic CD141+ DC and to a lesser extent CD1c+ DC numbers 10 days after the first Flt3L treatment (ie, day 19 of experiment, figure 6C). CD141+ DC (mean 1.33%±1.15 SEM of human CD45+ cells) and CD1c+ DC (mean 3.39%±2.26 SEM of human CD45+ cells) were also clearly detectable in two out of four tumors in Flt3L+PolyIC treated mice at this time point (online supplemental figure 7B).
However, when pembrolizumab, Flt3L, and PolyIC were combined as a tritherapy, tumor growth was significantly impaired compared with control and Flt3L+PolyIC groups (figure 6A, B). Spleens of mice treated with tritherapy or Flt3L+PolyIC also harbored significantly higher numbers of CD123+ pDCs by day 35 (figure 6D). Although there were no significant differences in the number or frequency of CD141+ DCs or CD1c+ DCs by day 35 (online supplemental figure 8), increased ratios of CD141+ DCs relative to CD1c+ DCs and CD11b+ myeloid cells was also observed (figure 6F).
A trend for an increased percentage of CD3+ and CD3+/CD8– (likely CD4+) T cells in tritherapy-treated and pembrolizumab-treated mice compared with Flt3L+PolyIC-treated and vehicle control mice was observed in spleens and tumors, although this did not reach statistical significance (online supplemental figure 8). Differences in the absolute number of CD56+ NK cells were also observed between groups; significantly decreased total numbers were observed in spleens of the pembrolizumab group compared with vehicle control and tritherapy groups (figure 6G).
Intratumoral injection of CD141+ DCs inhibits tumor growth
To provide more direct evidence that increasing CD141+ DC quantity and quality can improve responses to pembrolizumab, humanized mice were treated with pembrolizumab along with intratumoral injections of activated CD141+ DCs derived from autologous HSCs (figure 7A, B). In keeping with previous results (figure 6A, B), pembrolizumab alone did not abrogate tumor growth and this was not improved when combined with adoptive transfer of CD11c+ DCs. However, adoptive transfer of CD141+ DCs combined with pembrolizumab resulted in a significant reduction in tumor growth (figure 7C) that was associated with increased infiltration of CD8+ T cells (figure 7D).
Figure 7.

Intratumoral injection of activated CD141+ DCs increases efficacy of pembrolizumab. (A) Humanized mice were treated with anti-PD-1 with or without 2 x intratumoral injections of sorted CD141+ DCs activated with PolyIC for 2 hours on days 14 and 19 after tumor injection. (B) Expression of CD83 and CD40 by sorted, PolyIC activated CD141+ DC. (C) Tumor growth curves of mice. Shown are mean±SEM of 6–8 mice per group combined from two experiments using two different human HSC donors. (D) Percentage of CD3+CD8+ cells among human CD45+ cells within tumors on day 35 (n=4–5 mice per group). Data represent mean±SEM and statistical significance was determined using one-way analysis of variance with Bonferroni’s multiple comparisons test. *p<0.05, ***p<0.001. DCs, dendritic cells; Flt3L,fms-like tyrosine kinase-3 ligand; PD-1, HSCs, hematopoietic stem cells; programmed cell death protein-1; PolyIC, polyinosinic:polycytidylic acid.
Taken together, our results in a novel humanized mouse model identify increasing CD141+ DC quantity and quality as an attractive strategy to increase immune responses in advanced melanoma resistant to PD-1 blockade.
Discussion
In this study, we identified quantitative and qualitative systemic defects within the DC compartment in patients with advanced melanoma. CD141+ DCs were decreased in number and in their ability to upregulate HLA-DR and CD83 after activation with combined TLR3, TLR7, and TLR8 stimulation. While CD141+ DC counts prior to treatment with ICIs did not associate with responses, their numbers and capacity to activate continued to decline in non-responding patients over 6 months of treatment. Similar trends were observed within the CD1c+ DC and pDC compartment. Using a novel humanized mouse model, we further demonstrated that increasing numbers of activated CD141+ DCs is an attractive strategy to enhance responses to anti-PD-1 in human melanoma.
Human CD141+ DC gene signatures within tumor biopsies are associated with favorable prognosis in melanoma, as well as many other human cancers.16–18 27 28 Increased tumor CD141+ DC frequencies have also been associated with responses to anti-PD-1 in patients with melanoma.17 Although we were unable to obtain tumor biopsies from the patients recruited to this study, migration of CD141+ DCs to the tumor site is one possible explanation for their reduced frequencies in the circulation compared with healthy controls. However, our data suggest that circulating CD141+ DC frequencies do not predict responses to immunotherapy and are therefore unlikely to serve as surrogates for their tumor counterparts. Systemic dysregulation of DC development could also account for the reduced circulating CD141+ DC numbers in patients with advanced melanoma. In support of this, tumor-derived granulocyte colony-stimulating factor (G-CSF) skews myeloid differentiation towards granulocytes and myeloid-derived suppressor cells at the expense of CD141+ DCs and their precursors in breast and pancreatic cancer.29 Impairments in systemic cDC1 levels in a mouse pancreatic cancer model were found to be due to cDC1 apoptosis mediated by tumor-derived IL-6.14 Our data demonstrating increased abundance of granulocytes in patients with advanced melanoma is consistent with their previously reported association with poor prognosis30 31 and supports the notion of dysregulated myeloid development as a contributing factor to the reduced frequencies of CD141+ DCs. Although we did not observe differences in patient plasma Flt3L, IL-6, or G-CSF to support this, it is possible that these factors may act more locally within the bone marrow to regulate hematopoiesis and/or that other factors may be required. Reduced frequencies of circulating DCs have also been found in patients with other cancer types including multiple myeloma, mesothelioma, acute myeloid leukemia, breast, ovarian and prostate cancer.1 32 Systemic DC defects may therefore be a common feature of human malignancy, however patient age, disease type, severity and treatments will likely impact the degree of the defects.
Our data also revealed that CD141+ and CD1c+ DCs in patients with advanced melanoma expressed lower levels of HLA-DR compared with healthy donors. CD141+ DCs also expressed lower levels of the maturation marker CD83 after stimulation with PolyIC and R848. In contrast to CD1c+ DCs and pDCs, CD40 was only modestly upregulated by CD141+ DCs within 4 hours of activation and to a similar degree in patients with melanoma and healthy donors. The ability of DCs to upregulate maturation markers after activation did not associate with responses to immunotherapy prior to commencement of treatment, but declined in non-responding patients after 6 months. Whether these defects are intrinsic to the DCs or are mediated by factors within their environment remain to be determined. Despite this, patients with melanoma retained their ability to produce the cytokines IFNα, IFNλ and IL-12p70 after whole blood activation.
While we did not find significant differences in steady state plasma cytokines, IL-8 and IL-1β were significantly higher in patients with melanoma compared with healthy donors after activation. Interestingly, reduced steady state serum IL-8 has been associated with responses to anti-PD-1 in both melanoma and non-small-cell lung cancer33 while IL-1β, IL-6, and TNFα were increased in patients with melanoma responding to IFNα treatment.34 Although these findings were not replicated in our patient cohort, higher levels of plasma TNFα and IL-8 in the absence of additional ex vivo activation, and IFNγ and IFNβ after activation, were characteristic of patients responding to ICI immunotherapy. Similar but non-significant trends were observed in levels of IL-6 and CXCL10 that warrant follow-up in larger patient cohorts.
The critical role of mouse cDC1 in mediating tumor immunity and responses to ICIs is well established.1 5–15 These models have also demonstrated the potential of enhancing cDC1 quantity and quality, either directly by adoptive transfer, or indirectly via administration of Flt3L+a DC activation stimulus, as a means of improving immune responses to ICIs.1 5–15 Using a novel humanized mouse model comprised of human melanoma growing in the presence of a human immune system, we showed that Flt3L+PolyIC was effective at reducing human melanoma growth when combined with anti-PD-1 and that this was associated with increased proportions of human CD141+ DCs in the spleens. Adoptive transfer of human CD141+ DCs was also effective at limiting tumor growth when combined with anti-PD-1. Flt3L+PolyICLC (the stabilized analog of PolyIC, Hiltonol, Oncovir) administered as an in situ vaccine was shown to be well tolerated and induced systemic cancer remission when combined with radiotherapy in patients with indolent non-Hodgkin’s lymphoma, demonstrating that this approach is safe and feasible in humans, with potential to be combined with ICIs and/or other standard therapies.11 Clinical trials are also underway to investigate Flt3L+PolyICLC combined with radiation and anti-PD-1 in lymphoma, breast cancer, and HNSCC (NCT03789097). Adoptive transfer of human CD141+ DCs may also be feasible in the future with the development of methods to differentiate them in large numbers from CD34+ progenitors in vitro.35 36 This could be an attractive strategy to pursue in situations where Flt3L may be insufficient to expand DC numbers29 or where administration of Flt3L could be potentially detrimental as in the case of Flt3-expressing hematological malignancies.37 38 Adoptively transferred DCs have potential to be administered by a number of routes, including intratumorally.39 Collectively, these findings demonstrate the potential of enhancing human CD141+ DC quantity and quality as a strategy to improve response rates to ICIs in human patients with melanoma.
In summary, our study has revealed numerical and functional deficits in DC subsets in patients with advanced melanoma, particularly within the CD141+ DC compartment. Importantly, we provide evidence that CD141+ DCs may be harnessed in vivo for anti-tumor efficacy in a humanized experimental model of melanoma unresponsive to anti-PD-1 alone, thus serving as a foundation for future studies targeting human CD141+ DCs to boost immune responses in patients unresponsive to ICIs.
Acknowledgments
We thank Robyn Rodwell, staff at the Queensland Cord Blood Bank, Stephanie Diaz-Guilas (Mater Hospitals, Brisbane, Australia), Vanessa Bonazzi, Kalpana Patel, Michael Gartside (University of Queensland, Brisbane, Australia), Janine Thomas and Jennifer Addison (Queensland Health, Brisbane, Australia) for consenting donors and collecting patient samples, and staff at the Translational Research Institute Flow Core and Biological Research Facilities (Mater Research Institute, Brisbane, Australia).
Footnotes
Contributors: YSL and LO designed and performed experiments, analyzed data, and wrote the manuscript. CW, FEP, IMLR and K-AM contributed to experimental design, data acquisition and analysis. VA and AB provided patient samples and clinical expertise. KJR conceived and designed experiments, analyzed and interpreted data, and wrote the manuscript. All authors read and approved the final manuscript.
Funding: This work was supported by the Mater Foundation, Brisbane, Australia and the National Health and Medical Research Council of Australia (NHMRC). YSL was supported by the International Postgraduate Research Scholarship from the Australian Government and University of Queensland Centennial Scholarship. LO was supported by Mater Research Honours Scholarship and University of Queensland Australian Postgraduate Award Scholarship. FEP was supported by Worldwide Cancer Research UK (grant 15-0181). The Translational Research Institute is supported by a grant from the Australian Government.
Competing interests: No, there are no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
There are no data in this work.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Written informed consent was obtained for human sample acquisition in line with standards established by the Declaration of Helsinki. Study approval was granted by the Mater Human Research Ethics Committee (1407AP, 1586M) and Queensland Health Metro South Health Research Ethics Committee (HREC/10/QPAH/153 and HREC/16/QPAH/342). All animal experiments were approved by the University of Queensland Animal Ethics Committee and conducted in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes in addition to the laws of the USA and regulations of the Department of Agriculture.
References
- 1.Lee YS, Radford KJ. The role of dendritic cells in cancer. Int Rev Cell Mol Biol 2019;348:123–78. [DOI] [PubMed] [Google Scholar]
- 2.Wculek SK, Cueto FJ, Mujal AM, et al. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 2020;20:7–24. 10.1038/s41577-019-0210-z [DOI] [PubMed] [Google Scholar]
- 3.Radford KJ, Tullett KM, Lahoud MH. Dendritic cells and cancer immunotherapy. Curr Opin Immunol 2014;27:26–32. 10.1016/j.coi.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 4.Anguille S, Smits EL, Lion E, et al. Clinical use of dendritic cells for cancer therapy. Lancet Oncol 2014;15:e257–67. 10.1016/S1470-2045(13)70585-0 [DOI] [PubMed] [Google Scholar]
- 5.Laoui D, Keirsse J, Morias Y, et al. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat Commun 2016;7:13720. 10.1038/ncomms13720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmon H, Idoyaga J, Rahman A, et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016;44:924–38. 10.1016/j.immuni.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sánchez-Paulete AR, Cueto FJ, Martínez-López M, et al. Cancer immunotherapy with immunomodulatory Anti-CD137 and Anti–PD-1 monoclonal antibodies requires BATF3-Dependent dendritic cells. Cancer Discov 2016;6:71–9. 10.1158/2159-8290.CD-15-0510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Böttcher JP, Reis e Sousa C, Reis ESC. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer 2018;4:784–92. 10.1016/j.trecan.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garris CS, Arlauckas SP, Kohler RH, et al. Successful anti-PD-1 cancer immunotherapy requires T Cell-Dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity 2018;49:1148–61. 10.1016/j.immuni.2018.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancel J-C, Crozat K, Dalod M, et al. Are conventional type 1 dendritic cells critical for protective antitumor immunity and how? Front Immunol 2019;10:9. 10.3389/fimmu.2019.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammerich L, Marron TU, Upadhyay R, et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat Med 2019;25:814–24. 10.1038/s41591-019-0410-x [DOI] [PubMed] [Google Scholar]
- 12.Wculek SK, Amores-Iniesta J, Conde-Garrosa R, et al. Effective cancer immunotherapy by natural mouse conventional type-1 dendritic cells bearing dead tumor antigen. J Immunother Cancer 2019;7:100. 10.1186/s40425-019-0565-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegde S, Krisnawan VE, Herzog BH, et al. Dendritic cell paucity leads to dysfunctional immune surveillance in pancreatic cancer. Cancer Cell 2020;37:289–307. 10.1016/j.ccell.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JH, Huffman AP, Wattenberg MM, et al. Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J Exp Med 2020;217. 10.1084/jem.20190673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Slone N, Chrisikos TT, et al. Vaccine efficacy against primary and metastatic cancer with in vitro-generated CD103 + conventional dendritic cells. J Immunother Cancer 2020;8:e000474. 10.1136/jitc-2019-000474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spranger S, Dai D, Horton B, et al. Tumor-Residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 2017;31:711–23. 10.1016/j.ccell.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry KC, Hsu J, Broz ML, et al. A natural killer–dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments. Nat Med 2018;24:1178–91. 10.1038/s41591-018-0085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böttcher JP, Bonavita E, Chakravarty P, et al. Nk cells stimulate recruitment of cdc1 into the tumor microenvironment promoting cancer immune control. Cell 2018;172:1022–37. 10.1016/j.cell.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacquelot N, Roberti MP, Enot DP, et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun 2017;8:592. 10.1038/s41467-017-00608-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 21.Behren A, Anaka M, Lo P-H, et al. The Ludwig Institute for cancer research Melbourne melanoma cell line panel. Pigment Cell Melanoma Res 2013;26:597–600. 10.1111/pcmr.12097 [DOI] [PubMed] [Google Scholar]
- 22.Minoda Y, Virshup I, Leal Rojas I, et al. Human CD141+ dendritic cell and CD1c+ dendritic cell undergo concordant early genetic programming after activation in humanized mice in vivo. Front Immunol 2017;8:1419. 10.3389/fimmu.2017.01419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vuckovic S, Gardiner D, Field K, et al. Monitoring dendritic cells in clinical practice using a new whole blood single-platform TruCOUNT assay. J Immunol Methods 2004;284:73–87. 10.1016/j.jim.2003.10.006 [DOI] [PubMed] [Google Scholar]
- 24.Bakdash G, Buschow SI, Gorris MAJ, et al. Expansion of a BDCA1 + CD14 + Myeloid Cell Population in Melanoma Patients May Attenuate the Efficacy of Dendritic Cell Vaccines. Cancer Res 2016;76:4332–46. 10.1158/0008-5472.CAN-15-1695 [DOI] [PubMed] [Google Scholar]
- 25.Leal Rojas IM, Mok W-H, Pearson FE, et al. Human blood CD1c+ dendritic cells promote Th1 and Th17 effector function in memory CD4+ T cells. Front Immunol 2017;8:971. 10.3389/fimmu.2017.00971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson FE, et al. Activation of human CD141(+) and CD1c(+) dendritic cells in vivo with combined TLR3 and TLR7/8 ligation. Immunol Cell Biol 2018;96:390–400. [DOI] [PubMed] [Google Scholar]
- 27.Broz ML, Binnewies M, Boldajipour B, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014;26:638–52. 10.1016/j.ccell.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spranger S, Luke JJ, Bao R, et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell–inflamed tumor microenvironment in melanoma. Proc Natl Acad Sci U S A 2016;113:E7759–68. 10.1073/pnas.1609376113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer MA, Baer JM, Knolhoff BL, et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat Commun 2018;9:1250. 10.1038/s41467-018-03600-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol 2016;27:732–8. 10.1093/annonc/mdw016 [DOI] [PubMed] [Google Scholar]
- 31.Lino-Silva LS, Salcedo-Hernández RA, García-Pérez L, et al. Basal neutrophil-to-lymphocyte ratio is associated with overall survival in melanoma. Melanoma Res 2017;27:140–4. 10.1097/CMR.0000000000000333 [DOI] [PubMed] [Google Scholar]
- 32.Mastelic-Gavillet B, Sarivalasis A, Lozano LE, et al. Quantitative and qualitative impairments in dendritic cell subsets of patients with ovarian or prostate cancer. Eur J Cancer 2020;135:173–82. 10.1016/j.ejca.2020.04.036 [DOI] [PubMed] [Google Scholar]
- 33.Sanmamed MF, Perez-Gracia JL, Schalper KA, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol 2017;28:1988–95. 10.1093/annonc/mdx190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yurkovetsky ZR, Kirkwood JM, Edington HD, et al. Multiplex analysis of serum cytokines in melanoma patients treated with Interferon-α2b. Clin Cancer Res 2007;13:2422–8. 10.1158/1078-0432.CCR-06-1805 [DOI] [PubMed] [Google Scholar]
- 35.Balan S, Arnold-Schrauf C, Abbas A, et al. Large-Scale human dendritic cell differentiation revealing Notch-dependent lineage bifurcation and heterogeneity. Cell Rep 2018;24:1902–15. 10.1016/j.celrep.2018.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirkling ME, Cytlak U, Lau CM, et al. Notch Signaling Facilitates In Vitro Generation of Cross-Presenting Classical Dendritic Cells. Cell Rep 2018;23:3658–72. 10.1016/j.celrep.2018.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rickmann M, Krauter J, Stamer K, et al. Elevated frequencies of leukemic myeloid and plasmacytoid dendritic cells in acute myeloid leukemia with the FLT3 internal tandem duplication. Ann Hematol 2011;90:1047–58. 10.1007/s00277-011-1231-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau CM, Nish SA, Yogev N, et al. Leukemia-Associated activating mutation of FLT3 expands dendritic cells and alters T cell responses. J Exp Med 2016;213:415–31. 10.1084/jem.20150642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castiello L, Aricò E, D'Agostino G, et al. In situ vaccination by direct dendritic cell inoculation: the coming of age of an old idea? Front Immunol 2019;10:2303. 10.3389/fimmu.2019.02303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001963supp001.pdf (3.5MB, pdf)
Data Availability Statement
There are no data in this work.