Abstract
Background:
Multiple factors have been proposed to explain the increasing prevalence of type 1 diabetes mellitus (T1DM), including psychological stress. The prevalence of gender dysphoria (GD) in youth is also growing. Identifying environmental triggers, such as psychological minority stress experienced by youth with GD, that may influence the pathogenesis and management of T1DM could have important clinical implications.
Objective:
Determine the prevalence of concurrent diagnosis of T1DM and GD in adolescents evaluated at a university-based children’s hospital.
Subjects:
Age 10–21 years, diagnosis of T1DM and/or GD.
Methods:
An electronic data extraction was conducted at the University of Wisconsin Hospital and Clinics from November 1, 2007 to November 1, 2017. Prevalence rates were calculated for T1DM and GD. For adolescents with T1DM and GD, information related to diagnosis, treatment and psychiatric history was collected.
Results:
The prevalence for T1DM was 2.69 per 1000; the prevalence for GD was 0.42 per 1000. Eight adolescents had T1DM and GD. In adolescents with GD, the prevalence of T1DM was 9.4-fold higher than the prevalence of T1DM alone (24.77 vs 2.68 per 1000). Five adolescents were seen in GD clinic and their glycemic control initially improved after the first GD clinic visit.
Conclusions:
There was an increased prevalence of a concurrent diagnosis of T1DM in those with GD compared to the general population. Glycemic control improved after the first GD clinic visit in adolescents with T1DM and GD, which may be secondary to stress reduction.
Keywords: Diabetes mellitus, type 1, Gender dysphoria, Adolescent, Prevalence
Introduction
Type 1 diabetes mellitus (T1DM) often presents during childhood, with an estimated prevalence of 1.9–2.4 per 1000.1–3 While reasons for increasing prevalence of T1DM remain unclear, some studies have suggested that environmental triggers, including psychological stress, may initiate an autoimmune response to pancreatic β cells in genetically predisposed individuals.4,5
Interestingly, the prevalence of transgender, nonbinary and gender expansive/nonconforming (TNG) youth is also growing. National estimates vary widely and prevalence data is difficult to gather in this population, but the prevalence of those who identify as TNG has been estimated to be 7 per 1000 youth in the United States6 and 20 per 1000 middle and high school students in Wisconsin.7 There is no reported association between T1DM and gender identity in adolescents. However, a recent study in adults found a 2.3-fold higher prevalence of T1DM in patients seen in a transgender clinic.8 Given the overlapping challenges T1DM and TNG youth face, identifying an association between T1DM and TNG could further elucidate understanding of these two complex populations.
The objectives of this study are: 1) to determine the prevalence of T1DM and gender dysphoria (GD) in adolescent patients seen at an university-based children’s hospital; 2) to determine the prevalence of concurrent diagnosis of T1DM and GD in an adolescent cohort; and 3) to analyze timing of diagnosis, hemoglobin A1c levels and mental health comorbidities in these adolescent patients with T1DM who identify as TNG.
Methods
An electronic data extraction was conducted at the University of Wisconsin Hospital and Clinics from November 1, 2007 to November 1, 2017. Inclusion criteria included age 10–21 years, problem list diagnosis of T1DM (E10.*), and problem list diagnosis of GD (F64.*). Individual patients were identified, and prevalence rates were calculated for each diagnosis. For adolescents with both T1DM and GD, the following data were also collected: age at T1DM diagnosis, T1DM antibodies at diagnosis, hemoglobin A1c (HbA1c) in the last 6 months, sex assigned at birth, gender identity, age at first GD clinic visit, type of puberty blocker, type of gender-affirming hormone therapy, HbA1c before and after first GD clinic visit, HbA1c before and after puberty blocker initiation, HbA1c before and after gender-affirming hormone therapy initiation, psychiatric diagnoses and number of suicide attempts.
Results
Results were available for 749,284 individual patients during the review period. There were 2017 patients diagnosed with T1DM for a prevalence rate of 2.69 per 1000 and 315 patients diagnosed with GD for a prevalence rate of 0.42 per 1000 (Figure 1).
Figure 1.
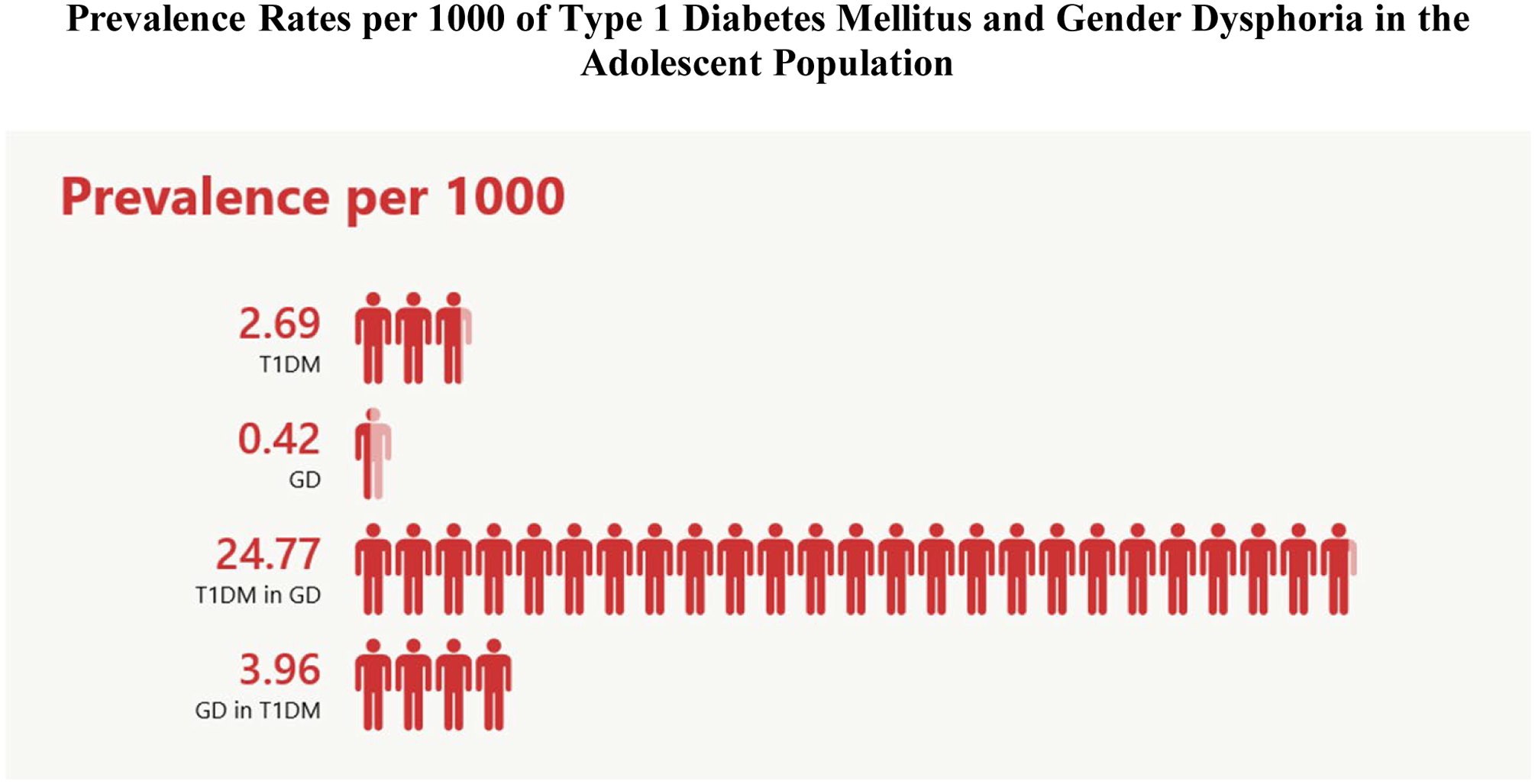
Prevalence rates per 1000 for type 1 diabetes mellitus (T1DM) in the adolescent population, gender dysphoria (GD) in the adolescent population, T1DM in the GD population and GD in the T1DM population.
Concurrent diagnosis of both T1DM and GD was found in 8 patients. The prevalence rate for T1DM in the non-GD population is 2.68 (95% CI 2.57–2.80) per 1000 vs. 24.77 (95% CI: 12.60–48.10) per 1000 in the GD population (p<0.0001). The difference in prevalence rate is 22.09 (95% CI: 5.14–39.03) per 1000. The prevalence of T1DM in the population of adolescents diagnosed with GD is 9.4-fold higher (95% CI: 4.7–19.1, p<0.0001) than the prevalence rate of T1DM alone in the overall adolescent patient group.
Most patients with both T1DM and GD were diagnosed with T1DM prior to their first interaction with the gender clinic (7/8). The average age at T1DM diagnosis was 9.9 years. The average age at the first GD clinic visit was 13 years although most adolescents endorsed feelings of GD earlier in life.
Four of eight patients with T1DM had documentation of positive antibody testing (Table 1). The remaining patients had either only two antibodies tested (both negative) or transferred care from an outside location with tests results that were not available. Factors that pointed toward a diagnosis of T1DM versus T2DM include younger age at diabetes diagnosis, normal weight, autoimmune family history and Caucasian ethnicity (Table 1).
Table 1.
Factors Related to Diagnosis in Adolescents with Type 1 Diabetes Mellitus and Gender Dysphoria
| Patient ID |  |
 |
 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|---|---|---|
| Age at T1DM Diagnosis (yrs) | 2 | 3 | 4 | 9 | 11 | 12 | 14 | 18 |
| Age of GD Onset (yrs) | ? | <5 | 9 | 8 | 8 | 8.5 | <5 | ? |
| Age at First GD Clinic Visit (yrs) | N/A | 11 | 16 | 17 | 17 | 10 | 15 | 18 |
| Autoimmune Family History | ? | Yes | Yes | Yes | No | No | No | No |
| BMI Classification | Norm. | Norm. | ? | Norm. | Norm. | Over weight | Norm. | Norm |
| T1DM Antibodies | Inc. | ? | ? | + | Inc. | + | + | + |
All adolescents in our study were Caucasian (N = 8).
Two adolescents were not seen in GD clinic but one adolescent was followed by their family practitioner for GD.
The adolescents with T1DM and GD varied in their gender identities and use of gender-affirming therapy (Table 2). Four adolescents were assigned female sex at birth and four adolescents were assigned male sex at birth. Three adolescents identified as transfeminine, three identified as transmasculine, one identified as gender fluid, and one identified as gender neutral. Four adolescents received gonadotropic agonist puberty blocking therapy. Two adolescents received estrogen and two adolescents received testosterone for gender-affirming hormone therapy.
Table 2.
Gender Dysphoria Characteristics in Adolescents with Type 1 Diabetes Mellitus and Gender Dysphoria
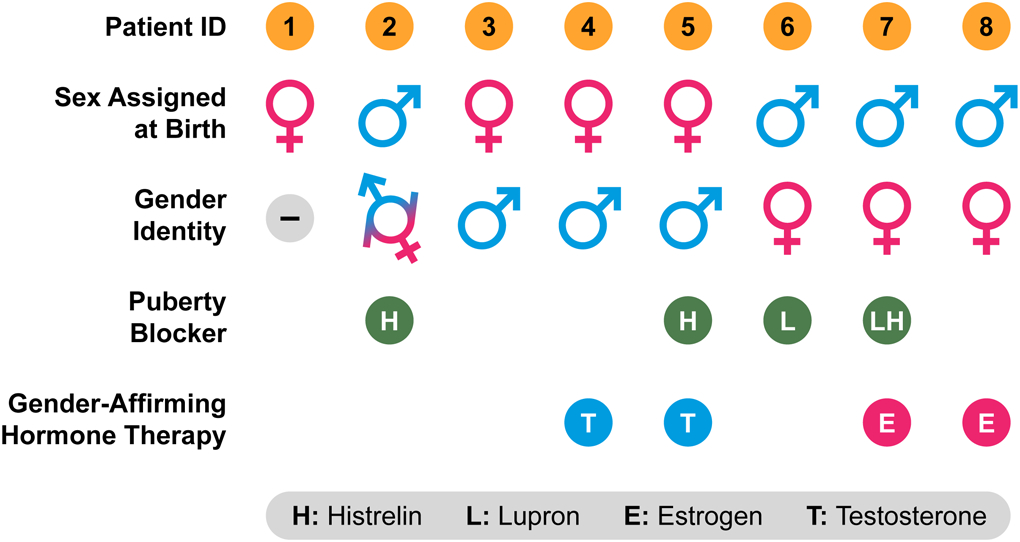
|
Glycemic control initially improved after the first GD clinic visit over a mean interval of 5 months (N = 5). There was no improvement in HbA1c in those that initiated puberty blocker therapy (N = 3) and gender-affirming hormone therapy (N = 5), with mean intervals of 4.5 months and 2.8 months respectively (Figure 2).
Figure 2.
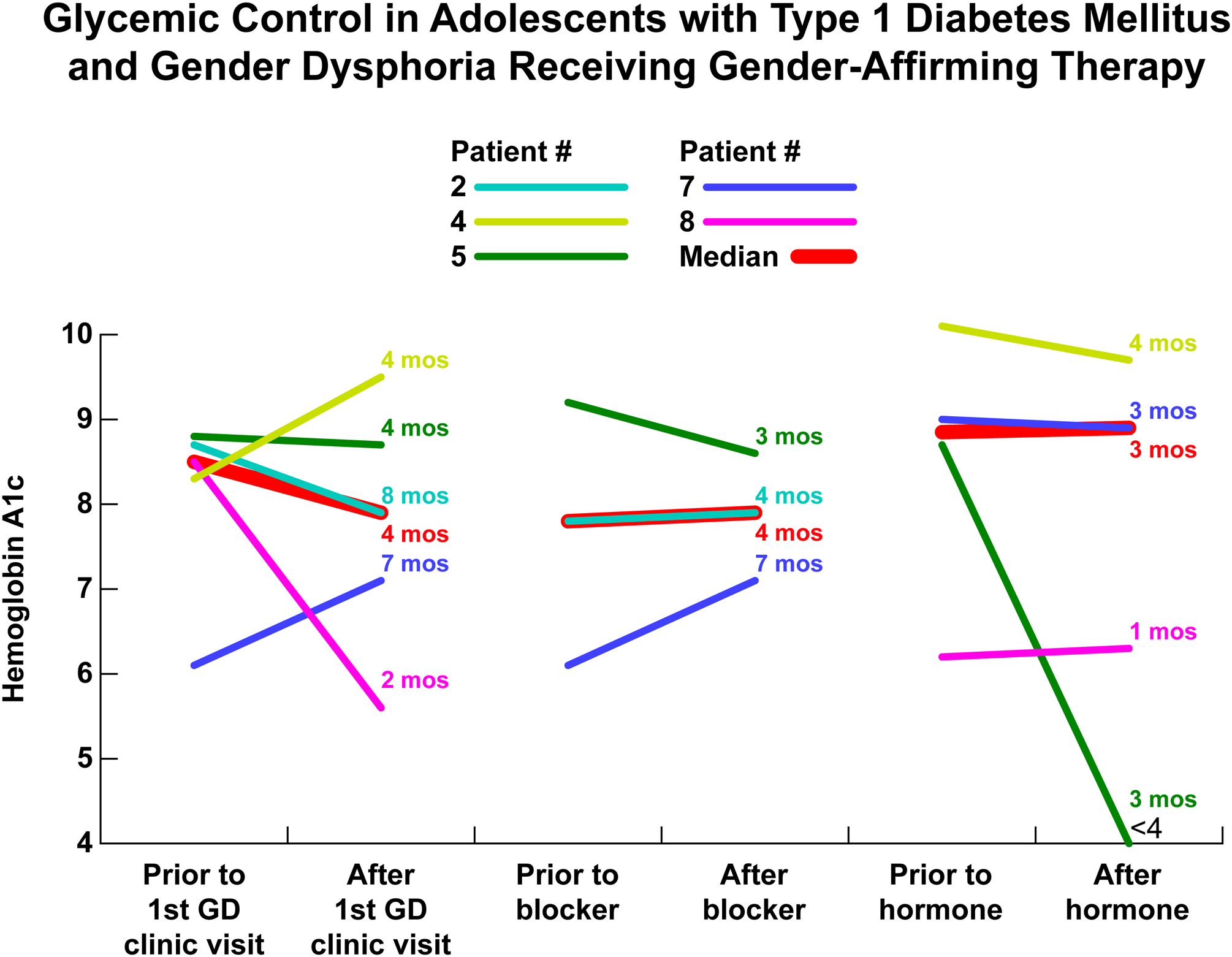
Hemoglobin A1c before and after (A) first gender dysphoria clinic visit with N = 5, (B) puberty blocker initiation with N = 3 and (C) gender-affirming hormone therapy initiation with N = 4.
Psychiatric diagnoses and suicide attempt occurrences are described in Table 3. Anxiety and depression were common psychiatric comorbidities in adolescents with T1DM and GD, as were suicide attempts.
Table 3.
Psychiatric Diagnoses and Suicidality in Adolescents with Type 1 Diabetes Mellitus and Gender Dysphoria
| Patient ID | Psychiatric Diagnoses | Suicidality |
|---|---|---|
 |
Anxiety | None |
 |
Unknown | Unknown |
 |
Anxiety Depression |
Unknown |
 |
Anxiety Depression |
Suicidal thoughts |
 |
Depression | Suicidal thoughts Overdose with Tylenol and alcohol Overdose with insulin |
 |
PTSD ADD Anxiety |
Overdose with insulin |
 |
ADHD Anxiety Depression Substance abuse |
None |
 |
ADHD Possible mood disorder (cutting behavior) Anxiety |
None |
Discussion
In this observational study, there was a significantly increased prevalence of a concurrent diagnosis of T1DM in those with GD compared to the general population. While results of an EMR review should be interpreted with caution, these findings support those of a prior adult study reporting a higher prevalence of T1DM in patients seen in a transgender clinic.8
In analyzing the clinical course of T1DM patients who received medical intervention related to GD, we found that glycemic control initially improved but was not sustained. This finding suggests that stress reduction due to initiation of gender-affirming hormone therapy leads to short-term improvement in diabetes control in adolescents with T1DM and GD. There may be two reasons why HbA1c was ultimately higher than at initial presentation. First, glycemic control in T1DM often worsens during adolescence due to puberty-related insulin resistance.9 Second, treatment adherence in T1DM often declines during adolescence; this is in part attributable to a decrease in parental oversight and an increase in the prevalence of psychiatric comorbidities.10–11 This is consistent with a recent study using the T1D Exchange registry that found mean HbA1c increased from 65 mmol/mol (8.1%) at age 5 years to 78 mmol/mol (9.3%) between ages 15–18 years.12
Explanation for a possible link between T1DM and GD remains speculative. An association between psychological stress and development of type 1 diabetes mellitus or other autoimmunity is well documented.13–20 Stress is known to cause the release of hormones such as cortisol and catecholamines that increase insulin resistance which, in turn, may increase stress on pancreatic β cells in genetically predisposed individuals. The effects of stress on the immune system may also accelerate autoimmune destruction of pancreatic β cells.21 Many adolescents with GD experience psychological stress secondary to body dissatisfaction, poor self-esteem, bullying, social stigma, and lack of social support.22–27 According to the 2015 U.S. Transgender Survey, 39% of respondents experienced serious psychological distress.28 In a 2013 study that sampled over 1000 adult transgender individuals, 44.1% reported having clinical depression and 33.2% reported having anxiety. These rates are higher than those seen in the general population.26 Thus, increased psychologic minority stress experienced by GD youth could contribute to development of diabetes-related autoimmunity. Psychological distress decreases after the initiation of gender-affirming hormone therapy.29–30 Furthermore, biological stress can also lessen with treatment with gender-affirming hormone therapy as evidenced by a decrease in the cortisol awakening response of patients with GD.29
Another possible explanation for the observed link between T1DM and GD is increased ascertainment of GD. First, adolescents with T1DM and GD have frequent exposure to a health care provider through separate clinic visits for these two diagnoses. Second, these adolescents have established a trusting relationship with their diabetes team. Therefore, they may feel comfortable discussing gender identity with their diabetes team and thus may be more likely to seek medical care related to gender identity. Lack of such a relationship with health care providers can impede or delay diagnosis of GD. A Wisconsin report found that only 20% of GD youth identified having a medical provider who was competent in gender related care. Only 49.5% of GD youth in this report have discussed gender identity with their primary care provider.7 The 2015 U.S. Transgender Survey found that 33% of respondents who saw a healthcare provider in the past year reported having at least 1 negative experience related to their gender identity. In this same survey, 23% of respondents did not see a provider when they needed to because of fear of mistreatment.28
A strength of this study is its large cohort and long review period, which allowed capture of a subset of patients with both T1DM and GD. Interestingly, this study found a lower prevalence rate of GD in adolescents compared to previous studies. First, potential confounders included reliance on problem list diagnoses which are not always up to date. Second, not all TNG youth may report their gender dysphoria due to fear of social stigma and mistreatment.31–33 Finally, not all transgender or gender nonconforming patients seek medical attention, have access to medical care, or have a documented diagnosis of gender dysphoria as there are barriers to having a gender dysphoria code listed in medical charts.31–33 However, diagnosis code search was complemented by additional chart review to improve accuracy.
Another limitation of this observational study is selection bias due to the study population being drawn from a tertiary center that provides medical care for GD youth. As such, the study population may not be representative of the general population. A small cohort with T1DM and GD also makes it difficult to draw wider reaching conclusions about these findings.
To our knowledge, this is the first study in adolescents that shows a possible association between T1DM and GD. Future research will include evaluating biomarkers of stress in adolescents with T1DM and GD and determining the prevalence of GD in other chronic autoimmune diseases.
Acknowledgments
We thank Dr. David B. Allen for his assistance editing this manuscript, Dr. Jens C. Eickhoff for his assistance with statistical analysis, and Robert J. Gordon for his assistance with graphic design.
Footnotes
The authors have no conflicts of interest or financial disclosures to declare.
References
- 1.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med. 2017;376(15):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menke A, Orchard TJ, Imperatore G, Bullard KM, Mayer-Davis E, Cowie CC. The prevalence of type 1 diabetes in the United States. Epidemiology. 2013;24(5):773–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egro FM. Why is type 1 diabetes increasing? J Mol Endocrinol. 2013;51(1):R1–13. [DOI] [PubMed] [Google Scholar]
- 5.Kappy MS, Allen DB, Geffner ME. Pediatric practice. Endocrinology. New York: McGraw-Hill Medical; 2010. [Google Scholar]
- 6.Herman JL, Flores AR, Brown TNT, Wilson BDM, Conron KJ. Age of Individuals who Identify as Transgender in the United States. In. Los Angeles, CA: The Williams Institute; 2017. [Google Scholar]
- 7.Botsford JC, Allen BJ, Andert BD, Budge SL, Rehm JL. Meeting the healthcare needs of transgender, nonbinary, and gender expansive/nonconforming youth in Wisconsin: A report of the 2017 Wisconsin Transgender Youth Community Needs Assessment. In:2018.
- 8.Defreyne J, De Bacquer D, Shadid S, Lapauw B, T’Sjoen G. Is Type 1 Diabetes Mellitus More Prevalent Than Expected in Transgender Persons? A Local Observation. Sex Med. 2017;5(3):e215–e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datye KA, Moore DJ, Russell WE, Jaser SS. A review of adolescent adherence in type 1 diabetes and the untapped potential of diabetes providers to improve outcomes. Curr Diab Rep. 2015;15(8):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster NC, Beck RW, Miller KM, et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technology & Therapeutics. 2019;21(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plamper M, Gohlke B, Woelfle J, et al. Interaction of Pubertal Development and Metabolic Control in Adolescents with Type 1 Diabetes Mellitus. J Diabetes Res. 2017;2017:8615769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rausch JR, Hood KK, Delamater A, et al. Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care. 2012;35(6):1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faresjö M The Link between Psychological Stress and Autoimmune Response in Children. Crit Rev Immunol. 2015;35(2):117–134. [DOI] [PubMed] [Google Scholar]
- 14.Karavanaki K, Tsoka E, Liacopoulou M, et al. Psychological stress as a factor potentially contributing to the pathogenesis of Type 1 diabetes mellitus. J Endocrinol Invest. 2008;31(5):406–415. [DOI] [PubMed] [Google Scholar]
- 15.Robinson N, Fuller JH. Role of life events and difficulties in the onset of diabetes mellitus. J Psychosom Res. 1985;29(6):583–591. [DOI] [PubMed] [Google Scholar]
- 16.Robinson N, Lloyd CE, Fuller JH, Yateman NA. Psychosocial factors and the onset of type 1 diabetes. Diabet Med. 1989;6(1):53–58. [DOI] [PubMed] [Google Scholar]
- 17.Sepa A, Ludvigsson J. Psychological stress and the risk of diabetes-related autoimmunity: a review article. Neuroimmunomodulation. 2006;13(5–6):301–308. [DOI] [PubMed] [Google Scholar]
- 18.Sepa A, Wahlberg J, Vaarala O, Frodi A, Ludvigsson J. Psychological stress may induce diabetes-related autoimmunity in infancy. Diabetes Care. 2005;28(2):290–295. [DOI] [PubMed] [Google Scholar]
- 19.Sipetić SB, Vlajinac HD, Kocev NI, Marinković JM, Radmanović SZ, Bjekić MD. The Belgrade childhood diabetes study: a multivariate analysis of risk determinants for diabetes. Eur J Public Health. 2005;15(2):117–122. [DOI] [PubMed] [Google Scholar]
- 20.Stojanovich L. Stress and autoimmunity. Autoimmun Rev. 2010;9(5):A271–276. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19–39. [PMC free article] [PubMed] [Google Scholar]
- 22.Bockting WO, Miner MH, Swinburne Romine RE, Hamilton A, Coleman E. Stigma, mental health, and resilience in an online sample of the US transgender population. Am J Public Health. 2013;103(5):943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall E, Claes L, Bouman WP, Witcomb GL, Arcelus J. Non-suicidal self-injury and suicidality in trans people: A systematic review of the literature. Int Rev Psychiatry. 2016;28(1):58–69. [DOI] [PubMed] [Google Scholar]
- 24.Olson-Kennedy J, Cohen-Kettenis PT, Kreukels BP, et al. Research priorities for gender nonconforming/transgender youth: gender identity development and biopsychosocial outcomes. Curr Opin Endocrinol Diabetes Obes. 2016;23(2):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson CM, Matthews A, Copps-Smith E, Conard LA. Suicidality, Self-Harm, and Body Dissatisfaction in Transgender Adolescents and Emerging Adults with Gender Dysphoria. Suicide Life Threat Behav. 2017;47(4):475–482. [DOI] [PubMed] [Google Scholar]
- 26.Reisner SL, Greytak EA, Parsons JT, Ybarra ML. Gender minority social stress in adolescence: disparities in adolescent bullying and substance use by gender identity. J Sex Res. 2015;52(3):243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toomey RB, Syvertsen AK, Shramko M. Transgender Adolescent Suicide Behavior. Pediatrics. 2018;142(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The Report of the 2015 U.S. Transgender Survey. In. Washington, DC: National Center for Transgender Equality; 2016. [Google Scholar]
- 29.Colizzi M, Costa R, Pace V, Todarello O. Hormonal treatment reduces psychobiological distress in gender identity disorder, independently of the attachment style. J Sex Med. 2013;10(12):3049–3058. [DOI] [PubMed] [Google Scholar]
- 30.Heylens G, Verroken C, De Cock S, T’Sjoen G, De Cuypere G. Effects of different steps in gender reassignment therapy on psychopathology: a prospective study of persons with a gender identity disorder. J Sex Med. 2014;11(1):119–126. [DOI] [PubMed] [Google Scholar]
- 31.Learmonth C, Viloria R, Lambert C, Goldhammer H, Keuroghlian AS. Barriers to insurance coverage for transgender patients. Am J Obstet Gynecol. 2018;219(3):272.e271–272.e274. [DOI] [PubMed] [Google Scholar]
- 32.Roberts TK, Fantz CR. Barriers to quality health care for the transgender population. Clin Biochem. 2014;47(10–11):983–987. [DOI] [PubMed] [Google Scholar]
- 33.Thompson HM. Patient Perspectives on Gender Identity Data Collection in Electronic Health Records: An Analysis of Disclosure, Privacy, and Access to Care. Transgend Health. 2016;1(1):205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]


