Abstract
Aims
Metabolic syndrome (MetS) increases the risk of diabetes mellitus (DM), cardiovascular disease (CVD), cancer, and mortality. Sarcopenia has been reported as a risk factor for MetS, non-alcoholic fatty liver disease, and CVD. To date, the association between sarcopenia and MetS has been investigated. However, there have been few studies on the dose-response relationship between sarcopenia and MetS. We investigated the association between sarcopenia and the prevalence of MetS. We also aimed to analyze the dose-response relationship between skeletal muscle mass and the prevalence of MetS.
Methods
We enrolled 13,620 participants from October 2014 to December 2019. Skeletal muscle mass was measured using bioelectrical impedance analysis (BIA). Appendicular skeletal muscle mass (ASM) was divided by body weight (kg) and was expressed as a percentage (ASM x 100/Weight, ASM%). The quartiles of ASM% were calculated for each gender, with Q1 and Q4 being the lowest and highest quartiles of ASM%, respectively. The quartiles of ASM% were calculated for each gender, with Q1 and Q4 being the lowest and highest quartiles of ASM%, respectively. Linear regression and logistic regression analyses were used to compare the clinical parameters according to ASM%, adjusted for age, sex, obesity, hypertension (HT), DM, dyslipidemia (DL), smoking, alcohol intake, and C-reactive protein (CRP). Multiple logistic regression analysis was performed to determine the risk of MetS in each group.
Results
A dose-response relationship was identified between ASM% and MetS. Sarcopenia was associated with an increased prevalence of MetS. After adjustment for age, sex, obesity, HT, DM, DL, smoking, alcohol intake, and CRP, sarcopenia remained significantly associated with MetS. For each 1 quartile increment in ASM%, the risk of MetS decreased by 56% (P< 0.001). After adjusting for age, sex, obesity, HT, DM, DL, smoking, alcohol intake, and CRP, the risk of MetS decreased by 25% per 1Q increment in ASM% (P < 0.001).
Conclusions
Sarcopenia by BIA is independently associated with the risk of MetS and has a dose-response relationship.
Introduction
Sarcopenia is defined as an age-related progressive loss of skeletal muscle mass [1,2]. With the global aging tendency of the world’s population, sarcopenia has become a worldwide issue [3,4]. Loss of skeletal muscle mass has been reported as a risk factor for metabolic syndrome (MetS) [5–8], non-alcoholic fatty liver disease [9,10], carotid atherosclerosis and cardiovascular disease (CVD) [4,11,12]. In addition, sarcopenia causes arterial stiffness and hypertension (HT) [13]. Sarcopenia can limit physical and daily-life activities [14]. Sarcopenia also increases morbidity [15], disability [16], medical costs [17], and mortality [18].
MetS is a global health problem and is closely related to diabetes, with a prevalence of 34.75% in the US in 2012 [19]. MetS increases the risk of diabetes mellitus (DM), CVD [20,21], chronic liver disease and hepatocellular carcinoma [22], other cancers, and mortality [23–25]. Till date, the association between sarcopenia and MetS has been investigated [5–8]. However, there have been few studies on the dose-response relationship between sarcopenia and MetS.
In the current study, we investigated the association between sarcopenia and the prevalence of MetS. In addition, we aimed to analyze a dose-response relationship between skeletal muscle mass and the prevalence of MetS.
Materials and methods
Study population
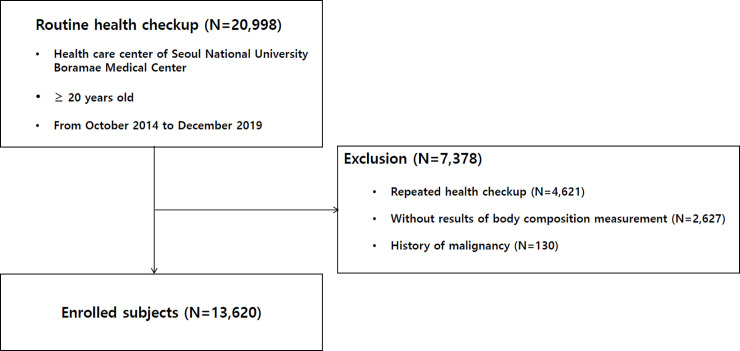
We recorded 20,998 participants from October 2014 to December 2019, as they underwent a voluntary routine health checkup at the health care center of Seoul National University Boramae Medical Center. All data were fully anonymized before we accessed them. After excluding 2,627 participants with insufficient data and 4,621 participants who underwent repeated checkups, only the data from the first examination were included. Moreover, after excluding 130 participants with a history of malignancy, 13,620 participants were enrolled in our study (Fig 1). This study was approved by the Institutional Review Board of Boramae Medical Center (IRB No. 10-2020-234). The requirement for written informed consent was waived due to the retrospective nature of our study. Our study was conducted in accordance with the Helsinki Declaration.
Fig 1. Enrollment flow chart of patients.
Data collection
The participants visited our health care center after an overnight 12-h fast. Clinical information and blood lab measurements were collected during the health checkup. Height and weight were measured when the subject was in a standing posture with a light examination gown and no shoes. Waist circumference (WC) was measured at the umbilicus level with the participants in a standing posture. Body composition analysis through bioelectrical impedance analysis (BIA) was performed with Inbody 720 (Biospace Co., Seoul, Korea) by a trained nurse following the manufacturer’s protocol [26]. With Inbody 720, skeletal muscle mass and visceral fat area (VFA) were automatically calculated. Clinical information was collected: age, sex, systolic and diastolic blood pressure (BP), smoking, alcohol drinking habits, and medical history including HT and diabetes. Tests were performed to determine the following: total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglycerides (TG), glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), uric acid, insulin level, and C-reactive protein (CRP).
Definitions
BMI was defined as weight (kg) divided by height squared (m2), and obesity was defined as BMI ≥ 25 kg/m2 based on the criteria for the Asia-Pacific region. Underweight was defined as BMI < 18.5 kg/m2 [27,28].
HT was defined as systolic BP ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, or the use of antihypertensive medication. DM was defined as fasting plasma glucose ≥ 126 mg/dL, glycated hemoglobin level ≥ 6.5%, or the use of anti-diabetic medication including insulin.
MetS was defined when three or more of the following criteria was met: 1) WC male ≥ 102 cm, female ≥ 88 cm, 2) TG ≥ 150 mg/dL or the use of medication, 3) HDL male < 40 mg/dL, female < 50 mg/dL or the use of medication; 4) systolic BP ≥ 130 mmHg, diastolic BP ≥ 85 mmHg, or the use of antihypertensive medication, and 5) fasting plasma glucose ≥ 100 mg/dL or the use of anti-diabetic medication [29,30].
Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as [fasting glucose (mg/dL) × fasting insulin (μU/mL)]/405 [31].
Appendicular skeletal muscle mass (ASM) was calculated as the sum of the lean skeletal muscle mass of the bilateral upper and lower limbs. ASM was divided by body weight (kg) and was expressed as a percentage (ASM x 100/Weight, ASM%). Sarcopenia was defined as ASM% < 29.0 in men and < 22.9 in women [32,33]. The quartiles of ASM% were calculated for each sex, with Q1 and Q4 being the lowest and highest quartiles of ASM%, respectively.
VFA was measured using Inbody 720 and was used to assess visceral obesity. Participants with VFAs ≥100 cm2 were defined as the visceral obesity group [34–36].
Comparison of Inbody 720 and computed tomography (CT) data
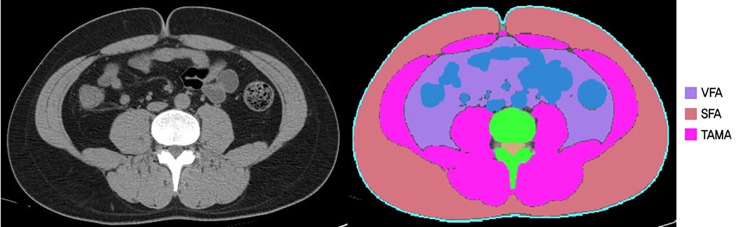
To evaluate the data of skeletal muscle mass measured by Inbody 720, we analyzed the correlation between BIA data and CT scans in participants who underwent body composition analysis using BIA and CT scans on the same day. Using CT, we measured VFA and total abdominal muscle area (TAMA) at the L3 vertebral level, which showed the highest correlation with visceral fat volume and whole body skeletal muscle in previous studies [37,38].
All abdominal CT scans were performed using a 64-slice multi-detector CT scanner (Brilliance 64 scanners; Philips Healthcare, Amsterdam, Netherlands). Pre-contrast CT images were analyzed using a commercially available segmentation software program (MEDIP Deep Catch v1.0.0.0, MEDICALIP Co. Ltd., Seoul, South Korea) to measure TAMA. After automatic segmentation, the reader selected the level of the inferior endplate of the L3 vertebra and extracted the TAMA at the corresponding level as previously described (Fig 2) [39]. The software contained 3D U-Net that was trained with 39,268 labeled CT images, providing an average dice similarity coefficient of 92.3% to 99.3% for muscle, abdominal visceral fat, and subcutaneous fat in the internal and external validation datasets. A clinically trained image analyst (DHL) reviewed and adjusted the results and finally a radiologist (SHY) confirmed the results.
Fig 2. Body morphometric evaluations of abdominal fat and muscle areas.
At the level of the inferior endplate of the L3 vertebra, a segmented axial computed tomography image showed the visceral fat area (VFA, cm2), subcutaneous fat area (SFA, cm2), and total abdominal muscle area (TAMA, cm2), including all muscles on selected axial images (psoas, paraspinals, transversus abdominis, rectus abdominis, quadratus lumborum, and internal and external obliques).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD). Categorical variables are presented as numbers and percentages. Linear regression and logistic regression analyses were used to compare the clinical parameters according to ASM%, adjusted for age, sex, obesity, HT, DM, DL, smoking, alcohol intake and CRP. Multiple logistic regression analysis was performed to determine the risk of MetS in each group. Crude odds ratios (ORs) were calculated with skeletal muscle mass at baseline. Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, and obesity. Model 3 was adjusted for age, sex, obesity, HT, DM, and DL. Model 4 was adjusted for age, sex, obesity, HT, DM, DL, smoking, and alcohol intake. Model 5 was adjusted for age, sex, obesity, HT, DM, DL, smoking, alcohol intake, and CRP. P-values less than 0.05 were considered statistically significant. All statistical analyses were conducted using IBM SPSS Statistics version 26 statistical software (IBM Corp., Armonk, NY).
Results
1. Clinical characteristics according to ASM% quartiles
The mean age of the study population was 48.1 ± 13.1 years, and 54.5% was male. ASM% was 26.6 ± 2.9, 29.1 ± 2.5, 30.7 ± 2.5, 33.3 ± 2.9 years in the Q1, Q2, Q3, and Q4, respectively (P < 0.001, Table 1). As ASM% increased, the mean age and mean BMI decreased. From Q1 to Q4 of ASM%, WC, systolic and diastolic BP, VFA, LDL, TG, AST, ALT, fasting glucose, HbA1c, uric acid, insulin level, CRP, and HOMA-IR also significantly decreased (P < 0.001, Table 1). HDL increased in order from Q1 to Q4. As ASM% increased from Q1 to Q4, the proportions of HT, DM, obesity, and MetS decreased significantly (P < 0.001 in all, Table 1).
Table 1. Clinical characteristics according to ASM% quartiles.
| Variables | Total | Q1 | Q2 | Q3 | Q4 | p for trend* |
|---|---|---|---|---|---|---|
| N = 13620 | N = 3412 | N = 3392 | N = 3409 | N = 3407 | ||
| Age (years) | 48.13±13.09 | 52.71±13.80 | 49.25±12.69 | 46.95±12.26 | 43.59±11.84 | <0.001 |
| Weight (kg) | 65.86±12.89 | 71.16±15.00 | 66.74±12.14 | 64.46±11.49 | 61.08±10.34 | <0.001 |
| Body mass index (BMI, kg/m2) | 23.71±3.42 | 26.64±3.59 | 24.25±2.56 | 22.89±2.38 | 21.04±2.22 | <0.001 |
| Waist circumference (cm) | 83.56±9.73 | 90.86±9.68 | 85.02±7.96 | 81.64±7.86 | 76.75±7.37 | <0.001 |
| Systolic blood pressure (mmHg) | 117.47±15.76 | 123.13±16.14 | 118.26±15.24 | 115.94±15.56 | 112.57±14.15 | <0.001 |
| Diastolic blood pressure (mmHg) | 79.10±10.98 | 81.76±11.35 | 79.86±10.77 | 78.53±10.82 | 76.25±10.19 | <0.001 |
| Visceral fat area (cm2) | 91.49±35.20 | 119.56±37.88 | 95.32±27.68 | 83.36±26.73 | 67.70±24.63 | <0.001 |
| ASM (kg) | 19.81±4.86 | 19.14±5.15 | 19.59±4.78 | 19.99±4.77 | 20.51±4.60 | <0.001 |
| ASM% | 29.94±3.63 | 26.64±2.85 | 29.06±2.53 | 30.71±2.46 | 33.33±2.87 | <0.001 |
| Cholesterol (mg/dL) | 196.36±36.21 | 200.40±39.13 | 198.88±36.36 | 196.30±35.40 | 189.86±32.76 | <0.001 |
| HDL (mg/dL) | 56.40±14.28 | 52.21±12.63 | 54.78±13.49 | 57.04±14.42 | 61.57±14.78 | <0.001 |
| LDL (mg/dL) | 118.20±33.61 | 122.38±36.06 | 120.98±34.05 | 118.19±32.82 | 111.32±30.20 | <0.001 |
| Triglyceride (mg/dL) | 110.40±76.84 | 131.84±86.41 | 117.59±84.46 | 106.92±74.15 | 85.25±48.68 | <0.001 |
| Glucose (mg/dL) | 94.44±19.96 | 100.28±23.93 | 95.45±20.38 | 92.95±17.30 | 89.09±15.49 | <0.001 |
| AST (IU/L) | 27.65±18.23 | 31.39±19.47 | 27.92±16.47 | 26.53±21.90 | 24.76±13.28 | <0.001 |
| ALT (IU/L) | 27.73±24.79 | 35.57±30.77 | 29.04±22.22 | 25.14±26.51 | 21.17±14.24 | <0.001 |
| Uric acid (mg/dL) | 5.25±1.34 | 5.52±1.43 | 5.28±1.30 | 5.16±1.31 | 5.02±1.25 | <0.001 |
| HbA1c (%) | 5.63±0.72 | 5.85±0.88 | 5.66±0.72 | 5.56±0.61 | 5.43±0.54 | <0.001 |
| Insulin | 9.65±5.78 | 12.44±7.84 | 9.74±4.40 | 7.80±2.91 | 6.80±2.78 | <0.001 |
| HOMA-IR | 2.42±1.65 | 3.25±2.13 | 2.46±1.31 | 1.83±0.73 | 1.61±1.13 | <0.001 |
| C-reactive protein (mg/dL) | 0.15±0.46 | 0.22±0.47 | 0.15±0.47 | 0.13±0.45 | 0.10±0.45 | <0.001 |
| Metabolic syndrome | 2238 (16.4) | 1165 (34.2) | 573 (16.8) | 386 (11.3) | 114 (3.3) | <0.001 |
| Hypertension | 4230 (31.1) | 1658 (48.7) | 1128 (33.1) | 899 (26.4) | 545 (16.0) | <0.001 |
| Diabetes mellitus | 1139 (8.4) | 498 (14.6) | 294 (8.6) | 226 (6.6) | 121 (3.6) | <0.001 |
| Obese status | <0.001 | |||||
| Obesity (BMI ≥ 25 kg/m2) | 4456 (32.7) | 2325 (68.3) | 1325 (38.9) | 676 (19.9) | 130 (3.8) | |
| Overweight (BMI 23–24.9 kg/m2) | 3266 (24.0) | 649 (19.1) | 1026 (30.1) | 1019 (29.9) | 572 (16.8) | |
| Normal (BMI 18.5–22.9 kg/m2) | 5345 (39.2) | 422 (12.4) | 1025 (30.1) | 1623 (47.7) | 2275 (66.8) | |
| Underweight (BMI < 18.5 kg/m2) | 553 (4.1) | 9 (0.3) | 29 (0.9) | 87 (2.6) | 428 (12.6) | |
| Smoking | 2381 (17.5) | 589 (17.3) | 584 (17.2) | 549 (16.1) | 659 (19.4) | 0.077 |
| Alcohol intake | 7224 (53.0) | 1660 (48.8) | 1822 (53.5) | 1827 (53.7) | 1915 (56.2) | <0.001 |
ASM, appendicular skeletal muscle mass; ASM%, ASMx100/Weight; HDL, high-density lipoprotein; LDL, low-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HbA1c, glycated hemoglobin; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; Data are presented as mean± SD or number (%).
*From linear and logistic regression without any adjustment.
2. Association between ASM% and MetS
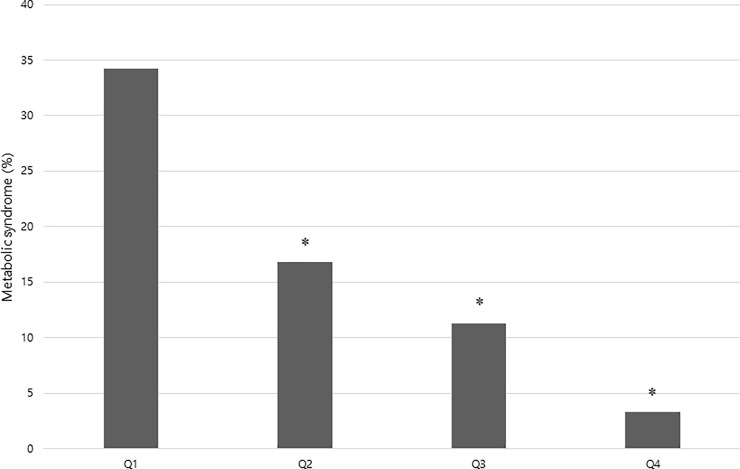
The prevalence of MetS was 34.2%, 16.8%, 11.3%, and 3.3% in Q1, Q2, Q3, and Q4 of ASM%, respectively (P for trend < 0.001, Table 1, Fig 3). Sarcopenia was associated with an increased prevalence of MetS (OR 5.306, 95% confidence interval [CI]; 4.656–6.046, P < 0.001). After adjustment for age, sex, obesity, HT, DM, DL, smoking, alcohol intake and CRP, sarcopenia remained significantly associated with MetS (OR 2.291, CI 1.874–2.801, P < 0.001, Model 5, Table 2).
Fig 3. Prevalence of metabolic syndrome according to ASM% (appendicular skeletal muscle mass x 100/Weight) quartiles.
*Significantly lower compared with the Q1 (P < 0.001).
Table 2. Association between metabolic syndrome and sarcopenia.
| Metabolic syndrome | |||
|---|---|---|---|
| OR | 95% CI | p value | |
| Crude | 5.306 | 4.656–6.046 | <0.001 |
| Model 1 | 4.414 | 3.847–5.065 | <0.001 |
| Model 2 | 2.254 | 1.950–2.605 | <0.001 |
| Model 3 | 2.325 | 1.903–2.840 | <0.001 |
| Model 4 | 2.328 | 1.905–2.844 | <0.001 |
| Model 5 | 2.291 | 1.874–2.801 | <0.001 |
Model 1: Adjusted for age, sex.
Model 2: Adjusted for age, sex, obesity.
Model 3: Adjusted for age, sex, obesity, hypertension, diabetes mellitus, dyslipidemia.
Model 4: Adjusted for age, sex, obesity, hypertension, diabetes mellitus, dyslipidemia, smoking, alcohol intake.
Model 5: Adjusted for age, sex, obesity, hypertension, diabetes mellitus, dyslipidemia, smoking, alcohol intake, CRP.
OR, odds ratio; CI, confidence interval; CRP, C-reactive protein.
In a stratified analysis according to visceral obesity, the association between sarcopenia and MetS was more prominent in participants without visceral obesity (OR 4.692 vs. OR 2.568, Table 3). In the stratified analysis according to obesity, the association between sarcopenia and MetS was more prominent in participants without obesity (OR 4.482 vs. OR 2.401, Table 3).
Table 3. Stratified association between metabolic syndrome and sarcopenia.
| Metabolic syndrome | |||
|---|---|---|---|
| OR | 95% CI | p value | |
| Visceral obesity | |||
| Yes | 2.568 | 2.218–2.973 | <0.001 |
| No | 4.692 | 3.230–6.815 | <0.001 |
| Obesity | |||
| Yes | 2.401 | 2.069–2.787 | <0.001 |
| No | 4.482 | 3.136–6.404 | <0.001 |
| Underweight | |||
| Yes | 17.222 | 0.115–343.283 | 0.186 |
| No | 5.078 | 4.455–5.787 | <0.001 |
| Sex | |||
| Male | 4.770 | 4.078–5.580 | <0.001 |
| female | 6.102 | 4.795–7.765 | <0.001 |
OR, odds ratio; CI, confidence interval; visceral obesity, VFAs ≥100 cm2; obesity, BMI ≥ 25 kg/m2; underweight, BMI < 18.5 kg/m2.
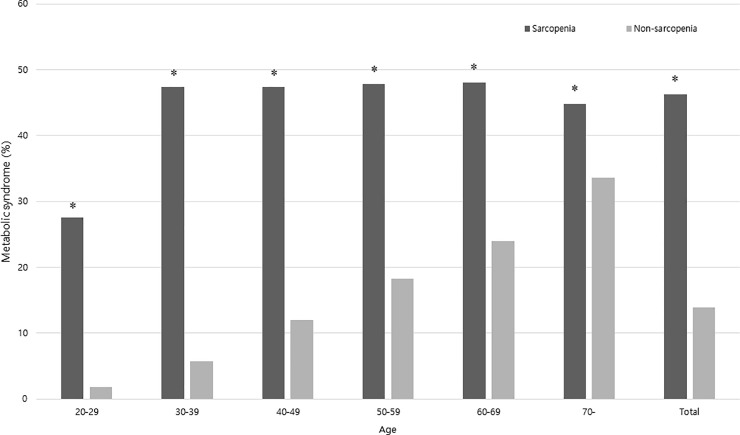
The prevalence of MetS according to sarcopenia was analyzed using age group stratification. In all age groups, the prevalence of MetS was significantly higher in the sarcopenia group (P < 0.05; Fig 4).
Fig 4. The prevalence of metabolic syndrome in a 10-year age strata according to the presence of sarcopenia.
*Significantly higher compared with the non-sarcopenia group (P < 0.05).
3. Association between sarcopenia and MetS with 4 or 5 criteria
Sarcopenia was associated with an increased prevalence of MetS with 4 or 5 criteria (OR 5.920, 95% CI; 4.974–7.045, P < 0.001). After adjustment for age, sex, obesity, HT, DM, DL, smoking, alcohol intake and CRP, sarcopenia remained significantly associated with MetS (OR 2.106, CI 1.681–2.639, P < 0.001, Model 5, Table 4) Sarcopenia was associated with an increased prevalence of severe MetS with 5 criteria (OR 10.453, 95% CI; 7.258–15.054, P < 0.001). After adjustment for age, sex, obesity, HT, DM, DL, smoking, alcohol intake and CRP, sarcopenia remained significantly associated with MetS (OR 3.073, CI 2.009–4.701, P < 0.001, Model 5, Table 4).
Table 4. Association between severe metabolic syndrome (4 or 5 criteria) and sarcopenia.
| Metabolic syndrome (4 or 5 criteria) | Metabolic syndrome (5 criteria) | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Crude | 5.920 | 4.974–7.045 | <0.001 | 10.453 | 7.258–15.054 | <0.001 |
| Model 1 | 5.073 | 4.222–6.096 | <0.001 | 10.285 | 6.921–15.286 | <0.001 |
| Model 2 | 2.375 | 1.960–2.878 | <0.001 | 3.605 | 2.442–5.323 | <0.001 |
| Model 3 | 2.195 | 1.756–2.745 | <0.001 | 3.243 | 2.130–4.940 | <0.001 |
| Model 4 | 2.182 | 1.744–2.730 | <0.001 | 3.170 | 2.078–4.835 | <0.001 |
| Model 5 | 2.106 | 1.681–2.639 | <0.001 | 3.073 | 2.009–4.701 | <0.001 |
Model 1: Adjusted for age, sex.
Model 2: Adjusted for age, sex, obesity.
Model 3: Adjusted for age, sex, obesity, hypertension, diabetes mellitus, dyslipidemia.
Model 4: Adjusted for age, sex, obesity, hypertension, diabetes mellitus, dyslipidemia, smoking, alcohol intake.
Model 5: Adjusted for age, sex, obesity, hypertension, diabetes mellitus, dyslipidemia, smoking, alcohol intake, CRP.
OR, odds ratio; CI, confidence interval; CRP, C-reactive protein.
4. Quantitative association between sarcopenia and MetS
A dose-response relationship was identified between ASM% and MetS (Fig 2). The risk of MetS significantly decreased as ASM% increased, compared with Q1 (P < 0.001 in all, Table 5). For each 1 quartile increment in ASM%, the risk of MetS decreased by 56% (OR per 1Q increment 0.443, 95% CI; 0.422–0.466, P<0.001). The risk of MetS significantly decreased even in the Q2 group compared with the Q1 group (OR 0.389, 95% CI; 0.347–0.436, P < 0.001). After adjusting for age, sex, obesity, HT, DM, DL, smoking, alcohol intake and CRP, ASM% remained associated with the risk of MetS (Model 5, Table 5). In Model 5, the risk of MetS decreased by 25% per 1Q increment in ASM% (OR per 1Q increment 0.754, 95% CI 0.699–0.814, P < 0.001).
Table 5. Risk of metabolic syndrome in each quartile of sarcopenia.
| Metabolic syndrome | |||
|---|---|---|---|
| OR | 95% CI | p value | |
| Unadjusted | |||
| Q1 | (Reference) | ||
| Q2 | 0.389 | 0.347–0.436 | <0.001 |
| Q3 | 0.246 | 0.216–0.279 | <0.001 |
| Q4 | 0.067 | 0.055–0.081 | <0.001 |
| Per 1Q | 0.443 | 0.422–0.466 | <0.001 |
| Model 1 | |||
| Q1 | (Reference) | ||
| Q2 | 0.424 | 0.377–0.476 | <0.001 |
| Q3 | 0.286 | 0.251–0.326 | <0.001 |
| Q4 | 0.086 | 0.070–0.105 | <0.001 |
| Per 1Q | 0.481 | 0.457–0.506 | <0.001 |
| Model 2 | |||
| Q1 | (Reference) | ||
| Q2 | 0.590 | 0.522–0.667 | <0.001 |
| Q3 | 0.514 | 0.446–0.593 | <0.001 |
| Q4 | 0.206 | 0.166–0.257 | <0.001 |
| Per 1Q | 0.645 | 0.609–0.684 | <0.001 |
| Model 3 | |||
| Q1 | (Reference) | ||
| Q2 | 0.624 | 0.530–0.735 | <0.001 |
| Q3 | 0.607 | 0.504–0.732 | <0.001 |
| Q4 | 0.384 | 0.293–0.505 | <0.001 |
| Per 1Q | 0.751 | 0.696–0.810 | <0.001 |
| Model 4 | |||
| Q1 | (Reference) | ||
| Q2 | 0.624 | 0.529–0.735 | <0.001 |
| Q3 | 0.608 | 0.504–0.733 | <0.001 |
| Q4 | 0.383 | 0.292–0.504 | <0.001 |
| Per 1Q | 0.751 | 0.696–0.810 | <0.001 |
| Model 5 | |||
| Q1 | (Reference) | ||
| Q2 | 0.630 | 0.534–0.742 | <0.001 |
| Q3 | 0.615 | 0.510–0.742 | <0.001 |
| Q4 | 0.388 | 0.295–0.510 | <0.001 |
| Per 1Q | 0.754 | 0.699–0.814 | <0.001 |
Model 1: Adjusted for age, sex.
Model 2: Adjusted for age, sex, obesity.
Model 3: Adjusted for age, sex, obesity, hypertension, diabetes mellitus, dyslipidemia.
Model 4: Adjusted for age, sex, obesity, hypertension, diabetes mellitus, dyslipidemia, smoking, alcohol intake.
Model 5: Adjusted for age, sex, obesity, hypertension, diabetes mellitus, dyslipidemia, smoking, alcohol intake, CRP.
OR, odds ratio; CI, confidence interval; CRP, C-reactive protein.
5. Correlation of skeletal muscle mass between Inbody 720 and CT
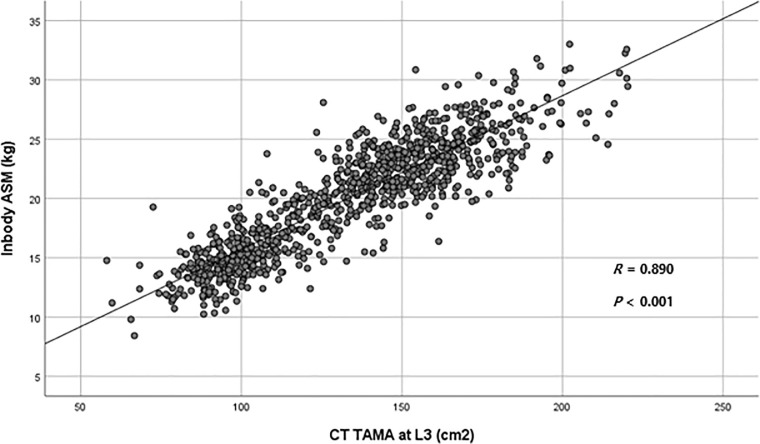
Among the population enrolled, CT scans were performed in 966 participants on the same day of Inbody 720. Thus, correlation analysis was conducted for these 966 participants. ASM measured by BIA was positively correlated with the TAMA measured by CT scan (R = 0.890, P < 0.001, Fig 5).
Fig 5. Correlation between the appendicular skeletal muscle mass (ASM) measured by Inbody 720 and the total abdominal muscle area measured by computed tomography (CT) scan.
ASM, Appendicular skeletal muscle mass; TAMA, Total abdominal muscle area; CT, Computed tomography.
Discussion
Our study showed that sarcopenia level measured by BIA was significantly associated with the risk of MetS in a dose-dependent manner. As ASM% increased from Q1 to Q4, the prevalence of MetS significantly decreased (Table 1, Fig 2). Not to mention the Q3 or Q4 groups, even individuals in the Q2 group had a significantly lower risk of MetS than those in the Q1 group (Table 5).
In the current study, sarcopenia was an independent risk factor for MetS regardless of age, sex, obesity, DM, HT, DL, smoking, alcohol intake and CRP levels (Table 2). The OR of MetS in participants with sarcopenia reached 2.266 after adjustment for age, sex, obesity, DM, HT, DL, smoking, alcohol intake and CRP levels. We adopted CRP as a variable as previous studies have shown the association between CRP and metabolic syndrome [40,41]. Our results are consistent with those of previous studies, which showed an association between sarcopenia and MetS [5–7,11,42]. We also analyzed the association between sarcopenia and more severe MetS with 4 or 5 criteria. The crude OR of severe MetS with 5 criteria in participants with sarcopenia was 10.453, which was higher than the 5.306 in the original MetS. After adjustment for age, sex, obesity, DM, HT, DL, smoking, alcohol intake and CRP levels, the OR of severe MetS with 5 criteria in subjects with sarcopenia was 3.119, which was higher than the 2.266 in the original MetS. We assume that severe MetS with 5 criteria may be more affected by skeletal muscle mass or sarcopenia. The strength of our study is that we demonstrated the dose-response relationship between sarcopenia and the risk of MetS. In our study, the risk of MetS significantly decreased for each 1 quartile increase of ASM%. Even after adjustment for age, sex, obesity, HT, DM, DL, smoking, alcohol intake and CRP, the risk of MetS significantly decreased by 25% per 1Q increase of ASM% (Table 5). The second strength of our study is that our study population included healthy individuals who voluntarily underwent routine health checkups. Thus, our results can be generalizable to the general healthy population. The large sample size is another strength of the current study.
We performed stratified analyses considering the possibility of other factors affecting the association between sarcopenia and MetS. In stratified analyses according to VFA, obesity, and sex, the association between sarcopenia and MetS was significant across all strata (Table 3). The association between sarcopenia and MetS seemed more prominent in participants with low visceral fat or in non-obese participants. These findings are consistent with those of previous studies [7,43]. In a previous study by Moon et al., sarcopenia was associated with insulin resistance, DM, and MetS in non-obese elderly subjects [7]. According to the results of previous and current studies, sarcopenia may be considered as a predictor of MetS susceptibility in the non-obese population. Considering the strong relationship between age and sarcopenia, the prevalence of MetS according to sarcopenia was analyzed using age group stratification (Fig 4). In all age groups, the prevalence of MetS was significantly higher in the sarcopenia group. This association between MetS risk and sarcopenia weakened as participants became older, though the association remained significant.
In the current study, skeletal muscle mass and VFA were measured using the BIA method (Inbody 720). The BIA method has strengths for use in clinical practice. Recently, BIA has been widely used with easy accessibility, quick assessment, safety, non-invasiveness, and cost-efficiency [27,44–46]. BIA has been reported to measure VFA and indicate the risk of MetS as precisely as CT [26,47]. In recent studies, BIA was used to assess skeletal muscle mass and to diagnose sarcopenia [48,49]. Our current study also showed that skeletal muscle mass measured by BIA was positively correlated with those calculated with CT scan. Based on the current study results and previous studies, BIA can be considered a valid option for measuring skeletal muscle mass in clinical practice. For measurement of skeletal muscle mass, CT, dual-energy X-ray absorptiometry (DEXA), and magnetic resonance imaging (MRI) may be other options. However, the use of CT and DEXA is limited due to the risk of radiation exposure, and MRI use is also limited because of cost [36].
Several mechanisms may affect the association between sarcopenia and MetS, including physical inactivity, insulin resistance, inflammation, and myokines [8,43]. Skeletal muscle is the main site of glucose uptake and utilization [50]. Thus sarcopenia is thought to increase insulin resistance and thereby induce DM and MetS [7]. However, in the current study, the data of HOMA-IR results were available only in a small sample size (N = 305). Thus, we failed to analyze the association between sarcopenia and MetS adjusted for insulin resistance in the current study.
Our study has some limitations. First, this study was limited by its cross-sectional and single-centered retrospective design. It was difficult to assess the causal relationship between sarcopenia and MetS. Further prospective longitudinal cohort studies need to be conducted to validate whether sarcopenia is the cause of MetS. Second, our study population included healthy participants who underwent routine health checkups in a health care center. Thus, the results of our study are not generalizable to the diseased population or patients. Third, muscle strength was not evaluated in the current study. However, with only skeletal muscle mass measured by BIA, we could assess the risk of MetS easily, quickly, safely, and cost-efficiently. Fourth, exercise was not included in the variables and could not be evaluated in the analysis.
In conclusion, our study demonstrated that sarcopenia by BIA is independently associated with the risk of MetS and might have a dose-response relationship. Future studies that assess causal relationship between sarcopenia and MetS are needed using the data of subjects who underwent repeated health checkup. By measuring sarcopenia using BIA, the risk of MetS can be assessed easily, safely, and cost-efficiently. BIA can be used as an easy, useful, and important guide to identify participants with the risk of MetS.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
MEDICALIP Co, Ltd. provided support in the form of salaries for author DHL, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schneider SM, Correia M. Epidemiology of weight loss, malnutrition and sarcopenia: A transatlantic view. Nutrition. 2020;69:110581. Epub 2019/10/18. 10.1016/j.nut.2019.110581 . [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990s–1s. Epub 1997/05/01. 10.1093/jn/127.5.990S . [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. Epub 2018/10/13. 10.1093/ageing/afy169 ; PubMed Central PMCID: PMC6322506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. Epub 2008/10/02. 10.1097/MCO.0b013e328312c37d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SH, Park JH, Park HY, Jang HJ, Kim HK, Park J, et al. Additional role of sarcopenia to waist circumference in predicting the odds of metabolic syndrome. Clin Nutr. 2014;33(4):668–72. Epub 2013/10/01. 10.1016/j.clnu.2013.08.008 . [DOI] [PubMed] [Google Scholar]
- 6.Park BS, Yoon JS. Relative skeletal muscle mass is associated with development of metabolic syndrome. Diabetes Metab J. 2013;37(6):458–64. Epub 2014/01/10. 10.4093/dmj.2013.37.6.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocr J. 2014;61(1):61–70. Epub 2013/10/04. 10.1507/endocrj.ej13-0244 [DOI] [PubMed] [Google Scholar]
- 8.Park SJ, Ryu SY, Park J, Choi SW. Association of Sarcopenia with Metabolic Syndrome in Korean Population Using 2009–2010 Korea National Health and Nutrition Examination Survey. Metab Syndr Relat Disord. 2019;17(10):494–9. Epub 2019/10/22. 10.1089/met.2019.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MJ, Kim EH, Bae SJ, Kim GA, Park SW, Choe J, et al. Age-Related Decrease in Skeletal Muscle Mass Is an Independent Risk Factor for Incident Nonalcoholic Fatty Liver Disease: A 10-Year Retrospective Cohort Study. Gut Liver. 2019;13(1):67–76. Epub 2018/07/25. 10.5009/gnl18070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59(5):1772–8. Epub 2013/09/03. 10.1002/hep.26716 . [DOI] [PubMed] [Google Scholar]
- 11.Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism. 2009;58(7):1013–22. Epub 2009/04/28. 10.1016/j.metabol.2009.02.027 . [DOI] [PubMed] [Google Scholar]
- 12.Seo DH, Lee YH, Suh YJ, Ahn SH, Hong S, Choi YJ, et al. Low muscle mass is associated with carotid atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2020;305:19–25. Epub 2020/07/01. 10.1016/j.atherosclerosis.2020.05.021 . [DOI] [PubMed] [Google Scholar]
- 13.Sanada K, Miyachi M, Tanimoto M, Yamamoto K, Murakami H, Okumura S, et al. A cross-sectional study of sarcopenia in Japanese men and women: reference values and association with cardiovascular risk factors. Eur J Appl Physiol. 2010;110(1):57–65. Epub 2010/04/15. 10.1007/s00421-010-1473-z . [DOI] [PubMed] [Google Scholar]
- 14.Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin Nutr. 2012;31(5):583–601. Epub 2012/07/20. 10.1016/j.clnu.2012.06.010 . [DOI] [PubMed] [Google Scholar]
- 15.Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care. 2005;28(10):2541–2. Epub 2005/09/28. 10.2337/diacare.28.10.2541 . [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–96. Epub 2002/05/25. 10.1046/j.1532-5415.2002.50216.x . [DOI] [PubMed] [Google Scholar]
- 17.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80–5. Epub 2003/12/23. 10.1111/j.1532-5415.2004.52014.x . [DOI] [PubMed] [Google Scholar]
- 18.Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36(1):228–35. Epub 2006/10/24. 10.1093/ije/dyl224 . [DOI] [PubMed] [Google Scholar]
- 19.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. Jama. 2015;313(19):1973–4. Epub 2015/05/20. 10.1001/jama.2015.4260 . [DOI] [PubMed] [Google Scholar]
- 20.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371(9626):1800–9. Epub 2008/05/27. 10.1016/S0140-6736(08)60768-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler J, Mooyaart EA, Dannemann N, Bamberg F, Shapiro MD, Ferencik M, et al. Relation of the metabolic syndrome to quantity of coronary atherosclerotic plaque. Am J Cardiol. 2008;101(8):1127–30. Epub 2008/04/09. 10.1016/j.amjcard.2007.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–8. Epub 2004/02/06. 10.1053/j.gastro.2003.10.065 . [DOI] [PubMed] [Google Scholar]
- 23.Braun S, Bitton-Worms K, LeRoith D. The link between the metabolic syndrome and cancer. Int J Biol Sci. 2011;7(7):1003–15. Epub 2011/09/14. 10.7150/ijbs.7.1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younis A, Younis A, Tzur B, Peled Y, Shlomo N, Goldenberg I, et al. Metabolic syndrome is independently associated with increased 20-year mortality in patients with stable coronary artery disease. Cardiovasc Diabetol. 2016;15(1):149. Epub 2016/10/30. 10.1186/s12933-016-0466-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002;288(21):2709–16. Epub 2002/12/04. 10.1001/jama.288.21.2709 . [DOI] [PubMed] [Google Scholar]
- 26.Ogawa H, Fujitani K, Tsujinaka T, Imanishi K, Shirakata H, Kantani A, et al. InBody 720 as a new method of evaluating visceral obesity. Hepatogastroenterology. 2011;58(105):42–4. Epub 2011/04/23. . [PubMed] [Google Scholar]
- 27.Lu CW, Yang KC, Chang HH, Lee LT, Chen CY, Huang KC. Sarcopenic obesity is closely associated with metabolic syndrome. Obes Res Clin Pract. 2013;7(4):e301–7. Epub 2013/12/07. 10.1016/j.orcp.2012.02.003 . [DOI] [PubMed] [Google Scholar]
- 28.Steering Committee 2000. The Asia-Pacific perspective: redefining obesity and its treatment. International Diabetes Institute: Melbourne. [Google Scholar]
- 29.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–7. Epub 2009/05/02. 10.1242/dmm.001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12(6):295–300. Epub 2006/01/06. 10.5551/jat.12.295 . [DOI] [PubMed] [Google Scholar]
- 31.Kuk JL, Ardern CI. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care. 2009;32(12):2297–9. Epub 2009/09/05. 10.2337/dc09-0574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YS, Lee Y, Chung YS, Lee DJ, Joo NS, Hong D, et al. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J Gerontol A Biol Sci Med Sci. 2012;67(10):1107–13. Epub 2012/03/21. 10.1093/gerona/gls071 . [DOI] [PubMed] [Google Scholar]
- 33.Lee YH, Kim JE, Roh YH, Choi HR, Rhee Y, Kang DR, et al. The combination of vitamin D deficiency and mild to moderate chronic kidney disease is associated with low bone mineral density and deteriorated femoral microarchitecture: results from the KNHANES 2008–2011. J Clin Endocrinol Metab. 2014;99(10):3879–88. Epub 2014/06/01. 10.1210/jc.2013-3764 . [DOI] [PubMed] [Google Scholar]
- 34.Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care. 2010;33(7):1652–4. Epub 2010/05/13. 10.2337/dc10-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zając-Gawlak I, Kłapcińska B, Kroemeke A, Pośpiech D, Pelclová J, Přidalová M. Associations of visceral fat area and physical activity levels with the risk of metabolic syndrome in postmenopausal women. Biogerontology. 2017;18(3):357–66. Epub 2017/03/21. 10.1007/s10522-017-9693-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeon HH, Lee YK, Kim DH, Pak H, Shin SY, Seo JH. Risk for metabolic syndrome in the population with visceral fat area measured by bioelectrical impedance analysis. Korean J Intern Med. 2020. Epub 2020/04/29. 10.3904/kjim.2018.427 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schweitzer L, Geisler C, Pourhassan M, Braun W, Glüer CC, Bosy-Westphal A, et al. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr. 2015;102(1):58–65. Epub 2015/05/29. 10.3945/ajcn.115.111203 . [DOI] [PubMed] [Google Scholar]
- 38.Ng AC, Wai DC, Tai ES, Ng KM, Chan LL. Visceral adipose tissue, but not waist circumference is a better measure of metabolic risk in Singaporean Chinese and Indian men. Nutr Diabetes. 2012;2(8):e38. Epub 2012/01/01. 10.1038/nutd.2012.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim DH, Lee SY, Park SJ, Lee YS. Relationships between Spinal Sarcopenia and Spinal Sagittal Balance in Older Women. Ann Geriatr Med Res. 2019;23(3):141–8. Epub 2020/08/04. 10.4235/agmr.19.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim S, Lee HK, Kimm KC, Park C, Shin C, Cho NH. C-reactive protein level as an independent risk factor of metabolic syndrome in the Korean population. CRP as risk factor of metabolic syndrome. Diabetes Res Clin Pract. 2005;70(2):126–33. Epub 2005/06/14. 10.1016/j.diabres.2005.02.020 . [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109(23):2818–25. Epub 2004/06/16. 10.1161/01.CIR.0000132467.45278.59 . [DOI] [PubMed] [Google Scholar]
- 42.Kim G, Lee SE, Jun JE, Lee YB, Ahn J, Bae JC, et al. Increase in relative skeletal muscle mass over time and its inverse association with metabolic syndrome development: a 7-year retrospective cohort study. Cardiovasc Diabetol. 2018;17(1):23. Epub 2018/02/07. 10.1186/s12933-018-0659-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Lin S, Gao T, Zhong F, Cai J, Sun Y, et al. Association between Sarcopenia and Metabolic Syndrome in Middle-Aged and Older Non-Obese Adults: A Systematic Review and Meta-Analysis. Nutrients. 2018;10(3). Epub 2018/03/17. 10.3390/nu10030364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85(1009):1–10. Epub 2011/09/23. 10.1259/bjr/38447238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unno M, Furusyo N, Mukae H, Koga T, Eiraku K, Hayashi J. The utility of visceral fat level by bioelectrical impedance analysis in the screening of metabolic syndrome—the results of the Kyushu and Okinawa Population Study (KOPS). J Atheroscler Thromb. 2012;19(5):462–70. Epub 2012/06/05. 10.5551/jat.11528 . [DOI] [PubMed] [Google Scholar]
- 46.Ranasinghe C, Gamage P, Katulanda P, Andraweera N, Thilakarathne S, Tharanga P. Relationship between Body Mass Index (BMI) and body fat percentage, estimated by bioelectrical impedance, in a group of Sri Lankan adults: a cross sectional study. BMC Public Health. 2013;13:797. Epub 2013/09/06. 10.1186/1471-2458-13-797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamakage H, Ito R, Tochiya M, Muranaka K, Tanaka M, Matsuo Y, et al. The utility of dual bioelectrical impedance analysis in detecting intra-abdominal fat area in obese patients during weight reduction therapy in comparison with waist circumference and abdominal CT. Endocr J. 2014;61(8):807–19. Epub 2014/06/17. 10.1507/endocrj.ej14-0092 . [DOI] [PubMed] [Google Scholar]
- 48.Park YS, Kim JW, Kim BG, Lee KL, Lee JK, Kim JS, et al. Sarcopenia is associated with an increased risk of advanced colorectal neoplasia. Int J Colorectal Dis. 2017;32(4):557–65. Epub 2016/12/26. 10.1007/s00384-016-2738-8 . [DOI] [PubMed] [Google Scholar]
- 49.Ida S, Watanabe M, Yoshida N, Baba Y, Umezaki N, Harada K, et al. Sarcopenia is a Predictor of Postoperative Respiratory Complications in Patients with Esophageal Cancer. Ann Surg Oncol. 2015;22(13):4432–7. Epub 2015/04/12. 10.1245/s10434-015-4559-3 . [DOI] [PubMed] [Google Scholar]
- 50.Klip A, Pâquet MR. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 1990;13(3):228–43. Epub 1990/03/01. 10.2337/diacare.13.3.228 . [DOI] [PubMed] [Google Scholar]