Abstract
The positive affect of rewards is an important contributor to well-being. Reward involves components of pleasure ‘liking’, motivation ‘wanting’, and learning. ‘Liking’ refers to the hedonic impact of positive events, with underlying mechanisms that include hedonic hotspots in limbic brain structures that amplify ‘liking’ reactions. ‘Wanting’ refers to incentive salience, a motivational process that makes reward cues attractive and able to trigger craving for their reward, mediated by larger dopamine-related mesocorticolimbic networks. Under normal conditions, ‘liking’ and ‘wanting’ cohere. However, ‘liking’ and ‘wanting’ can be dissociated by alterations in neural signaling, either induced in animal neuroscience laboratories or arising spontaneously in addictions and other affective disorders, which can be detrimental to positive well-being.
Introduction
Positive affect occurs during emotional reactions that typically occur consciously as subjective experiences. But positive affective reactions also exist as measurable objective processes, which in some conditions can be unconscious [1-3]. In this review, we will focus on neural mechanisms of basic core processes of positive affect, namely of reward ‘liking’ and ‘wanting’. These core processes can be objectively measured, but also contribute to subjective experiences of positive affect when elaborated into consciousness. The brain mechanisms of ‘liking’ and ‘wanting’ discussed here contribute to diverse sensory, social and cultural pleasures, and perhaps even to overall happiness [2,3].
Reward is a psychological composite that contains three core components: 1) ‘liking’ or pleasure, 2) ‘wanting’ or the motivation to obtain pleasure, and 3) learning and prediction of future values. We use the term ‘liking’ to refer to positive hedonic impact or core affective process within pleasure that can be objectively measured. We put ‘liking’ in quotation marks to distinguish the core process from the ordinary meaning of the word liking as conscious pleasure. Similarly, we use the term ‘wanting’ to refer to the core process of incentive salience, a mesocorticolimbic motivational process that makes rewards and their cues attention-grabbing, attractive, and able to motivate pursuit and consumption. ‘Wanting’ contributes urgency to many consciously experienced desires, but ‘wanting’ can occur without conscious awareness in some conditions [1,2].
‘Wanting’ and ‘liking’ evolved together, and are mediated by intertwined brain mechanisms, so the two processes usually arise together in normal life. But ‘liking’ and ‘wanting’ can also come apart in some conditions, because their neural mechanisms are separable [4-6]. Separation can produce intense and narrowly focused ‘wants’, such as addictions, and even produce maladaptive ‘wanting what hurts’, as discussed below.
Hedonic ‘liking’ reactions
Human neuroimaging studies indicate that overlapping hedonic brain systems are activated by diverse sensory pleasures and by higher order social, cognitive and cultural pleasures such as music [2,3,7,8]. Therefore, insights into how hedonic circuitry generates the hedonic impact or ‘liking’ of sensory pleasures such as food, which comes mostly from animal studies, may also give insights into understanding how diverse other pleasures are generated in both animals and humans. From an evolutionary perspective, neural circuitry that evolved originally for sensory pleasures has likely been co-opted by human evolution into service of higher-order cultural pleasures, and serves as a mechanism to weigh the value of one reward to another.
A useful way to objectively measure sensory ‘liking’ reactions comes from affective orofacial expressions elicited by sweet versus bitter tastes in human infants, non-human primates, and rodents [9,10]. For example, in all these a squirt of sweet sugar water in the mouth elicits positive ‘liking’ facial expressions, including lip licking. Conversely, a squirt of bitter quinine elicits negative ‘disgust’ expressions, including mouth gapes and headshakes.
Those ‘liking’ versus ‘disgust’ expressions reflect the hedonic impact of tastes, and are not mere reflexes to sweet or bitter sensation. For example, hunger increases ‘liking’ for sweetness whereas satiety reduces ‘liking’, sodium appetite states switch reactions to intense saltiness from ‘disgust’ to ‘liking’, and learned taste preferences or learned taste aversions can similarly reverse ‘liking’ and ‘disgust’ expressions to a particular taste [11].
Neural mechanisms of ‘liking’
A coordinated network of small brain ‘hedonic hotspots’, when one is appropriately stimulated, interact together to enhance ‘liking’ reactions (Figure 1). Hedonic hotspots exist in the orbitofrontal cortex (OFC) and insula cortex, nucleus accumbens (NAc), ventral pallidum (VP), and the pontine parabrachial nucleus. In a hotspot, a microinjection of opioid-stimulating drugs, endocannabinoid-stimulating drugs, orexin, GABA or benzodiazepines can enhance ‘liking’. Each hotspot is only 1 to several cubic millimeters volume in the rat brain, or approximately 1 to several cubic centimeters when translated to the size of a human brain, and comprises only a portion of its containing structure. Microinjections of the same drugs outside of the hotspots, even if within the same brain structure, fail to increase ‘liking’ reactions to sucrose and can even suppress ‘liking’ when administered to certain ‘hedonic coldspot’ sites that reduce positive hedonic impact. Evidence suggests activation of one hotspot also recruits activation of other hotspots unanimously, so that the entire network comes on as a unified circuit. Preventing unanimous recruitment after an opioid microinjection in a hotspot, for instance by simultaneously microinjecting an opioid blocking drug in another hotspot, prevents enhancement of ‘liking’ reactions, suggesting that circuit unanimity is required for hedonic enhancement [12].
Figure 1:
Brain circuitry underlying ‘wanting’ (distributed dopamine-related circuitry in dark green) versus ‘liking’ (smaller network of discrete hedonic hotspots in yellow, where opioid, endocannabinoid and related stimulations can enhance the hedonic impact of pleasant rewards).
Regarding loss of hedonic function, although all hotspots are capable of enhancing expressions of ‘liking’ when appropriately stimulated, only the posterior VP hotspot appears to be necessary for normal ‘liking’ [13]. Disrupting neural activity in the VP hotspot, either by pharmacological inhibition or via neurochemical lesion that destroys VP neurons, eliminates normal ‘liking’ and replaces with excessive ‘disgust’ reactions even to sweetness, by releasing activity within negative ‘disgust’ generating circuitry (i.e., disinhibition) [13,14].
Neural mechanisms of incentive salience ‘wanting’
‘Liking’ is the hedonic kernel of pleasant reward, but is only one part of reward. Incentive salience, or ‘wanting’, is a mesolimbic process that makes stimuli in the world become attractive, attention-grabbing, ‘wanted’, and able to trigger urges for associated rewards. Incentive salience gives motivational oomph to conscious desires for pleasures, and zest for life, but too-intense ‘wanting’ for maladaptive targets can also oppose an individual with addiction who has a sincere cognitive desire to abstain (Figure 2).
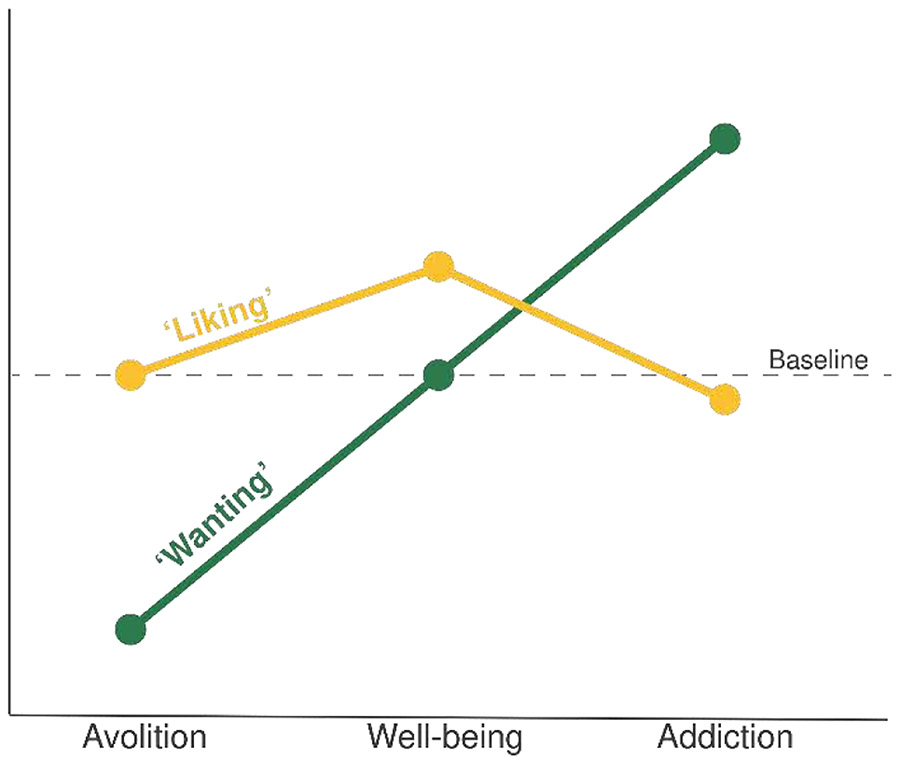
Figure 2:
Conceptual representation of the relative ratios of ‘liking’ versus ‘wanting’ in avolition, normal well-being, and addiction states. ‘Liking’ (yellow) and ‘wanting’ (green) are shown relative to baseline levels (dotted black line). In avolition or motivational anhedonia, a selective deficit in ‘wanting’ (green line) drains value from life rewards, even if hedonic, ‘liking’ reaction (yellow line) remains intact. In well-being (center), ‘liking’ and ‘wanting’ are in balance, so that healthy ‘liking’ reactions contribute a positive hedonic component to happiness, and ‘wanting’ adds zest to life in the form of motivational value. In addiction (right), ‘wanting’ becomes excessive due to incentive sensitization of mesocorticolimbic dopamine-related systems that mediate incentive salience, even if ‘liking’ declines. A similar selective exaggeration of excessive ‘wanting’ may also produce ‘wanting what hurts’ induced by amygdala optogenetic stimulation that co-opts the mesocorticolimbic ‘wanting’ system.
Incentive salience ‘wanting’ processes can also be measured objectively, and obey a signature set of psychological rules: 1) Incentive salience is attributed to Pavlovian cues that predict reward (conditioned stimuli; CSs). Cues thus become powerful triggers of motivation to seek and consume their associated reward. For example, the smell of food cooking can trigger an urge to eat, and similarly, drug cues and contexts can trigger addicted individuals to relapse even when they consciously wish to abstain. Cue-triggered urges to obtain reward are often measured in animal studies using Pavlovian-Instrumental Transfer (PIT) or in cue-triggered relapse procedures, where a Pavlovian CS elicits cue-triggered ‘wanting’ to instrumentally pursue reward UCS. 2) Reward cues also become attractive, as well as triggers, so that a Pavlovian CS often elicits approach and manipulation. Cue attraction is often measured in sign-tracking, autoshaping and attention-capture studies, and attractive CSs may even sometimes be consumed similarly to their UCS reward. 3) When absent, ‘wanted’ reward cues may be actively sought, which is sometimes studied using instrumental conditioned reinforcement procedures, where individuals must work to obtain the cue [5,15-17]. 4) The intensity of incentive salience triggered by a given cue is modulated by relevant physiological states such as appetite, satiety, stress, drug intoxication, etc. Similarly, current brain states, including mesolimbic sensitization, can powerfully modulate the intensity of cue-triggered incentive salience [6,11,18-25].
Addiction
The incentive-sensitization theory of addiction posits enduring mesolimbic hyper-reactivity to drug cues, induced by sensitization of dopamine-related circuitry, to cause excessive cue-triggered ‘wanting’ to take drugs in individuals with addiction [26]. This is due to alterations in dopamine neurons as well as related changes in pre-synaptic neurons in ventral tegmentum, post-synaptic neurons in NAc, and neostriatum, and cortico-limbic glutamate neurons that interact with dopamine [27-30]. The persistence of neural sensitization leaves sensitized individuals vulnerable to cue-triggered relapse even after long periods of abstinence [31,32]. Incentive-sensitization can create a dissonance between mesolimbic ‘wanting’ to relapse and cognitive wanting to abstain when encountering drug cues, which becomes even further exacerbated during stress or emotional arousal states that heighten mesolimbic responsiveness to drug cues [33]. Endogenous incentive-sensitization with mesolimbic hyper-reactivity has also been suggested to occur spontaneously in especially vulnerable individuals to cause food, gambling, sex, and related behavioral addictions [34-39].
Translational applications of ‘wanting’ vs ‘liking’ to human addictions.
The incentive-sensitization theory of addiction was proposed in 1993, and throughout the 1990s, was almost entirely based on objective ‘liking’ and ‘wanting’ measures in animals. Beginning around 2000, human studies began to separately tease apart subjective ratings of wanting versus liking for drug or food rewards, and to simultaneously manipulate dopamine levels in people as they rated rewards. It became clear that dopamine manipulations altered subjective reward wanting but not subjective reward liking [40,41]. Similarly, evidence emerged that drugs can sensitize human dopamine systems too, and that dopamine sensitization may produce increases in wanting ratings but not euphoria or liking ratings [42,43]. Thus, incentive-sensitization, though discovered in rodents, seems to have significant translational applications to human addiction. A therapeutic implication is that addiction therapies may need to focus on addressing excessive ‘wants’.
Addiction might be comparable to the situation of Tantalus, a greedy king in Greek mythology, was punished by the gods by eternal temptation while in a state of extreme hunger and thirst. In the myth, Tantalus’s thirsty torture is increased by the temptation of standing up to his neck in a sparkling pool of cool drinkable water, but the water recedes whenever he dips his head to try to drink. Similarly, a tree full of delicious fruit hangs just above his head, but the fruit spoils and rots each time he tries to reach for a bite. Tantalus is the origin of the English word ‘tantalize’, meaning to tempt to the point of torment. In some ways, this story may reflect the experiences of individuals with addiction who are tortured by their intense ‘wanting’ that persists even if the drug is no longer ‘liked’, even after withdrawal is gone, and even despite a cognitive intention to abstain.
Recent insights into ‘wanting’ mechanisms
In recent studies we used optogenetic manipulations of NAc and amygdala to explore brain mechanisms that focus intense ‘wanting’ narrowly onto particular targets. These use virus microinjections carrying a photoreceptor gene, such as channelrhodopsin (ChR2), into targeted neurons in a brain structure. Laser light delivered by implanted optic fibers subsequently stimulates gene-expressing neurons. For example, we found that optogenetic activation of NAc shell neurons that express either D1-type dopamine receptors or D2-type dopamine receptors can be rewarding at least in some circumstances [44]. Conversely, and somewhat paradoxically local pharmacological inhibition of NAc neurons can also generate increases in incentive motivation in rats to consume reward, such as promoting eating and food intake, presumably by altering downstream targets in hypothalamus, VP, and ventral tegmentum. NAc-induced increase in incentive motivation depends on endogenous local D1 dopamine signals in NAc, and can also be prevented by opposing the drug-induced inhibition of NAc neurons with local optogenetic excitation at the same site [45].
Narrow focusing of maladaptive ‘wanting’
A hallmark of addictive motivation, besides intensity, is narrowness of focus on targets of addiction. For instance, motivation to pursue the addicted target may take precedence over the pursuit of life’s other rewards, so that health, success, and well-being may suffer. Recently, we probed neural mechanisms that may play an important role in narrowly focusing the assignment of maladaptively-intense incentive salience on a particular target. For example, in one experiment, we paired optogenetic laser stimulation of neurons in the central nucleus of the amygdala (CeA) associatively with earning either sucrose or intravenous cocaine. Rats whose CeA stimulation was paired with sucrose consequently became ‘sucrose addicts’ that vigorously pursued only sucrose and ignored the chance to earn cocaine. Other rats whose CeA stimulation was paired with cocaine became ‘cocaine addicts’ that pursued only cocaine and ignored sucrose [46]. Similarly, if rats are faced with two identical sucrose options, or with two cocaine options, but only one of those two equal rewards is paired with CeA stimulation, the rats subsequently pursue only their laser-paired sucrose reward [47] or laser-paired cocaine reward [48] and ignore the equal alternative. Yet most of these rats did not apparently ‘want’ CeA optogenetic laser much itself: most rats would not self-stimulate laser activation of their CeA by touching a neutral object that delivered a brief laser pulse, or by returning to a place where CeA laser was delivered. Rather than becoming intensely ‘wanted’ itself, CeA laser made its associatively-paired sucrose or cocaine reward become intensely and narrowly ‘wanted’, by recruiting activation of mesolimbic circuitry that generates incentive salience [46]. Others have shown that CeA activation can make a paired sucrose reward ‘wanted’ over a larger alternative sucrose reward, and that rats persisted in pursuing CeA-paired sucrose even when their lever presses were punished by electric shock [49].
A neural mechanism for dangerous desire: ‘wanting’ what hurts
Is it possible to even ‘want what hurts you’? If so, this would be perhaps the most conclusive proof of principle demonstration that ‘wanting’ can become independent of ‘liking’ [46]. This demonstration was recently provided in our laboratory, again using optogenetic stimulation of the CeA, but this time paired with encounters of a painful target: an electrified metal shock-rod protruding a few centimeters from one wall of a large chamber, which if voluntarily touched delivered a mild electric shock [46]. No rat was ever forced to touch the shock-rod, but normal rats typically explored and touched the shock-rod once or twice out of curiosity, and then avoided it. They subsequently remained as far as possible from the shock-rod, and emitted defensive behaviors toward it, such as burying it. By contrast, rats with paired CeA optogenetic-stimulations became maladaptively attracted to the shock-rod, via recruitment of mesolimbic dopamine-related circuitry. CeA rats eagerly hovered over the shock-rod and touched it repeatedly, and so subjected themselves voluntarily to multiple shocks. These CeA-stimulated rats would even repeatedly climb over an occluding barrier to reach the shock-rod, and again receive several shocks.
A separate test indicated that shock-rod cues had become attractive via incentive salience. These CeA-stimulated rats were willing to learn a new nosepoke response and to work hard to earn presentations of a shock-associated auditory cue, which had also been paired with shock-rod encounters (i.e., instrumental conditioned reinforcement test) [46]. In other words, shock-rod cues had taken on incentive salience and become ‘wanted’ in themselves.
Yet the CeA study also showed that the same amygdala laser stimulations could flip valence to negative, oppositely magnifying fearful freezing and avoidance reactions in a different Pavlovian fear conditioning situation (i.e., where an auditory cue predicted inescapable footshock), even in the same CeA rats that were attracted to their shock-rod [46]. These findings show that even negative painful stimuli can gain incentive salience to become maladaptively ‘miswanted’. They also show that CeA recruitment of mesocorticolimbic circuitry can take multiple affective modes, either positive or negative in valence. Thus, overlapping circuitry may dynamically participate as shared components in both ‘wanting’ and ‘fear’, depending on situational factors.
Conclusion
Key aspects of positive affect in reward include, ‘liking’, ‘wanting’, and learning components, which are shared by diverse life pleasures, and may even contribute to overall happiness. For example, Aristotle suggested that happiness consists of both hedonia or pleasure, which may have roots in ‘liking’, and eudaimonia or meaningful fulfillment [2,50,51]. Neural generators of pleasurable ‘liking’ reactions may be relevant particularly to understanding brain mechanisms for the hedonic half of happiness. For example, nucleus accumbens activation correlates with subjective well-being in older adults [52], and meditating Buddhist monks show increased insula activity [53,54]. A form of happiness involving ‘liking’ without ‘wanting’ might be described by ‘sukha’, a Buddhist term for a state of bliss and happiness experienced during meditation that is free from desires [55]. However, mesolimbic ‘wanting’, although not intrinsically pleasurable in itself, in proper balance may also add zest to life by contributing motivationally significant properties that make pleasures desirable. Yet excessive ‘wanting’ can impair well-being and cause maladaptive addictions, or even ‘wanting what hurts’. In conclusion, a balance of ‘liking’ and ‘wanting’ is important for positive emotion and well-being.
Highlights.
‘Liking’ and ‘wanting’ are important components of positive affect.
Excessive neural hyper-reactivity of ‘wanting’ brain systems can lead to addiction via incentive-sensitization in human addicts, and produce maladaptive ‘wanting what hurts’ in laboratory experiments.
A proper balance of ‘liking’ and ‘wanting’ is important for positive affect and well-being.
Acknowledgements
This work was supported by National Institutes of Health grants MH063649, DA015188, and T32DA7268.
Footnotes
Conflict of interest statement
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Winkielman P, Gogolushko Y: Influence of suboptimally and optimally presented affective pictures and words on consumption-related behavior. Front Psychol 2018, 8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kringelbach ML, Berridge KC: The affective core of emotion: Linking pleasure, subjective well-being, and optimal metastability in the brain. Emot Rev 2017, 9:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stark EA, Vuust P, Kringelbach ML: Music, dance, and other art forms: New insights into the links between hedonia (pleasure) and eudaimonia (well-being). Prog Brain Res 2018, 237:129–152. [DOI] [PubMed] [Google Scholar]
- [4].DiFeliceantonio AG, Coppin G, Rigoux L, Edwin Thanarajah S, Dagher A, Tittgemeyer M, Small DM: Supra-additive effects of combining fat and carbohydrate on food reward. Cell Metab 2018, 28:33–44.e3. [DOI] [PubMed] [Google Scholar]
- [5].Grigutsch LA, Lewe G, Rothermund K, Koranyi N: Implicit ‘wanting’ without implicit ‘liking’: A test of incentive-sensitization-theory in the context of smoking addiction using the wanting-implicit-association-test (W-IAT). J Behav Ther Exp Psychiatry 2019, 64:9–14. [DOI] [PubMed] [Google Scholar]
- [6].Massaccesi C, Korb S, Skoluda N, Nater UM, Silani G: Effects of appetitive and aversive motivational states on wanting and liking of interpersonal touch. Neuroscience 2020, doi: 10.1016/j.neuroscience.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sachs M, Habibi A, Damasio H: Reflections on music, affect, and sociality. Prog Brain Res 2018, 237:153–172. [DOI] [PubMed] [Google Scholar]
- [8].Ferreri L, Mas-Herrero E, Zatorre RJ, Ripollés P, Gomez-Andres A, Alicart H, Olivé G, Marco-Pallarés J, Antonijoan RM, Valle M, et al. : Dopamine modulates the reward experiences elicited by music. Proc Natl Acad Sci U S A 2019, 116:3793–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Steiner JE: Innate, discriminative human facial expressions to taste and smell stimulation. Ann N Y Acad Sci 1974, 237:229–233. [DOI] [PubMed] [Google Scholar]
- [10].Grill HJ, Norgren R: The taste reactivity test i. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 1978, 143:263–279. [DOI] [PubMed] [Google Scholar]
- [11].Robinson MJF, Berridge KC: Instant transformation of learned repulsion into motivational “wanting.” Curr Biol 2013, 23:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Morales I, Berridge KC: ‘Liking’ and ‘wanting’ in eating and food reward: Brain mechanisms and clinical implications. Physiol Behav 2020, 227:113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ho CY, Berridge KC: Excessive disgust caused by brain lesions or temporary inactivations: Mapping hotspots of the nucleus accumbens and ventral pallidum. Enr J Neurosci 2014, 40:3556–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Khan HA, Urstadt KR, Mostovoi NA, Berridge KC: Mapping excessive “disgust” in the brain: Ventral pallidum inactivation recruits distributed circuitry to make sweetness “disgusting.” Cogn Affect Behav Neurosci 2020, 20:141–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bindra D: How adaptive behavior is produced: A perceptual-motivational alternative to response-reinforcement. Behav Brain Sci 1978, 1:4191. [Google Scholar]
- [16].Colaizzi JM, Flagel SB, Joyner MA, Gearhardt AN, Stewart JL, Paulus MP: Mapping sign tracking and goal-tracking onto human behaviors. Neurosci Biobehav Rev 2020, 111:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Toates FM: Motivational Systems. Cambridge University Press; 1986. [Google Scholar]
- [18].Glynn RM, Rosenkranz JA, Wolf ME, Caccamise A, Shroff F, Smith AB, Loweth JA: Repeated restraint stress exposure during early withdrawal accelerates incubation of cue-induced cocaine craving. Addict Biol 2018, 23:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Clay JM, Parker MO: The role of stress-reactivity, stress-recovery and risky decision-making in psychosocial stress-induced alcohol consumption in social drinkers. Psychopharmacology (Berl) 2018, 235:3243–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Clay JM, Adams C, Archer P, English M, Hyde A, Stafford LD, Parker MO: Psychosocial stress increases craving for alcohol in social drinkers: Effects of risk-taking. Drug Alcohol Depend 2018, 185:192–197. [DOI] [PubMed] [Google Scholar]
- [21].Fuentes S, Carrasco J, Hatto A, Navarro J, Armario A, Monsonet M, Ortiz J, Nadal R: Sex-dependent impact of early-life stress and adult immobilization in the attribution of incentive salience in rats. PLoS One 2018, 13:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].González-Marín M del C, Coune F, Naassila M: Vulnerability to ethanol sensitization predicts higher intake and motivation to self-administer ethanol: Proof of the incentive salience sensitization theory? Addict Biol 2020, 25. [DOI] [PubMed] [Google Scholar]
- [23].Overby PF, Daniels CW, Del Franco A, Goenaga J, Powell GL, Gipson CD, Sanabria F: Effects of nicotine self-administration on incentive salience in male Sprague Dawley rats. Psychopharmacology (Berl) 2018, 235:1121–1130. [DOI] [PubMed] [Google Scholar]
- [24].Soussignan R, Schaal B, Jiang T: Watching happy faces potentiates incentive salience but not hedonic reactions to palatable food cues in overweight/obese adults. Appetite 2019, 133:83–92. [DOI] [PubMed] [Google Scholar]
- [25].Robinson MJF, Caplan KA, Knes AS, Rodríguez-Cruz HO, Clibanoff C, Freeland CM: Reward uncertainty attributes incentive value to reward proximal cues, while amphetamine sensitization reverts attention to more predictive reward distal cues. Prog Neuro-Psychopharmacology Biol Psychiatry 2020, 97. [DOI] [PubMed] [Google Scholar]
- [26].Robinson TE, Berridge KC: The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev 1993, 18:247–291. [DOI] [PubMed] [Google Scholar]
- [27].Engeli EJE, Zoelch N, Hock A, Nordt C, Hulka LM, Kirschner M, Scheidegger M, Esposito F, Baumgartner MR, Henning A, et al. : Impaired glutamate homeostasis in the nucleus accumbens in human cocaine addiction. Mol Psychiatry 2020, doi: 10.1038/s41380-020-0828-z. [DOI] [PubMed] [Google Scholar]
- [28].Wolf ME: Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 2016, 17:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Velasquez-Martinez MC, Santos-Vera B, Velez-Hernandez ME, Vazquez-Torres R, Jimenez-Rivera CA: Alpha-1 adrenergic receptors modulate glutamate and gaba neurotransmission onto ventral tegmental dopamine neurons during cocaine sensitization. Int J Mol Sci 2020, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kawa AB, Valenta AC, Kennedy RT, Robinson TE: Incentive and dopamine sensitization produced by intermittent but not long access cocaine self-administration. Eur J Neurosci 2019, 50:2663–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Metrik J, Aston ER, Kahler CW, Rohsenow DJ, McGeary JE, Knopik VS, MacKillop J: Cue-elicited increases in incentive salience for marijuana: Craving, demand, and attentional bias. Drug Alcohol Depend 2016, 167:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Singer BF, Fadanelli M, Kawa AB, Robinson TE: Are cocaine-seeking “habits” necessary for the development of addiction-like behavior in rats? J Neurosci 2018, 38:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH: Exacerbated craving in the presence of sress and drug cues in drug-dependent patients. Neuropsychopharmacology 2018, 43:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Oginsky MF, Goforth PB, Nobile CW, Lopez-Santiago LF, Ferrario CR: Eating “junk-food” produces rapid and long-lasting increases in NAc CP-AMPA receptors: Implications for enhanced cue-induced motivation and food addiction. Neuropsychopharmacology 2016, 41:2977–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zeeb FD, Li Z, Fisher DC, Zack MH, Fletcher PJ: Uncertainty exposure causes behavioural sensitization and increases risky decision-making in male rats: Toward modelling gambling disorder. J Psychiatry Neurosci 2017, 42:404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, Fibiger HC: Sexual behavior enhances central dopamine transmission in the male rat. Brain Res 1990, 530:345–348. [DOI] [PubMed] [Google Scholar]
- [37].Robinson MJF, Fischer AM, Ahuja A, Lesser EN, Maniates H: Roles of “wanting”‘ and ‘liking’” in motivating behavior: Gambling, food, and drug addictions.’” In Behavioral Neuroscience of Motivation. Edited by Simpson EH, Balsam PD. Springer International Publishing; 2016:105–136. [DOI] [PubMed] [Google Scholar]
- [38].Hellberg SN, Russell TI, Robinson MJF: Cued for risk: Evidence for an incentive sensitization framework to explain the interplay between stress and anxiety, substance abuse, and reward uncertainty in disordered gambling behavior. Cogn Affect Behav Neurosci 2019, 19:737–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fordahl SC, Locke JL, Jones SR: High fat diet augments amphetamine sensitization in mice: Role of feeding pattern, obesity, and dopamine terminal changes. Neuropharmacology 2016, 109:170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brauer LH, De Wit H: High dose pimozide does not block amphetamine-induced euphoria in normal volunteers. Pharmacol Biochem Behav 1997, 56:265–272. [DOI] [PubMed] [Google Scholar]
- [41].Leyton M, Aan Het Rot M, Booij L, Baker GB, Young SN, Benkelfat C: Mood-elevating effects of d-amphetamine and incentive salience: The effect of acute dopamine precursor depletion. J Psychiatry Neurosci 2007, 32:129–136. [PMC free article] [PubMed] [Google Scholar]
- [42].Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C: Modeling sensitization to stimulants in humans: An [11C] raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry 2006, 63:1386–1395. [DOI] [PubMed] [Google Scholar]
- [43].Strakowski SM, Sax KW: Progressive behavioral response to repeated d-amphetamine challenge: Further evidence for sensitization in humans. Biol Psychiatry 1998, 44:1171–1177. [DOI] [PubMed] [Google Scholar]
- [44].Cole SL, Robinson MJF, Berridge KC: Optogenetic self-stimulation in the nucleus accumbens: D1 reward versus D2 ambivalence. PLoS One 2018, 13:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Baumgartner HM, Cole SL, Olney JJ, Berridge KC: Desire or dread from nucleus accumbens inhibitions: Reversed by same-site optogenetic excitations. J Neurosci 2020, 40:2737–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Warlow SM, Naffziger EE, Berridge KC: The central amygdala recruits mesocorticolimbic circuitry for pursuit of reward or pain. Nat Commun 2020, 11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Robinson MJF, Warlow SM, Berridge KC: Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another. J Neurosci 2014, 34:16567–16580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Warlow SM, Robinson MJF, Berridge KC: Optogenetic central amygdala stimulation intensifies and narrows motivation for cocaine. J Neurosci 2017, 37:8330–8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tom RL, Ahuja A, Maniates H, Freeland CM, Robinson MJF: Optogenetic activation of the central amygdala generates addiction-like preference for reward. Eur J Neurosci 2019, 50:2086–2100. [DOI] [PubMed] [Google Scholar]
- [50].Zeng Z, Chen H: Distinct associations of hedonic and eudaimonic motives with well-being: Mediating role of self-control. Int J Environ Res Public Health 2020, 17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Aristotle: Nicomachean Ethics. Hackett Pub Co.; 1999. [Google Scholar]
- [52].Heller AS, van Reekum CM, Schaefer SM, Lapate RC, Radler BT, Ryff CD, Davidson RJ: Sustained striatal activity predicts eudaimonic well-being and cortisol output. Psychol Sci 2013, 24:2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ: Regulation of the neural circuitry of emotion by compassion meditation: Effects of meditative expertise. PLoS One 2008, 3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lutz A, Greischar LL, Perlman DM, Davidson RJ: BOLD signal in insula is differentially related to cardiac function during compassion meditation in experts vs. novices. Neuroimage 2009, 47:1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ekman P, Davidson RJ, Ricard M, Wallace BA: Buddhist and psychological perspectives on emotions and well-being. Curr Dir Psychol Sci 2005, 14:59–63. [Google Scholar]