Abstract
Peroxidation of plasma membranes, characterized by oxidative attack of lipidic carbon-carbon double-bonds in unsaturated fatty acids, has been identified as an important biochemical event in multiple pathological conditions, including neurodegenerative diseases, atherosclerosis, diabetes, preeclampsia, aging, cancer, etc. Changes to the lipid bilayer structure as a result of lipid peroxidation may lead to lipid membrane malfunction, and consequently initiate further downstream biochemical cascades. However, how lipid peroxidation modulates the mechanical properties of lipid membranes remains largely controversial. In this study, we investigate the peroxidation of lipids with polyunsaturated fatty acid tails using molecular dynamics simulations. By systematically varying the oxidation site, we find that lipid peroxidation alters the biophysical properties of bilayer membrane in a peroxidation site-specific manner. Specifically, our results suggest that peroxidation at sites in the bilayer interior disturbs and softens the membrane, whereas peroxidation at sites near the membrane-water interface results in a more ordered and stiffer membrane. Such a peroxidation site-specific modulation of lipid membrane mechanics provides an explanation for the contradictory results obtained in previous experiments. Our study paves the way for an improved understanding of the initiation of the downstream cellular dysfunction caused by lipid peroxidation.
Keywords: Peroxidation, Lipid bilayer, Elastic modulus, Molecular simulation, Ferroptosis
Graphical Abstract
1. Introduction
Lipid peroxidation is characterized by an oxidative attack of lipidic carbon-carbon double-bonds in unsaturated fatty acids, often leading to severe damage and cell membrane dysfunction [1]. The most common oxidants are reactive oxygen species (ROS) which can be generated by intracellular metabolic changes or extracellular stimuli, such as ultraviolet or ionizing radiation, heavy metals, and certain drugs [1–6]. Peroxidation reactions can be catalysed by intracellular enzymes, such as lipoxygenases [6] and cyclooxygenases [7], in the presence of molecular oxygen. Under normal physiological conditions, cells may survive mild oxidative stress through the activation of their antioxidant defence mechanisms. However, under higher peroxidation rates, the repair mechanisms are overwhelmed and cells die by apoptosis or necrosis [8]. 4-hydroxynonenal, one of the lipid peroxidation secondary products, may act as a signaling molecule to activate stress response pathways and at low levels confer protection to the cell, but at high levels may inhibit gene expression and promote cell death [8]. The brain is especially vulnerable to lipid peroxidative damage due to its high concentration of polyunsaturated fatty acids (PUFAs). Lipid peroxidation has been implicated in several neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease [9]. It also contributes to many other pathological conditions, including atherosclerosis, diabetes, inflammation, asthma, preeclampsia, aging, and cancer [10–16]. The iron-catalysed lipid peroxidation of phosphatidylethanolamine (PE) phospholipids with PUFAs by lipoxygenases has been found associated with another form of programmed cell death called ferroptosis [17,18]. Our recent investigations observed formation of large membrane blebs on human trophoblasts undergoing ferroptosis, suggesting a dramatic change to the plasma membrane mechanics associated with placental ferroptotic damage [19]. Despite its wide pathological implications, it remains largely elusive how lipid peroxidation contributes to cellular dysfunction and cell death. It thus becomes critical to precisely define the phospholipid membrane damage, as it lays the foundation for understanding possible downstream injuries.
The effects of lipid peroxidation on the biophysical properties of membranes have been investigated using both experimental and computational approaches. By embedding photosensitizers into giant unilamellar vesicles (GUVs) of either 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC) or 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), Weber et al. showed that the GUVs expanded in area by up to about 15% after oxidizing the lipids by UV-irradiating the GUVs [20]. Furthermore, the elastic modulus of the membrane, a measure of the resistance of the membrane to stretching, decreased linearly with the fraction of oxidized lipids in the GUVs. Although lipid peroxidation made the membrane more stretchable, neither membrane integrity nor permeability to sugar was affected. The surface area increase per lipid of peroxidized POPC lipid membranes along with a decrease in the membrane thickness has been experimentally confirmed by small angle X-ray scattering, where 95% of the polar hydroperoxide (OOH) groups formed at the oxidation sites lied in the membrane polar region, probably forming hydrogen-bonds to carbonyl and phosphate groups [16]. Computer simulations using atomistic and coarse-grained (CG) lipid models suggest that the migration of hydroperoxide groups to the membrane-water interface accounts for the increase in the area per lipid and decrease in the membrane thickness [21–24].
Although it has been generally accepted that lipid peroxidation tends to result in a softer membrane, several experimental studies have suggested an opposite effect [25–27]. Dobretsov et al. found that lipid peroxidation lowered the membrane fluidity of liposomes of phosphatidylcholine (PC) and PE mixtures, suggesting the rigidification of the bilayer membrane [25]. Choe et al. added peroxidized liposomes into microsomal membranes from rat livers and reported a substantial increase in the microsomal membrane rigidity [26]. Using liposomes with increasing amounts of 1-stearoyl-2-arachidonoyl phosphatidylcholine (SAPC) that could be oxidized chemically by an oxidant/catalyst pair, Borst et al. showed that membrane fluidity significantly decreased with the increase in the oxidized SAPC [27]. Recently, Bour et al. found that GUVs of DOPC lipids with double-bonds located at carbon-6 (C6) showed no clear peroxidation-induced swell-burst cycles that had been observed with native DOPC containing double-bonds at carbon-9 (C9), suggesting that C6-OOH DOPC membrane might be mechanically different with its C9-OOH counterpart [28]. Considering that the location of possible peroxidation sites varies depending on the location of carbon-carbon double bonds in the lipids, it is important to understand the possible effect of peroxidation site on the modulation of the membrane biophysical properties, which may provide a way to understand the conflicting observations discussed above.
To precisely understand how the peroxidation site modulates the structural and mechanical properties of lipid membranes, we have carried out CG molecular dynamics (MD) simulations of PE and PC phospholipids with C18:0/20:4 fatty acid tails (namely, SAPE and SAPC lipids). It has been reported that the four carbon-carbon double bonds of SAPE/PC lipids allow peroxidation to occur at four different sites, including C5, C8, C12 and C15 [18]. Our simulations confirm that lipid peroxidation alters membrane structure and mechanical properties in an oxidation site-dependent manner. We find that, although the membrane expands in all cases, peroxidation at C12 and C15 softens the membrane whereas peroxidation at C5 and C8 makes the membrane more rigid. Our simulations indicate that such a distinct trend is closely associated with the change in lipid packing order as a result of the migration of OOH groups towards the polar interface, where peroxidation at sites deep in the membrane interior (i.e., C12 and C15) tends to destabilize the membrane; whereas the membrane becomes more ordered when peroxidation occurs near the membrane-water interface (i.e., C5 and C8). Our study thus bridges the gap in understanding the effect of oxidation site on the biophysical and mechanical properties of lipid bilayer, and thus may illuminate the downstream effects in many pathological conditions affecting the cell membrane.
2. Materials and Methods
MD simulations were carried out for SAPE and SAPC lipid bilayers using the CG MARTINI force-field version 2.2 implemented in the GROMACS version 2018.2 software [29–31]. The initial configurations and simulation set-up files for the lipid bilayers were generated using the Martini Bilayer Maker [32] within the CHARMM-GUI web-based platform [33]. Planar bilayers were generated with 1024 lipids per monolayer (27 nm × 27 nm in area). Periodic boundary conditions were applied, with bilayer images separated by 8 nm of water in the membrane normal direction (4 nm thick water layer both above and below the bilayer). After energy minimization steps, MD simulations were carried out with progressively reduced restraints on lipid head-group positions as the simulation time-step increased from 2 to 20 fs. Unrestrained simulations were then carried out for 2 µs to equilibrate the bilayer and obtain the steady-state configuration. Electrostatic interactions were computed using Coulomb interaction with reaction-field approximation beyond the cut-off distance of 1.1 nm with a dielectric constant of 15 [34], accounting for the long range interactions. Van der Waals interactions were computed using cut-off method with the same distance of 1.1 nm. System temperature was maintained at 310 K with the Velocity-rescale thermostat, whereas pressure was maintained at 1 bar with the Parrinello-Rahman barostat with semi-isotropic coupling (X-Y plane coupled separately from the Z or bilayer normal direction) with time-constant of 12 ps and compressibility of 3 × 10−4 bar−1.
From the configurations of SAPE or SAPC bilayers after 2 µs of equilibration, we constructed bilayers with varying fractions of peroxidized lipids by linking OOH particles to the CG lipid tail particles containing carbon atoms connected by double bonds, following a previously published protocol [22]. This procedure was carried out automatically using an in-house Python script, which adds a MARTINI P2 type bead representing OOH particle at 0.33 nm from the corresponding CG tail particle along a vector that is the sum of bond vectors on the two sides of that tail particle. MD simulations were then carried out for the bilayers following the same procedure as for un-peroxidized bilayers, detailed above.
Various physical and mechanical properties of the SAPE/PC bilayers were measured using the configurations of 100 snapshots taken from the 1–2 µs simulation. The area per lipid was computed as the simulation box area divided by the number of lipids in each monolayer. The bilayer thickness at each snapshot was computed from the mean separation distance of the peaks in the frequency distributions of the z-coordinates of PO4 particles in the bilayer head-groups. The process is then repeated for all the 100 configurations taken to obtain the averaged bilayer thickness and associated error estimate. The degree of disorder of the lipids in the bilayers was quantified by calculating the order parameter , where 〈θ〉 is an average for all lipids and for each angle between a selected lipidond and the bilayer normal, as well as over simulation time [35]. The order parameter was calculated using an in-house TCL script, running on the Visual MD software [36]. The elastic modulus of each bilayer was calculated based on the Hooke’s law which states that the elastic modulus is the proportionality constant relating the membrane tension σ to the area strain (A(σ) — A(σ = 0))/A(σ = 0) for small σ (or correspondingly small area strain). We obtained the membrane tension-area strain curve by stretching the bilayer to different tension levels via specifying a negative value for pressure PLat in the bilayer plane [37]. The membrane tension was then calculated from the external pressure applied to the system as σ = Lz(Pz — PLat) [37], where Lz and Pz are the size of the simulation box and the pressure in the direction perpendicular to the bilayer plane. We varied PLat from −5 bar to −30 bar while maintaining Pz at 1 bar. The elastic modulus KA was then taken as the slope of the linear regression fit to the membrane tension-area strain curve up to an area strain of 10% [35]. Unlike the area fluctuation method which is known to show strong system size-dependence (larger membranes allow larger wavelength undulations which enhances the deviation between true and projected areas) [38,39], the elastic modulus obtained with the Hooke approach is independent of the system size (at least up to a system with 4000 lipids per monolayer which is 4× of our system size) [38].
3. Results and Discussion
3.1. SAPE/PC membranes peroxidized at different sites show distinct biophysical properties
As demonstrated in Fig. 1(a), SA tails consist of an 18-carbon saturated tail and a 20-carbon polyunsaturated tail with four double-bonds. Peroxidation can occur at four different carbon sites, namely at C5, C8, C12 and C15 [6]. After peroxidation at a certain carbon site, an OOH group is covalently linked to the carbon and the double-bond shifts one position down (up) the chain if linked carbon is the first (second) atom in the double-bond [21]. We use the MARTINI force-field to simulate both native and peroxidized SAPE and SAPC lipids, where the lipid structure is mapped to a CG model by grouping four atoms into one particle in general. The CG model of SAPE is shown in Fig. 1(b) and replacing the NH3 particle with a NC3 particle would lead to the CG model for SAPC. The four peroxidation sites, C5, C8, C12 and C15, are included in the D1A, D2A, D3A and D4A particles, respectively. Following a previously published protocol [22], the OOH group is modelled as a P2 type particle in MARTINI force field and is linked to one of the “D” particles at a distance of 0.33 nm from the “D” particle along a vector that is the summation of bond vectors on the two sides of the “D” particle. Figure 1(c) shows the equilibrated configurations of both the native and the fully oxidized SAPE bilayers at different oxidation sites. We qualitatively observe that the flexibility of the lipid bilayer increases (or persistent length of the membrane decreases) as the oxidation site moves towards the bilayer interior from C5 to C15.
Figure 1.
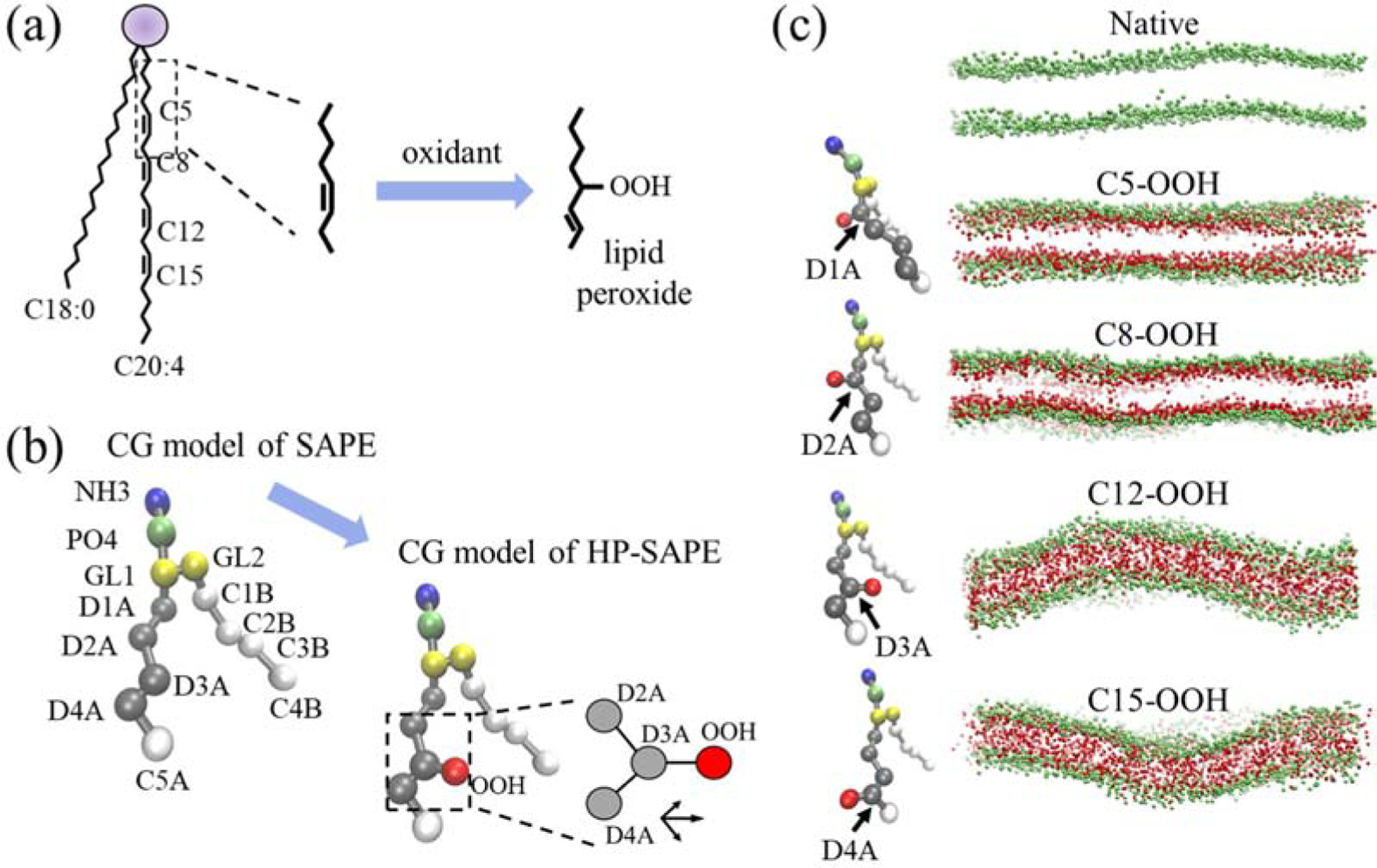
CGMD simulations of peroxidation of poly-unsaturated phospholipids. (a) Phospholipids with C18:0/C20:4 (SA) fatty acid tails have four double-bonds along its unsaturated fatty acid tail. The purple circle represents the hydrophilic head-group and glycerol linkage atoms. Peroxidation occurs when an oxidant adds an OOH group at a double-bonded carbon (C5 in this example), with the double-bond shifted by one carbon position. C5, C8, C12 and C15 label the four possible peroxidation sites. (b) CG models of native and hydro-peroxidized (HP) SAPE phospholipids with about four non-hydrogen atoms reduced to one CG particle, where NH3 and PO4 are head-group particles, GL1/GL2 are glycerol linkage group particles, and “D” and “C” are particles containing carbon atoms connected by double bonds and single bonds, respectively, with the OOH particle linked to a “D” particle (D3A as example) in the HP-SAPE model. Inset shows determination of D3A-OOH bond vector as the vector sum of D2A-D3A and D4A-D3A vectors. (c) Configurations of native SAPE and HP-SAPE bilayers after 2 μs simulation with all lipids oxidized at the indicated oxidation site. Only PO4 and OOH particles are shown for clarity.
To quantify how the location of OOH groups changes during the simulation, we examined the vertical distance between the OOH particle of each oxidized lipid and the bilayer-water interface before and after the equilibration. We define ∆z = zPO4 — zOOH, where zPO4 and zOOH are the spatial coordinates of the PO4 and OOH particles of each individual lipid in the direction perpendicular to the lipid bilayer plane, respectively. Therefore, all the distributions of the measured vertical distance exhibit two peaks (Fig. 2(a)), where the positive one corresponds to the OOH particle location in the upper leaflet while the negative one in the lower leaflet. During the simulations, the polar OOH groups tend to migrate towards the head-group region in all cases (i.e. the two peaks shifting towards zero distance). Histograms obtained from 0.9–1 μs are similar to those between from 1.9–2 μs, indicating that systems have reached equilibrated states after 1 μs (Fig. S1). At the start of the simulation, the mean distance of each OOH particle from PO4 particle (taken as the average of the absolute values of the means of the two distributions about zero in Fig. 2(a)) increases from C5 to C15 site (Fig. 2(b)). After equilibration, both C5-OOH and C8-OOH reside at locations with an average distance of 0.5 nm below the PO4 particles. Surprisingly, C15-OOH becomes even closer to the head-group region than C12-OOH. Although the migration of OOH groups towards the head-group region is energetically favored as the polar OOH particle would be able to form hydrogen-bonds with head-group particles as well as solvent, it is penalized by the bending of the fatty acid chain. The relatively small change in the distance of C12-OOH from PO4 is likely due to the fact that the C12-OOH particles are located farther away from the lipid-water interface than C8-OOH at the initial state and their migration towards the interface requires the fatty acid chain to bend more significantly than C15-OOH.
Figure 2.
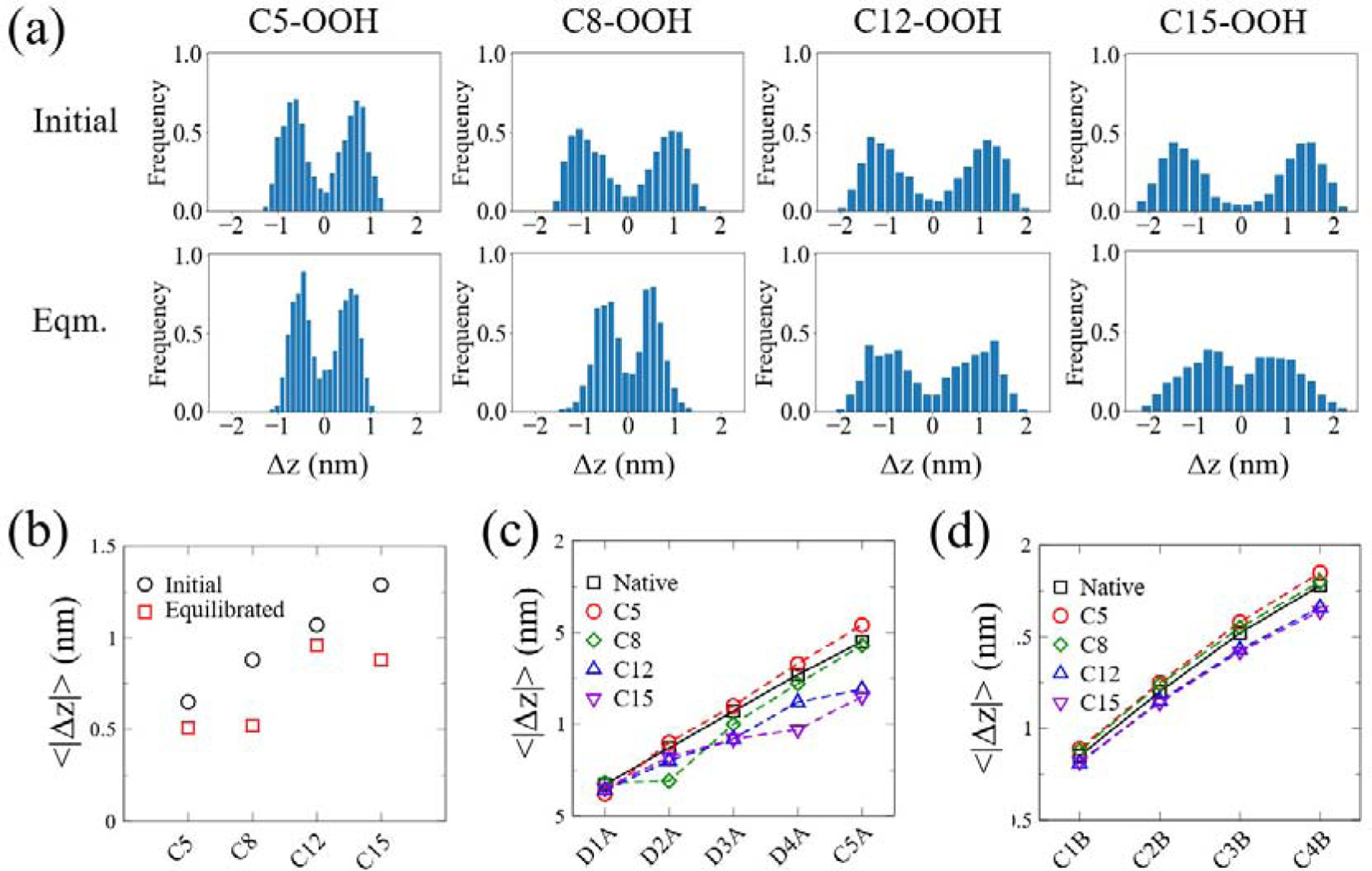
Migration of OOH and fatty acid tail particles towards bilayer-water interface of fully oxidized HP-SAPE bilayers. (a) Frequency distributions of the distance between PO4 particles (taken as reference) and OOH particles in the bilayer normal direction, ∆z, at both initial (right after linking OOH particles to lipids in the equilibrated SAPE bilayer; top) and equilibrated (after 2 μs; bottom) states. (b) Mean z-coordinate distances of PO4 particles from OOH particles (for each oxidization site) for initial and equilibrated states, quantified from the histograms in (a) as the average value of the (absolute) means of each distribution about zero. (c-d) Mean distances of PO4 particles from each particle along the unsaturated (c) and saturated (d) tails after equilibration. Means are taken over 1.9–2 μs. Error bars are omitted as standard errors are only about 1% of the means.
Next, we examined how peroxidation at each site affects the locations of each unsaturated and saturated tail particles. As indicated by Fig. 2(c), the migration of OOH groups drags the directly linked “D” particles towards the head-group region in all cases, causing the tilting of the fatty acid tail. For the C5-OOH bilayer, all carbon particles that are not directly linked to the OOH groups tend to move further away from the head-group region, suggesting an increase in the bilayer thickness. For the C8-OOH bilayer, the carbon particles in the unsaturated tail become closer to the head-group region, while those in the saturated tail moves slightly towards the interior region. In contrast, peroxidation at C12 and C15 drives nearly all the carbon particles move towards the lipid-water interface, suggesting a decrease in the bilayer thickness.
The tilting of the unsaturated fatty acid tails leads to changes in the lipid tail packing order. The variation of the order parameters of bonds along unsaturated and saturated tails are shown in Fig. 3(a–b) (see Fig. S2 for corresponding plots for SAPC bilayer which show similar trends). Figure 3(a) shows that, across all four sites, the P2 value of the bond involving particles linked to the OOH group is significantly lower than their counterpart in the native bilayer whereas the next bond along the chain shows a sharp increase in P2 value. The sharp increase is more prominent for C5 (linked to D1A) and C8 (linked to D2A) with P2 values reaching close to 0.3, whereas those of C12 and C15 only reach 0.1 which is close to that of the native bilayer. Although the P2 values of the bonds along the saturated tails of C12 and C15 oxidized bilayer are consistently lower than those of the un-oxidized bilayer, the P2 values of the first few bonds for C5 or C8 bilayer are slightly higher than their native counterparts (Fig. 3(b)). To reveal how the fraction of oxidized lipids affects the overall lipid packing order, we have averaged the P2 values over C-C bonds along both lipid tails and found that C5 oxidation leads to an increase in the overall lipid packing order while oxidation at other sites results in an opposite trend (Fig. 3(c) for SAPE and Fig. S2(c) for SAPC). From C8 to C15, the reduction in the overall lipid packing order becomes more significant as the peroxidation site moves towards the bilayer interior. The change in the overall lipid packing order increases proportionally with the increase in the fraction of the oxidized lipids.
Figure 3.
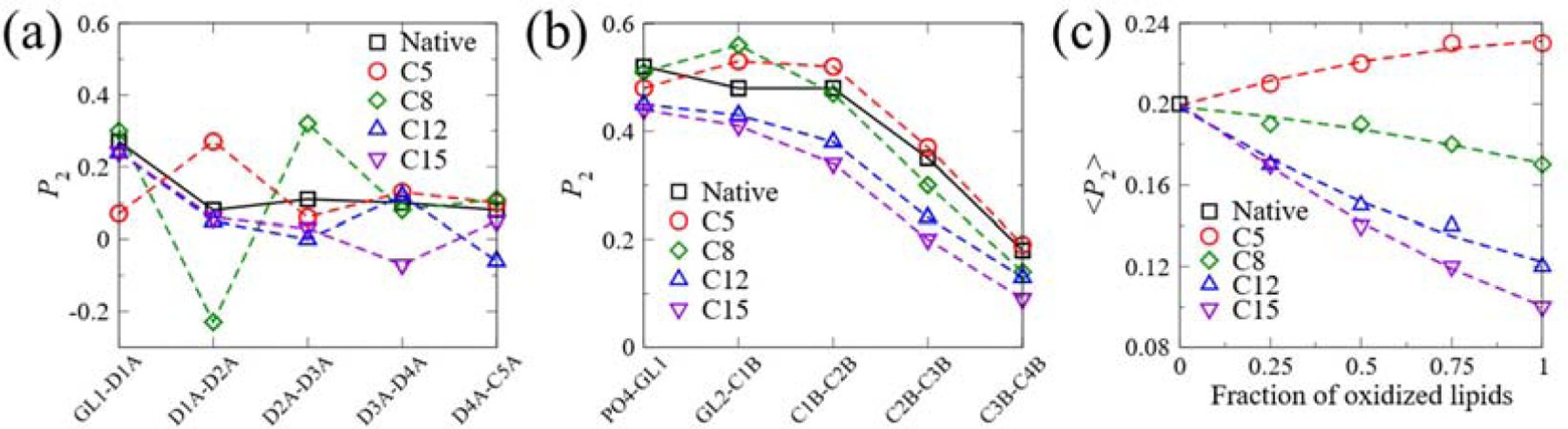
The effect of site-specific peroxidation of SAPE lipid tails on the ordering of the lipid tails. (a-b) Order parameter P2 of the bonds between neighboring CG beads along unsaturated (a) and saturated (b) fatty acid tails of SAPE lipids in fully oxidized bilayers. (c) Averaged order parameter P2 as a function of the fraction of oxidized lipids.
The distinct conformation of oxidized lipids results in significant change in the bilayer structures. As shown in Fig. 4(a), the area per lipid increases monotonically with the increase in the fraction of oxidized lipids, and the extent of the increase exhibits a strong oxidation site-dependence. Oxidation at C15 leads to the largest increase in the area per lipid, while oxidation at C5 causes the least increase. The increase in area per lipid can be attributed to the following two schemes: reduced lipid tail order and the presence of OOH groups within the head-group region. Although the increase of the lipid tail order of the bilayer peroxidized at C5 tends to reduce the area per lipid, the migration of OOH groups to the head-group region outperforms the effect of lipid tail order, resulting in a slight increase in the area per lipid. Since the bilayer thickness is primarily determined by the lipid tail order only, we observe that the effects of lipid peroxidation on bilayer thickness follow a similar trend as the averaged order parameter. The bilayer thickness decreases monotonically with the increase in the fraction of oxidized lipids when oxidation occurs at C8, C12 and C15 (Fig. 4(b)). Nevertheless, C5 oxidation results in a slightly thicker bilayer than the native one, and the thickness increases with the fraction of oxidized lipids. The same trend is observed for SAPC bilayers (Fig. S3(a–b)).
Figure 4.
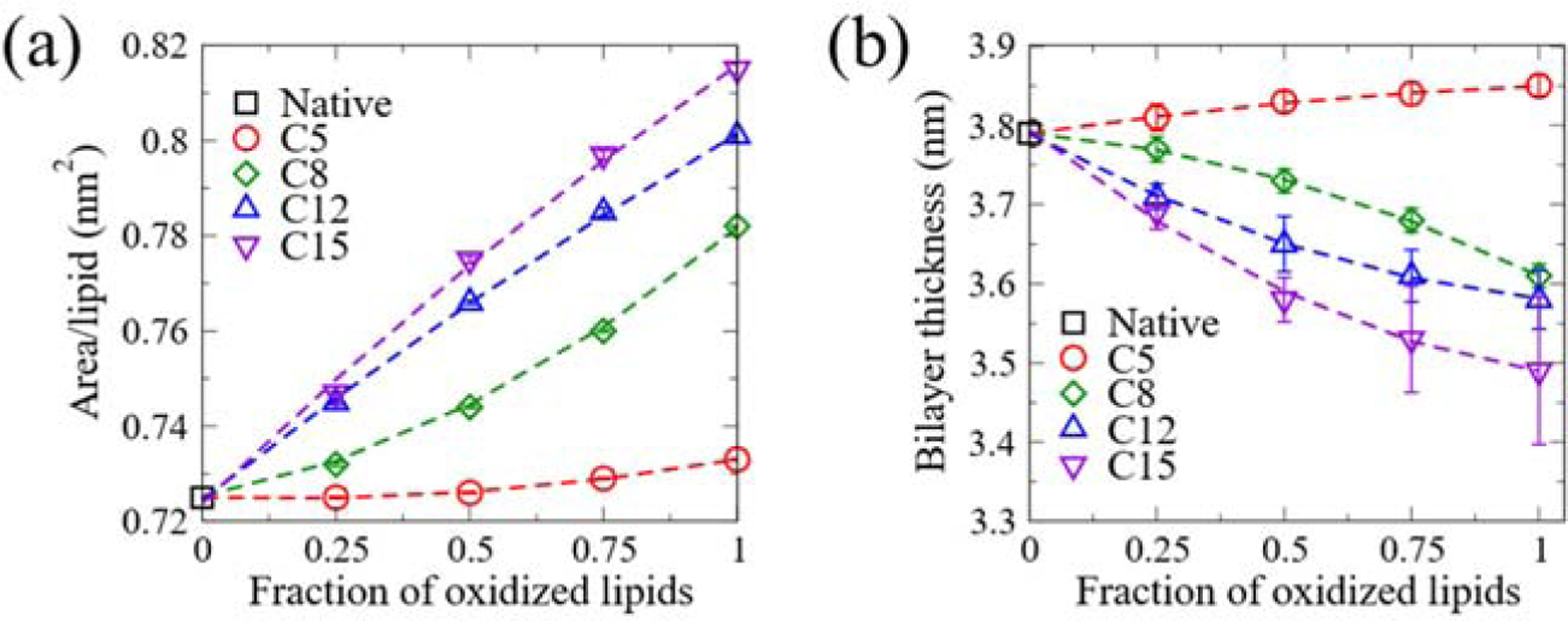
The effect of lipid peroxidation on the physical properties of the SAPE bilayer. (a) The area per lipid of the SAPE bilayer as a function of the fraction of oxidized lipids. (b) The bilayer thickness of the SAPE bilayer as a function of the fraction of oxidized lipids.
3.2. Effects of oxidation site on the mechanical properties of SAPE/PC bilayers
Changes to the lipid membrane structure due to lipid peroxidation consequently lead to changes in the mechanical properties of the membrane. We have virtually stretched the membrane to different area strain levels and calculated the corresponding membrane tension (see Materials and Methods for more details). As demonstrated in Fig. 5(a), the membrane tension increases monotonically with the area strain. Peroxidation at C12 or C15 makes the bilayer less resistant to stretching than the native one as the same membrane tension results in a higher area strain. Conversely, oxidation at C5 or C8 makes the bilayer more resistant to stretching than the native one as a higher membrane tension is needed to generate the same amount of area strain. C12 or C15 oxidized bilayer shows a linear behavior of area strain to membrane tension implying they behave as elastic materials over the range of area strain up to 25%. In contrast, C5 or C8 oxidized bilayers start to deviate from the linear behavior after an area strain of 10%.
Figure 5.

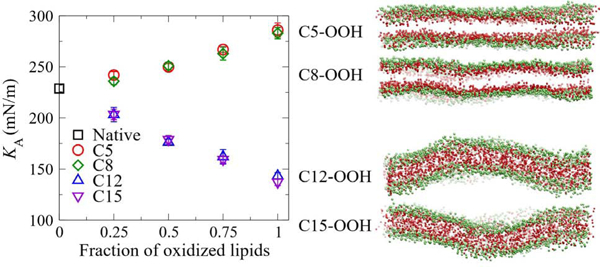
The effect of lipid peroxidation on the mechanical property of the SAPE bilayer. (a) The membrane tension of native SAPE and fully HP-SAPE bilayers as a function of the membrane area strain during stretching simulations. Elastic moduli are extracted as the slope of the least-squares regression line fitted to low area strain data points (up to about 0.1). (b) The effect of the fraction of oxidized lipids in the SAPE bilayer on the elastic modulus.
The variations of KA with the fraction of oxidized lipids in bilayers with lipids peroxidized at different sites are shown in Fig. 5 (b), which clearly indicates that lipid oxidation regulates the elastic modulus of the membrane in an oxidation site-dependent manner. Oxidation at C12 or C15 makes the SAPE bilayer progressively softer as the fraction of oxidized lipids increases, whereas C5 or C8 oxidation has an opposite effect. Fully oxidized C12/C15-oxidized membranes are about 40% more stretchable than native, un-oxidized membrane, whereas C5/C8-oxidized membranes are about 25% less stretchable than native. The change in the elastic modulus is linearly proportional to the fraction of oxidized lipids. The same trend has also been observed for SAPC bilayers (Fig. S4(b)). A linear decrease of KA with the fraction of oxidized lipids agrees with the trend reported in the experimental study by Weber et al., where POPC (C16:0/C18:1) and DOPC (C18:1/C18:1) lipids were oxidized at C9 [20]. The same trend has also been reported in another computational study [22]. As a new form of programmed cell death, ferroptosis involves SAPE peroxidation at C12/C15 sites by the iron-dependent 15-lipoxygenases [18]. The reduced membrane stiffness may help explain the origin of downstream events during ferroptosis, such as membrane macro-blebbing of human trophoblasts undergoing ferroptosis [19].
A stiffer C5-OOH membrane relative to its native counterpart can be expected from its more ordered fatty acid tails (Fig. 3(c)). Surprisingly, the KA value of C8-OOH membrane follows the same trend as that of C5-OOH, although its overall fatty acid tail ordering is lower than that of the native membrane. The OOH particles in the fully oxidized C8-OOH bilayer are at a similar distance from head-group PO4 particles as in the C5-OOH bilayer (Fig. 2(b)), implying that inter-molecular hydrogen-bonds at the head-group region in C8-OOH bilayer might also be enhanced. This may account for the enhanced ordering of their GL2-C1B bonds (Fig. 3(b)). Hence, our results suggest that the enhancement of inter-molecular hydrogen-bonds at the head-group region by OOH particles in both C5 and C8-OOH bilayers might be key to determining the elastic modulus of the bilayers. Such an effect on membrane stiffening by oxidation at C5 or C8 sites may provide an explanation for the peroxidation induced-membrane stiffening phenomena observed in previous experiments [25–27]. In the study by Borst et al. using liposomes of DOPC and SAPC mixtures, the increased membrane viscosity suggests that lipid peroxidation makes the membrane stiffer [27]. Therefore, we postulate that the oxidant likely only reached C5/C8 sites in SAPC lipids. Considering that the DOPC C9 oxidation site is buried deeper in the bilayer interior than SAPC C5/C8 sites, our notion is validated by the observation that DOPC viscosity did not significantly change upon peroxidation. Bour et al. observed that liposomes of DOPC variant with oxidation site at C6 showed no clear swell-burst cycles that were observed with DOPC C9 oxidation [28]. Such a distinct morphological response suggests that DOPC membranes with C6 oxidation might be stiffer, which is consistent with our prediction.
4. Conclusions
Peroxidation of PUFA-containing phospholipids is the first step in the oxidative damage to cell membranes. We have carried out CGMD simulations of phospholipid bilayers consisting of PUFA tails to decipher how the location of the peroxidation site in the fatty acid tail affects the structural and mechanical properties of the respective membrane. Our simulations demonstrate that the location of PUFA-peroxidation plays an important role in modulating the mechanics of the peroxidized membrane. Specifically, peroxidation at sites buried in the membrane interior disrupts the lipid-lipid packing as the polar hydroperoxide group migrates towards the membrane-water interface. This makes the membrane softer. On the other hand, peroxidation at sites close to the membrane-water interface enhances the lipid-lipid packing in the membrane interior and thus makes the membrane stiffer. Our results suggest that the contrasting effects of lipid peroxidation on the membrane stiffness observed in previous experiments may result from the difference in the location of the peroxidation site. Our study highlights the importance of considering peroxidation site-specific effects when interpreting experimental investigations involving oxidation of membranes containing PUFAs, which may pave the way for a more accurate understanding of the initiation of any lipid peroxidation-induced downstream biochemical events in various pathological conditions.
Supplementary Material
Acknowledgments
Y.S., K.J.H. and C.H. acknowledge the financial support by the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant R01HD086325). K.J.H. would like to acknowledge financial support from Nanyang Technological University (start-up grant M4082428.050). C.H. would also like to acknowledge financial support from Nanyang Technological University (start-up grant M4082352.050) and Singapore Ministry of Education Academic Research Fund Tier 1 (M4012229.050). The computational work for this article was fully performed on resources of the National Supercomputing Centre, Singapore (https://www.nscc.sg).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Gaschler MM, Stockwell BR, Lipid peroxidation in cell death, Biochem. Biophys. Res. Commun 482 (2017) 419–425. 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Girotti AW, Mechanisms of lipid peroxidation, J. Free Radicals Biol. Med 1 (1985) 87–95. 10.1016/0748-5514(85)90011-X. [DOI] [PubMed] [Google Scholar]
- [3].Moldovan L, Moldovan NI, Oxygen free radicals and redox biology of organelles, Histochem. Cell Biol 122 (2004) 395–412. 10.1007/s00418-004-0676-y. [DOI] [PubMed] [Google Scholar]
- [4].Kawamura K, Qi F, Kobayashi J, Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production, J. Radiat. Res 59 (2018) ii91–ii97. 10.1093/jrr/rrx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Arudi L, Sutherland MW, A study of the reactivity of HO2/O2-with unsaturated fatty acids, J. Biol. Chem 258 (1983) 4759–4761. [PubMed] [Google Scholar]
- [6].Haeggström JZ, Funk CD, Lipoxygenase and leukotriene pathways: Biochemistry, biology, and roles in disease, Chem. Rev 111 (2011) 5866–5896. 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- [7].Rouzer CA, Marnett LJ, Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases, Chem. Rev 103 (2003) 2239–2304. 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- [8].Ayala A, Muñoz MF, Argüelles S, Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal, Oxid. Med. Cell. Longev 2014 (2014). 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pamplona R, Borras C, Jové M, Pradas I, Ferrer I, Viña J, Redox lipidomics to better understand brain aging and function, Free Radic. Biol. Med 144 (2019) 310–321. 10.1016/j.freeradbiomed.2019.03.016. [DOI] [PubMed] [Google Scholar]
- [10].Ito F, Sono Y, Ito T, Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: Oxidative stress in diabetes, atherosclerosis, and chronic inflammation, Antioxidants 8 (2019) 72. 10.3390/antiox8030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhong S, Li L, Shen X, Li Q, Xu W, Wang X, Tao Y, Yin H, An update on lipid oxidation and inflammation in cardiovascular diseases, Free Radic. Biol. Med 144 (2019) 266–278. 10.1016/j.freeradbiomed.2019.03.036. [DOI] [PubMed] [Google Scholar]
- [12].Rådmark O, Werz O, Steinhilber D, Samuelsson B, 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1851 (2015) 331–339. 10.1016/j.bbalip.2014.08.012. [DOI] [PubMed] [Google Scholar]
- [13].Raijmakers MTM, Dechend R, Poston L, Oxidative stress and preeclampsia: Rationale for antioxidant clinical trials, Hypertension 44 (2004) 374–380. 10.1161/01.HYP.0000141085.98320.01. [DOI] [PubMed] [Google Scholar]
- [14].Wenzel SE, Tyurina YY, Zhao J, St. Croix CM, Dar HH, Mao G, Tyurin VA, Anthonymuthu TS, Kapralov AA, Amoscato AA, Mikulska-Ruminska K, Shrivastava IH, Kenny EM, Yang Q, Rosenbaum JC, Sparvero LJ, Emlet DR, Wen X, Minami Y, Qu F, Watkins SC, Holman TR, VanDemark AP, Kellum JA, Bahar I, Bayır H, Kagan VE, PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals, Cell 171 (2017) 628–641.e26. 10.1016/j.cell.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS, Mitochondrial oxidative stress in aging and healthspan, Longev. Heal 3 (2014) 6. 10.1201/b21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bebber CM, Müller F, Clemente LP, Weber J, von Karstedt S, Ferroptosis in cancer cell biology, Cancers (Basel) 12 (2020) 164. 10.3390/cancers12010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Baylr H, Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis, Nat. Chem. Biol 13 (2017) 81–90. 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Anthonymuthu TS, Kenny EM, Shrivastava I, Tyurina YY, Hier ZE, Ting HC, Dar HH, Tyurin VA, Nesterova A, Amoscato AA, Mikulska-Ruminska K, Rosenbaum JC, Mao G, Zhao J, Conrad M, Kellum JA, Wenzel SE, Vandemark AP, Bahar I, Kagan VE, Baylr H, Empowerment of 15-lipoxygenase catalytic competence in selective oxidation of membrane ETE-PE to ferroptotic death signals, HpETE-PE, J. Am. Chem. Soc 140 (2018) 17835–17839. 10.1021/jacs.8b09913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kajiwara K, Beharier O, Choon-Peng C, Goff JP, Ouyang Y, Croix CMS, Huang C, Kagan VE, Hsia KJ, Sadovsky Y, Ferroptosis induces membrane blebbing in placental trophoblasts, J. Cell Sci Submitted (2020). [DOI] [PMC free article] [PubMed]
- [20].Weber G, Charitat T, Baptista MS, Uchoa AF, Pavani C, Junqueira HC, Guo Y, Baulin VA, Itri R, Marques CM, Schroder AP, Lipid oxidation induces structural changes in biomimetic membranes, Soft Matter 10 (2014) 4241–4247. 10.1039/c3sm52740a. [DOI] [PubMed] [Google Scholar]
- [21].Wong-Ekkabut J, Xu Z, Triampo W, Tang IM, Tieleman DP, Monticelli L, Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study, Biophys. J 93 (2007) 4225–4236. 10.1529/biophysj.107.112565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guo Y, Baulin VA, Thalmann F, Peroxidised phospholipid bilayers: Insight from coarse-grained molecular dynamics simulations, Soft Matter 12 (2016) 263–271. 10.1039/c5sm01350j. [DOI] [PubMed] [Google Scholar]
- [23].Siani P, de Souza RM, Dias LG, Itri R, Khandelia H, An overview of molecular dynamics simulations of oxidized lipid systems, with a comparison of ELBA and MARTINI force fields for coarse grained lipid simulations, Biochim. Biophys. Acta - Biomembr 1858 (2016) 2498–2511. 10.1016/j.bbamem.2016.03.031. [DOI] [PubMed] [Google Scholar]
- [24].Agmon E, Solon J, Bassereau P, Stockwell BR, Modeling the effects of lipid peroxidation during ferroptosis on membrane properties, Sci. Rep 8 (2018) 1–11. 10.1038/s41598-018-23408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dobretsov GE, Borschevskaya TA, Petrov VA, Vladimirov YA, The increase of phospholipid bilayer rigidity after lipid peroxidation, FEBS Lett 84 (1977) 125–128. 10.1016/0014-5793(77)81071-5. [DOI] [PubMed] [Google Scholar]
- [26].Choe M, Jackson C, Yu BP, Lipid peroxidation contributes to age-related membrane rigidity, Free Radic. Biol. Med 18 (1995) 977–984. 10.1016/0891-5849(94)00217-8. [DOI] [PubMed] [Google Scholar]
- [27].Borst JW, Visser NV, Kouptsova O, Visser AJWG, Oxidation of unsaturated phospholipids in membrane bilayer mixtures is accompanied by membrane fluidity changes, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1487 (2000) 61–73. 10.1016/S1388-1981(00)00084-6. [DOI] [PubMed] [Google Scholar]
- [28].Bour A, Kruglik SG, Chabanon M, Rangamani P, Puff N, Bonneau S, Lipid unsaturation properties govern the sensitivity of membranes to photoinduced oxidative stress, Biophys. J 116 (2019) 910–920. 10.1016/j.bpj.2019.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E, GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers, SoftwareX. 1 (2015) 19–25. 10.1016/j.softx.2015.06.001. [DOI] [Google Scholar]
- [30].Páll S, Abraham MJ, Kutzner C, Hess B, Lindahl E, Tackling exascale software challenges in molecular dynamics simulations with GROMACS, in: Markidis S, Laure E (Eds.), Solving Softw. Challenges Exascale, Springer, Cham, 2015: pp. 3–27. 10.1007/978-3-319-15976-8_1. [DOI] [Google Scholar]
- [31].Pronk S, Páll S, Schulz R, Larsson P, Bjelkmar P, Apostolov R, Shirts MR, Smith JC, Kasson PM, van der Spoel D, Hess B, Lindahl E, GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit, Bioinformatics 29 (2013) 845–854. 10.1093/bioinformatics/btt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Qi Y, Ingo HI, Cheng X, Lee J, Marrink SJ, Im W, CHARMM-GUI Martini Maker for coarse-grained simulations with the Martini force field, J. Chem. Theory Comput 11 (2015) 4486–4494. 10.1021/acs.jctc.5b00513. [DOI] [PubMed] [Google Scholar]
- [33].Jo S, Kim T, Iyer VG, Im W, CHARMM-GUI: a web-based graphical user Interface for CHARMM, J. Comput. Chem 29 (2008) 1859–1865. 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- [34].Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, de Vries AH, The MARTINI forcefield: coarse grained model for biomolecular simulations, J. Phys. Chem. B 111 (2007) 7812–7824. [DOI] [PubMed] [Google Scholar]
- [35].Chng C-P, Sadovsky Y, Hsia KJ, Huang C, Curvature-regulated lipid membrane softening of nano-vesicles, Extrem. Mech. Lett (2020) Under review. [DOI] [PMC free article] [PubMed]
- [36].Humphrey W, Dalke A, Schulten K, VMD: Visual molecular dynamics, J. Mol. Graph 14 (1996) 33–38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- [37].Leontiadou H, Mark AE, Marrink SJ, Molecular dynamics simulations of hydrophilic pores in lipid bilayers, Biophys. J 86 (2004) 2156–2164. 10.1016/S0006-3495(04)74275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chacón E, Tarazona P, Bresme F, A computer simulation approach to quantify the true area and true area compressibility modulus of biological membranes, J. Chem. Phys 143 (2015) 034706. 10.1063/1.4926938. [DOI] [PubMed] [Google Scholar]
- [39].Doktorova M, LeVine MV, Khelashvili G, Weinstein H, A new computational method for membrane compressibility: bilayer mechanical thickness revisited, Biophys. J 116 (2019) 487–502. 10.1016/j.bpj.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


