Clinically relevant SARS-CoV-2 vaccines induce protective responses in nonhuman primates and humans.
Abstract
Multiple preventive vaccines are being developed to counter the coronavirus disease 2019 pandemic. The leading candidates have now been evaluated in nonhuman primates (NHPs) and human phase 1 and/or phase 2 clinical trials. Several vaccines have already advanced into phase 3 efficacy trials, while others will do so before the end of 2020. Here, we summarize what is known of the antibody and T cell immunogenicity of these vaccines in NHPs and humans. To the extent possible, we compare how the vaccines have performed, taking into account the use of different assays to assess immunogenicity and inconsistencies in how the resulting data are presented. We also review the outcome of challenge experiments with severe acute respiratory syndrome coronavirus 2 in immunized macaques, while noting variations in the protocols used, including but not limited to the virus challenge doses. Press releases on the outcomes of vaccine efficacy trials are also summarized.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic rages unabated and may continue to do so until there is a safe, effective, and widely used protective vaccine. Multiple vaccines to prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and/or COVID-19 disease are now progressing through preclinical testing and phase 1/2a human trials, while some are already in phase 2b/3 efficacy trials in and outside the United States (www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines and https://clinicaltrials.gov/ct2/results?recrs=&cond=Covid19&term=vaccine&cntry=&state=&city=&dist=) (Table 1) (1–32). Several of these mid- to late-stage vaccines are part of the U.S. government’s Operation Warp Speed (OWS), which has been reviewed elsewhere (33–35). Multiple vaccine candidates produced in China are also well advanced in the evaluation and approval process (1, 2, 12, 14, 15, 22–24, 29, 30). Phase 1/2 trial data on the Russian-made Sputnik V vaccine have now been published (28).
Table 1. SARS-CoV-2 vaccines under evaluation in NHPs and phase 1/2 human trials.
| Vaccine name* | Sponsor† | Design principle‡ | NHP studies (citation) | Human trials (citation) |
| PiCoVacc/CoronaVac§ | Sinovac | Inactivated virus | (1) | (14) |
| BBIBP-CorV | Sinopharm/BIBP | Inactivated virus | (2) | (15) |
| ChAdOx1 nCoV-19 (AZD1222) | AstraZeneca | ChAdeno virus–S protein | (3) | (16, 17) |
| Ad26.COV2 | Janssen | Ad26 virus–S protein | (4, 5) | (18) |
| Various constructs | Not applicable║ | DNA–S protein | (6) | |
| INO-4800 | INOVIO | DNA–S protein | (7) | |
| mRNA-1273 | Moderna | mRNA–S protein | (8) | (19, 20) |
| NVX-CoV2373 | Novavax | Recombinant S protein | (9, 10) | (21) |
| Unnamed | Sinopharm/WIBP | Inactivated virus | (22) | |
| Ad5-nCoV | CanSinoBIO | Ad5 virus–S protein | (23, 24) | |
| BNT162b1¶ | Pfizer/BioNTech | mRNA-RBD | (11) | (25–27) |
| BNT162b2 | mRNA–S protein | |||
| Gam-COVID-Vac (Sputnik V) | Gamaleya Center | Ad26 + Ad5 virus–S protein | (28) | |
| S trimer | Clover Biopharmaceuticals | Recombinant S protein | (12) | |
| KMS-1 | IMB, CAMS, and PUMC | Inactivated virus | (29, 30) | |
| MRT5500 | Sanofi Pasteur | mRNA–S protein | (13) | |
| CoVLP | Medicago | S protein virus–like particles | (31) | |
| CVnCoV | Curevac | mRNA–S protein | (32) |
*Some vaccines have alternative names or corporate designations. We use the same names as in the papers cited. The entries in this column are arranged in approximate order of appearance of the first relevant paper on a preprint server or journal website. The citations are arranged so that the papers on the nonhuman primate (NHP) studies are all numbered before those on human trials.
†The five companies highlighted in bold in this and subsequent tables are part of the U.S. government’s OWS program or, in the case of Pfizer/BioNTech, have close ties to it. As this program rapidly evolves, readers should consult appropriate websites (e.g., https://medicalcountermeasures.gov/app/barda/coronavirus/COVID19.aspx) for updated information. In some cases, the companies have academic partners. For example, Moderna is the corporate partner of the National Institutes of Health’s Vaccine Research Center, where the mRNA construct was designed, while the AstraZeneca vaccine (also known as AZD1222) similarly involves the Oxford University in the United Kingdom, and Medicago’s CoVLP vaccine program is a collaboration with McGill University in Canada. BIBP, Beijing Institute of Biological Products; WIBP, Wuhan Institute of Biological Products. Both these organizations are part of the Sinopharm consortium. The Gamaleya Center in Moscow has multiple partners within the Ministry of Health of the Russian Federation. IMB, Institute of Medical Biology; CAMS, Chinese Academy of Medical Sciences; PUMC, Peking Union Medical College.
‡The SARS-CoV-2 components of these vaccines are all based on the S protein or, in the case of the Pfizer/BioNTech now abandoned BNT162b1, the S protein’s receptor-binding domain (RBD). The adenovirus, mRNA, and DNA vaccines express the full-length S protein. Truncated variants have been studied as comparator immunogens (4, 6). The recombinant protein vaccines are based on stabilized S full-length S proteins. The inactivated virus vaccines all include S proteins together with other viral components. For full details of the immunogens, including modifications made to the S proteins, the primary papers should be consulted.
§The Sinovac vaccine was named PiCoVacc at the preclinical stage and then renamed CoronaVac when it moved into human trials.
║The DNA vaccines tested in the macaque study are not known to be part of a clinical development program; we include this paper in the review because it has a macaque challenge component and is therefore relevant to the comparison with other such studies.
¶Although both vaccines were studied at phase 1, only BNT162b2 was advanced into phase 2/3.
All the vaccines are either based solely on the viral Spike (S) protein, which is administered by various methods including expression from nonreplicating adenoviruses and nucleic acid vectors or as recombinant proteins, or are inactivated viruses that include the S protein together with all other structural viral proteins (Table 1). The vaccines are all based on S proteins containing D614, which was the dominant strain when they were designed. A variant virus with G614 in its S protein has since emerged to dominance globally because of its greater transmissibility (36–38). However, this D614G change does not affect sensitivity to neutralization by sera from infected or vaccinated people, or to neutralizing monoclonal antibodies (MAbs), and therefore is not problematic for vaccine efficacy (36–38). Some of the more recent papers reviewed below include neutralization data using the G614 virus. All the adenovirus, mRNA, and DNA vaccine candidates listed in Table 1 involve full-length S proteins; variants with truncations of the transmembrane region and/or the cytoplasmic tail were tested as comparators in two macaque studies (4, 6). The recombinant proteins from Novavax and Clover Biopharmaceuticals are based on full-length S proteins (9, 10, 12, 21). Many S protein constructs incorporate two proline substitutions in the S2 region (K986P and V987P) that stabilize the expressed trimer in the prefusion structure that is considered to be optimally immunogenic for the induction of neutralizing antibodies (NAbs), while minimizing non-NAb responses (39). The same method was used to stabilize the respiratory syncytial virus F (fusion) protein and improve its immunogenicity (40, 41). In one macaque study of adenovirus serotype 26 (Ad26) virus variants, the NAb response to the two-proline mutant S protein was stronger than to other constructs that contained or lacked stabilizing changes, truncations, or alternative leader sequences (4). A mouse immunogenicity experiment that also compared Ad26 virus variants led to a similar conclusion (42). Comparative experiments in mice led to the inclusion of the same double proline change (and a furin cleavage site knockout) in the Sanofi Pasteur mRNA vaccine (13). Recombinant S protein immunogens with the same combination of mutations provided the strongest protection against virus challenge in a mouse model, when compared with proteins that lacked these stabilizing changes (43). In a macaque comparison of DNA vaccines expressing various S protein constructs, the authors reported that a soluble S protein that contained the two proline substitutions together with a cleavage site knockout and a trimerization domain (S.dTM.PP) was better than the corresponding truncated S protein (S.dTM) at conferring protection from SARS-CoV-2 challenge (6).
Experimentation and precedent therefore support the use of stabilizing changes that maintain the SARS-CoV-2 S protein trimer in its prefusion conformation. However, the S protein expressed in the AstraZeneca/Oxford University ChAdOx1 nCoV-19 vaccine is a wild-type sequence that does not include any stabilizing changes (3, 16, 17). The Sputnik V rAd5 and rAd26 adenovirus vectors express a full-length S protein, but the published report does not mention whether stabilizing mutations were added (28). Similarly, it was not stated whether the S protein in the CanSinoBIO Ad5-nCoV vaccine was stabilized (23, 24). Whether the absence of stabilizing changes affects the performance of these various adenovirus vaccines is not known.
The Pfizer/BioNTech BNT162b1 vaccine was based on the S protein’s receptor-binding domain (RBD), but its development was terminated after phase 1/2 trials, in favor of the BNT162b2 construct that expresses the complete, stabilized S protein (Table 1) (11, 25–27). All the mRNA vaccines are encapsulated within liposomes of unpublished composition, which accounts for their thermal fragility and need for storage and shipment in freezers at various temperatures.
Table 4. Vaccine immunogenicity in human phase 1 and/or phase 2 trials.
| Vaccine name (citation) | Design | Vaccine dose* | Binding antibody titer† | NAb titer‡ | T cell response§ |
| Sinovac CoronaVac (14) | Inactivated virus | 6 μg × 2 | GM titer, ~2500 | RV GM CPE titer, ~64 | ND |
| Sinopharm/WIBP unnamed, phase 1 (15) |
Inactivated virus | 8 μg × 2 (days 0 and 28) | ND | RV GM titer, 228.7 | ND |
| Sinopharm/WIBP unnamed, phase 2 (15) |
Inactivated virus | 8 μg × 2 (days 0 and 21) | ND | RV GM titer, 282.7 | ND |
| Sinopharm/WIBP unnamed, phase 1 (22) |
Inactivated virus | 10 μg × 3║ | GM EP (whole virus), 311 | RV GM ID50, 297 | ND |
| Sinopharm/WIBP unnamed, phase 2 (22) |
Inactivated virus | 5 μg × 2 | GM EP (whole virus), 215 | RV GM ID50, 247 | ND |
| CanSinoBIO Ad5-nCoV (23) | Ad5 virus | 1.5 × 1011 VP × 1 | GM titer, 596.4 | RV GM titer, 34; PV GM titer, 45.6 |
GM, ~580 (day 14) |
| CanSinoBIO Ad5-nCoV (24) | Ad5 virus | 1.0 × 1011 VP × 1 | GM titer (RBD), 656.5 | RV GM titer, 19.5; PV GM titer, 61.4 |
ND |
|
AstraZeneca ChAdOx1 nCoV-19 (16) |
ChAdeno virus | 5 × 1010 VP × 1 | Median EU, 157.1 | RV median ID50, 201; PV median ID50, 87.9 |
Median, 856 (day 14); median, 424 (day 56) |
|
AstraZeneca ChAdOx1 nCoV-19 (16) |
ChAdeno virus | 5 × 1010 VP × 2 | Median EU, 997.5 | RV median ID50, 372; PV median ID50, 450.9 |
Median, 1642.3 (day 14); median, 528.7 (day 35); median, 614 (day 56) |
|
AstraZeneca ChAdOx1 nCoV-19 (17) |
ChAdeno virus | 3.5 × 1010–6.5 × 1010 VP × 2 | Median AU, 16,170–20,713 |
RV median ID80, 144–193 |
Median, 797–1187 (day 14) |
| Janssen Ad26.COV2 (18) | Ad26 virus | 1 × 1011 VP × 1 | GM EU, 695 | RV GM ID50, 243 | Median CD4+, ~0.1%** (day 15); median CD8+, ~0.09%** (day 15) |
|
Moderna mRNA-1273 (19, 20) |
mRNA | 250 μg × 2 (19) and 100 μg × 2 (20) |
GM EP, 1,261,975 (19) and ~1000,000 RBD (20) |
PV GM ID50, 373.5 (19) and ~300 (20) |
Median CD4+, ~800; median CD8+, ~40 (day 43) (19); median CD4+, ~1600 (day 43) (20) |
|
Pfizer/BioNTech BNT162b1 (25) |
mRNA RBD | 30 μg × 2 | GM EU (RBD), 16,166 | RV GM ID50, 267 | ND |
|
Pfizer/BioNTech BNT162b1 (26) |
mRNA RBD | 50 μg × 2 | GM EU (RBD), 25,006 | RV GM ID50, 578; PV GM ID50, 3100 |
CD4+ median, ~2000; CD8+ median, ~2600 (day 29) |
|
Pfizer/BioNTech BNT162b1¶ (27) |
mRNA RBD | 30 μg × 2 | GM EU (S1), 6580–23,516 | RV GM ID50, 101–267 | ND |
|
Pfizer/BioNTech BNT162b2¶ (27) |
mRNA S protein | 30 μg × 2 | GM EU (S1), 7895–9136 | RV GM ID50, 149–361 | ND |
| Novavax NVX-CoV2373 (21) | S protein | 5 μg × 2 | GM EU, 63,160 | RV GM ID>99, 3906 | ND |
| Gamaleya Center Gam-COVID-Vac (28)# |
Ad26 + Ad5 virus | 1 × 1011 VP of each virus |
GM EP (S1), 53,006 | RV GM CPE67, 49.25 | NA†† |
| IMB, CAMS, PUMC KMS-1 (29), phase 1 |
Inactivated virus KMS-1 |
100–150 unspecified units |
GM titer, 2000–4000 | RV GM CPE titer, ~20 | AM, 250 (middle dose); AM, 50 (high dose) |
| IMB, CAMS, PUMC KMS-1 (30), phase 2 |
Inactivated virus KMS-1 |
150 unspecified high dose |
GM EP, 2432 | RV GM CPE titer, 21.39 | ND |
| CoVLP (31), phase 1 | S protein virus–like particles |
3.75 μg × 2 | GM ED50, ~300,000 | PV GM ID50, ~2200; RV GM ID50, ~630 |
GM, ~500 |
| CVnCoV (32), phase 1 | mRNA–S protein | 12 μg × 2 | Median EP, 5463 | RV median CPE50, 113 | ND |
*The number of immunizations is also given. VP, viral particles.
†Antibody binding was measured against S proteins (except when RBD, S1 protein, or inactivated, purified whole virus was used instead, as stated) in IgG ELISAs, and the values are listed as median effective dilution (ED50), EP, or ELISA units derived from comparison with a standard curve (EU); AU, arbitrary units; titer, unspecified method of the titer determination. The samples were obtained at a time corresponding approximately to the peak response after the final (or only) immunization. Tabulated values reported for (17) are the ranges of medians in the age groups 18 to 55, 56 to 69, and ≥70 years taken together.
‡Neutralization was quantified in PV or RV assays as indicated. The potency was measured as ID50, ID>99, or CPE67 values 2 weeks after the final (or only) immunization, which corresponds approximately to the peak response. The values reported for (15, 23, 24) are given simply as GM titers, as in the original text where the cutoff was not defined.
§T cell responses were measured in ELISpot IFN-γ assays and recorded as SFCs per 106 cells or intracellularly stained cells [in percentage measured by cytokine flow cytometry (CFC)], which are PBMCs except where subpopulations of CD4+ and CD8+ are indicated, after stimulation with different SARS-CoV-2 S protein–derived peptides. The days between immunization (day 0) and sampling are also listed (in parentheses).
║The binding antibody and NAb titer ranges were similar in the lower-dose (2.5 and 5.0 μg) groups 14 days after the third dose. In other studies, one dose stood out as giving stronger responses and was chosen for tabulation.
¶The ranges listed for BNT162b1 and BNT162b2 are the GM values for the age groups 65 to 85 years (lower value) and 18 to 55 years (higher value).
#Data are for the frozen/thawed stock subcomponent of the phase 2 combination vaccine trial.
**IFN-γ or interleukin-2 (IL-2) measured by intracellular cytokine staining (ICS).
††NA, not applicable (data were not presented in the relevant quantitative format; see text).
It is of considerable scientific and public interest to know the immunogenicity of the leading vaccines in absolute and, to the extent possible, comparative terms. Here, we have reviewed antibody and T cell immune response data derived from published studies of vaccines that were tested in nonhuman primates (NHPs) and then progressed into human phase 1/2 trials or that are in human trials without a prior NHP experiment (Tables 2 to 4). We have also evaluated macaque vaccine challenge experiments, including how they were performed, as the outcomes are relevant to understanding the protective potential of SARS-CoV-2 vaccines (Table 3). The NHP experiments are described in (1–13), and the human trials are described in (14–32).
Table 2. Vaccine immunogenicity in NHP studies.
| Vaccine name (citation) | Vaccine dose* | Binding antibody titer† | NAb titer‡ | T cell response§ |
| Sinovac PiCoVacc (1) | 6 μg × 3 | GM EP, ~12,800 | RV GM ID50, ~50 | ND║ |
| Sinopharm/BIPP BBIBP-CorV (2) |
8 μg × 2 | ND | RV GM ID50, ~230 | ND |
|
AstraZeneca ChAdOx1 nCoV-19 (3) |
2.5 × 1010 VP × 2 | Median EP, ~28,000¶ | RV median ID50, ~280¶ | ND |
| Janssen Ad26.COV 2S.PP (4) | 1 × 1011 VP × 1 | Median EP, ~4000 | PV median ID50, 408; RV median ID50, 113 |
Median, ~80 (day 28) |
| Janssen Ad26.COV 2S.PP (5) | 5 × 1010 VP × 2 | GM EU, ~7500 | PV GM ID50, ~3000 | GM, ~200 (day 70) |
| DNA, full-length S protein (6) | 5 mg × 2 | Median EP, ~140 | PV median ID50, ~200; RV median ID50, ~40 |
Median, ~80 (day 35) |
| INOVIO INO-4800 (7) | 1 mg × 2 | GM EP, ~130,000 | PV GM ID50, ~1000 | AM, ~140 (day 42); AM, ~30 (day 84) |
| Moderna mRNA-1273 (8) | 100 μg × 2 | Log AUC, 4–5 | PV GM ID50, 1862; RV GM ID50, 3481 |
ND |
| Novavax NVX-CoV2373 (9) | 5 μg × 2 | GM ED50, 174,000 | RV GM ID>99, 17,000 | ND |
| Novavax NVX-CoV2373 (10) | 25 μg × 2 | GM ED50, 469,739 | RV GM CPE100, 23,040 | ND |
| Pfizer/BioNTech BNT162b2 (11) | 100 μg × 2 | GM EU, 34,668 | RV GM ID50 1689 | GM, ~750 (days 28 and 42) |
|
Clover Biopharmaceuticals S trimer (12) |
30 μg × 2 | GM EP, 17,497 | PV GM ID50, ~5227; RV GM CPE50, ~20,234 |
ND |
| Sanofi Pasteur MRT5500 (13) | 135 μg × 2 | GM EP, ~200,000 | PV GM ID50, ~2871; RV GM ID50, ~1877 |
GM, 30 to 40 (day 42) |
*Only results for the optimal dose, i.e., the strongest responses without unacceptable side effects, are recorded. When the number of immunizations differed between groups, the one inducing the strongest response was chosen. VP, virus particle.
†Antibody binding was measured in S protein immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) 2 weeks after the last immunization, and the values are listed as median effective dilution (ED50), end point (EP), or ELISA units derived from comparison with a standard curve (EU); GM, geometric mean; AUC, area under the curve.
‡Neutralization was quantified in pseudo-virus (PV) or replicating virus (RV) assays, as indicated. The potency was measured as median inhibitory dilution (ID50) or ID>99 values [CPE100 in (10) is the approximate equivalent of ID>99 in (9)].
§T cell responses were measured in enzyme-linked immune absorbent spot (ELISpot) interferon-γ (IFN-γ) assay as spot forming cells (SFC) per 106 cells after stimulation with different SARS-CoV-2 S-derived peptides. The days between immunization (day 0) and sampling are also listed (in parentheses). AM, arithmetic mean.
║ND, not done (no data were presented in the paper).
¶Data are for the two-dose (prime boost) group.
Table 3. Antibody responses at the time of challenge and degree of protection in NHP studies.
| Vaccine name (citation) |
Binding antibody titer near time of challenge* |
NAb titer near time of challenge† |
Dose and route of challenge‡ |
Time from last immunization to challenge |
Viral load reductions§ |
| Sinovac PiCoVacc (1) | GM EP, ~12,800 (1 day BC) |
RV GM ID50, ~50 (1 day BC) |
1 × 106 TCID50 IT | 22 days | TS AM||, ~1.8; AS AM, ~4.7 |
| Sinopharm/BIBP BBIBP-CorV (2) |
ND | RV GM ID50, ~230 (day of challenge) |
1 × 106 TCID50 IT | 14 days | TS AM, ~5.0¶; AS AM, ~2.9 |
|
AstraZeneca ChAdOx1 nCoV-19 (3) |
Median EP ~6300# (28 days BC) |
RV median ID50, ~60 (28 days BC) |
(1.6 + 0.8 + 0.8 + 0.2) × 106 TCID50 IT-IN-OR-OC** |
14 days | BAL median, ~1.7; INS median, ~1.5 |
| Janssen Ad26.COV 2S.PP (4) | Median EP, ~4000 (14 days BC) |
PV median ID50, 408; RV median ID50, 113 (14 days BC) |
1 × 105 TCID50 IT-IN | 42 days | BAL median, 3.2 (0/6 detectable); INS median, 3.9 (1/6 detectable) |
| Full-length S protein (6) | Median EP, ~160 (7 days BC) |
PV median ID50, ~40; RV median ID50, ~200 (7 days BC) |
1.2 × 108 VP = 1.1 × 104 PFU IT-IN | 21 days | BAL median, 3.1; INS median, 3.7 |
| INOVIO INO-4800 (7) | GM EP, ~3200 (14 days BC) |
PV GM ID50, ~260 (14 days BC) |
1.1 × 104 PFU IT-IN | 77 days | BAL median, ~1.5; INS median, ~0.20 |
| Moderna mRNA-1273 (8) | Log AUC, 4 to 5 (14 days BC) |
PV GM ID50, 1862; RV GM ID50, 3481 (14 days BC) |
7.6 × 105 PFU; 1 × 106 TCID50 IT-IN |
28 days | BAL median, ~4.0; INS median, ~3.0 |
| Novavax NVX-CoV2373 (10) | GM ED50, 469,739 (day of challenge) |
RV GM CPE100, 23,040 (day of challenge) |
1.04 × 104 PFU IT-IN | 35 days | BAL median, ~2.6 (0/4 detectable); INS median, ~2.6 (0/4 detectable) |
|
Pfizer/BioNTech BNT162b2 (11) |
GM EU, 6317 (20 days BC) |
RV GM ID50, 310 (20 days BC) |
1.05 × 106 PFU IT-IN | 55 days | BAL GM, ~3.0 (0/6 detectable); INS GM, ~1.5 (0/6 detectable); OPS GM, ~2.5 (2/6 detectable) |
| Clover Biopharmaceuticals S trimer (12) |
GM EP, 17,497 (day of challenge) |
PV GM ID50, ~5227; RV GM CPE50, ~20,234 (day of challenge) |
2.6 × 106 TCID50 IT (60%) IN (40%) |
14 days | TS GM, ~1.7; AS GM, ~1.5; ITS GM, ~1.7; INS GM, ~0.5 |
*Antibody binding was measured in S protein IgG ELISA, and the values are listed as ED50, EP, or ELISA units derived from comparison with a standard curve (EU). The data are derived from the time point (listed in days) closest to the time of challenge. BC, before challenge.
†Neutralization was quantified in PV or RV assays, as indicated, and the potency was measured as ID50 or CPE100 values.
‡Challenge dose {in plaque-forming units (PFU) or tissue culture infectious dose yielding infection in 50% of wells [median tissue culture infectious dose (TCID50)] and route of challenge}; only in (8) were both PFU and TCID50 given. IT, intratracheal; IN, intranasal; OR, oral; OC, ocular.
§Protection was measured as median log reductions in subgenomic RNA copies per milliliter [except for (2, 11) where viral RNA data are listed]. The viral load (VL) data were derived from bronchoalveaolar lavages (BALs), intranasal swabs (INSs), throat swabs (TSs), oropharyngeal swabs (OPSs), or anal swabs (ASs), at times when VLs were approximately at their peak levels after challenge. In some studies, more substantial protective effects could be detected after the peak values began to decline (see the primary papers for details).
║AM, arithmetic mean of the VL log values.
¶Because viral RNA declined without any discernable peak in the control animals, only RNA measurements for day 7 (the last time point sampled) are listed.
#Data are for the two dose (prime boost) group.
**The macaques were challenged simultaneously via four different routes (IT-IN-OR-OC) with the various doses listed in the same order in the brackets.
The Chinese government authorized the CanSinoBIO Ad5-nCoV vaccine for use on military personnel in June 2020, presumably on the basis of the phase 1/2 trial data (23, 24). In August 2020, the Russian government approved an Ad5 and Ad26 adenovirus vector vaccine, Gam-COVID-Vac (also referred to as Sputnik V), after minimal safety testing and with no evidence of protective efficacy, several weeks before phase 1/2 trial data were published (28). Reservations have been expressed about the suitability of Ad5 vaccines for use in areas of high HIV-1 incidence, based on the risks of increased HIV-1 acquisition (44). This concern would apply to both the CanSinoBIO Ad5-nCoV and the Gam-COVID-Vac vaccines (23, 24, 28). The approval processes that will be applied to the vaccines of the U.S. government–supported OWS program are outlined in brief elsewhere (33).
The immunogenicity of some of the >150 vaccine candidates now in preclinical development worldwide has been tested in small animals and, in some cases, NHPs. These reports are beyond the scope of this review, although we and others have summarized several previously (34, 35, 45). Small animal immunogenicity studies that directly relate to the vaccine candidates we review here are described in several of the papers on NHP experiments and human trials and also in (42, 46–50).
The first indications of vaccine efficacy emerged during November, 2020, with the appearance of seven press releases. “Science by press release” is a far from ideal mechanism for the dissemination of important data but is understandable in the context of the COVID-19 pandemic. The information in the press releases was generally quite limited, and, of course, the data were not peer reviewed. In some cases, more questions were raised than answers given. Nonetheless, it was clear that significant and meaningful levels of efficacy were being accomplished. None of the press releases reported severe safety problems. We summarize what is now known near the end of this review.
ASSESSING ANTIBODY RESPONSES TO VACCINE CANDIDATES
Antibodies induced by the S protein–based immunogens are generally measured in two ways. Immunoassays, usually but not always enzyme-linked immunosorbent assays (ELISAs), quantify antibody binding to the S protein or fragments thereof, such as the RBD. Neutralization assays assess the abilities of NAbs to inhibit SARS-CoV-2 infection of target cells (51, 52). The binding and NAb assays both have value, and titers derived from them generally correlate reasonably well. However, neutralization assays quantify antibodies that block infection, while ELISAs and other binding antibody assays also detect antibodies that lack these properties (non-NAbs) (Figs. 1 to 3). Other assays are sometimes used, for example, to detect antibodies that inhibit the binding of the S protein or its RBD to a soluble form of the angiotensin-converting enzyme 2 (ACE2), which is the entry receptor for SARS-CoV-2. We restrict our discussion to binding antibodies and NAbs, with some exceptions. A repeated occurrence in the papers we summarize is the use of COVID-19 convalescent sera or plasma as comparators for vaccine-induced antibody responses. We have ignored all of these datasets. The serum/plasma panels differ among the various studies, and the range of antibody titers seen in COVID-19 patients can span a 5-log range and vary considerably also during convalescence (34, 35, 51, 53–55). Accordingly, we have not found the convalescent serum panels helpful when gauging the relative immunogenicity of the various vaccine candidates. There is a compelling need to now assemble and use a standard panel containing neutralizing MAbs and/or validated convalescent plasma or purified immunoglobulin G (IgG) preparations for these comparisons (34, 35, 51).
Fig. 1. The measurement of antibody binding and virus neutralization in vitro.
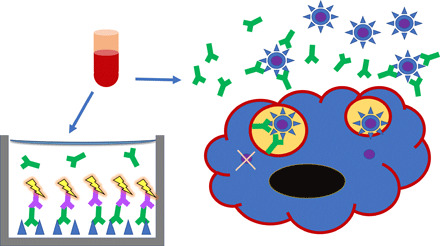
Blood samples are obtained from patients or experimental animals and serum is separated. (Left) Serum antibody binding is usually measured by ELISA: S proteins (blue triangles) or RBDs are immobilized in wells, S-specific antibodies (green) in titrated sera are allowed to bind, and they are then detected with labeled anti-antibodies (purple with yellow flash) (51). (Right) Neutralization is measured as antibody-mediated inhibition of viral infectivity in cell culture assays. A susceptible cell is shown with blue cytoplasm, black nucleus, and red cell membrane. PVs carry a signal gene but cannot form infectious progeny, whereas RVs cause cytopathicity (51, 52). Virus particles are shown as blue circles with triangular spikes, the latter representing the S protein as in the ELISA. The internal viral core is purple. Antibodies in green bind to the S protein on virions in suspension. Some extracellular virions are prevented from receptor binding and cellular uptake by antibody binding to the S protein. Two virions are shown in endosomes. One has antibodies bound to the S protein, which prevents fusion of the viral and endosomal membranes, thereby preventing entry of the viral core into the cytoplasm.
Fig. 3. The quantification of neutralization.
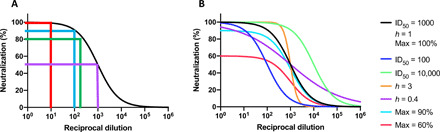
(A) Different degrees of inhibitory reciprocal dilutions are recorded for neutralization assays (purple, ID50; green, ID80; blue, ID90; red, ID99). (B) Neutralization curves differ not only in midpoints (ID50) but also in plateau of maximum neutralization (max %) and slope (Hill coefficient, h) (51, 52, 56). Token values for these three quantities are given for the black curve; one quantity at the time is varied for the other curves as indicated by the color code. Markedly different curves can therefore generate similar AUC values. The relationship between antibody binding to surface viral proteins and neutralization depends on binding strength (affinity), concentration, and the occupancy of NAb on the virion that is required for neutralization (52). What neutralizing titers are sufficient for protecting organisms from infection depends on viral dose and other factors and tends to fall in the range ID50 100 to 1000 (34, 51, 52, 56). When h = 1, the ID99 value is ~100 ID50. Vaccine-mediated protection in vivo not only is dominated by neutralization for many viruses but also can be influenced by non-NAb antiviral effects and cytotoxic T cell responses, as well as by innate immunity and other host factors.
Fig. 2. Different kinds of binding titers.
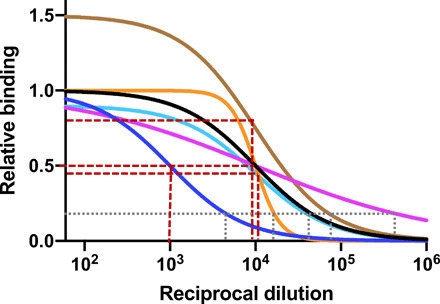
End-point titers and ED50 values are measured in ELISAs. Binding curves with different maxima, midpoints (or half-maximal values), and slopes (Hill coefficients, h) are depicted. Brown stippled lines show the derivation of ED50 at half the plateau values on the y-axis values; gray stippled lines show the derivation of the end-point titers, where the curve crosses a stipulated common cutoff value; all of these titer values are read on the x axis. The ED50 values are products of antibody concentration and affinity, the maxima reflect the number of antigenic epitopes, and the slopes indicate antibody heterogeneity or binding cooperativity.
As we have noted previously, different research groups use different assays and measure antibody binding and virus neutralization differently, which greatly complicates comparisons of datasets (34). The different measurements made in the original papers are explained in Table 5 and Figs. 1 to 3. How binding and NAb titer and protection data derived from animal experiments that use different infectious doses of challenge virus can be related to each other has been modeled and discussed elsewhere (51, 52, 56). The range of SARS-CoV-2 vaccine–induced antibody titers seen in groups of NHPs and humans generally exceeds 100-fold and can be as high as 1000-fold. As noted previously, the existence of such a wide range of responses has implications for the proportion of a population that a vaccine can protect (34). The titer spreads reported in the various primary papers are worth comparing from this perspective.
Table 5. Comparisons of vaccine-induced antibody responses across different studies.
| Antibody binding assays (usually ELISAs) are performed as serum titrations or at only a single dilution. |
| End-point titers or 50% binding titers (ED50, effective serum dilution factor giving 50% of maximum binding) or units relative to standard curves or arbitrary units are reported. |
| NAbs are measured against either replicating viruses (RV assays) or S protein–pseudo-typed viruses, which do not complete an infection cycle (PV assays). |
| Usually, PV assays are a few-fold more sensitive than RV assays and therefore generate higher titer values. |
| NAb data are reported as 50% neutralizing titers (ID50, inhibitory serum dilution factor giving 50% infectivity) or the more stringent neutralization ID80 or ID>99 values (see Fig. 3). |
| Instead of titers, AUCs are sometimes measured. |
| In some primary papers, titers are listed in tables, figures, or the text as multidigit values. We repeat those numbers in this review, although using more than two significant figures is rarely justified. |
| When we have had to make estimates, the values we list in the text and tables are preceded by an ~ symbol. |
| We differentiate median and GM or AM values as was done in the primary papers. |
Antibody responses in the various studies are usually measured for only a short period after the final immunization or, in the case of some macaque experiments, after the virus challenge. The same constraint applies also to the T cell data. In one study where evaluations were carried out for longer than is normal, rhesus macaques were immunized twice with the Pfizer/BioNTech BNT162b2 mRNA vaccine. The peak anti–S1 protein antibody titers then declined ~5-fold over a 28-day period, while NAb replicating virus (RV) median inhibitory dilution (ID50) titers also dropped ~5-fold in the high-dose (100 μg) group (11). We estimate that the early-phase half-life of these antibody titers is only 1 to 2 weeks. The lack of knowledge of the longevity of SARS-CoV-2 vaccine–induced immune responses in humans is a substantial gap that will need filling.
In almost all of the papers we review, antibody responses are measured only in serum. There has been very little attention paid, to date, to mucosal immune responses, which seems unfortunate given how SARS-CoV-2 is transmitted and where it predominantly replicates. Accordingly, we cannot address mucosal immunity in this review, other than by noting that one recent preclinical study of a chimpanzee adenovirus vaccine (different from the AstraZeneca clinical candidate) in mice highlights how important inducing and characterizing mucosal immune responses might turn out to be (50).
Briefly, it is often difficult to inspect two different papers on vaccines A and B and conclude with certainty that one induces the superior immune response. Knowledge of how vaccines of different designs generally perform can help form judgments, but there must always be caveats.
ASSESSING T CELL RESPONSES TO VACCINE CANDIDATES
T cell responses to vaccine immunogens are generally measured by quantifying the amount of cytokine expressed by a T cell after specific antigenic stimulation from a peptide, protein, or vector-delivered antigen. The enzyme-linked immune absorbent spot (ELISpot) assay is most often used, or variants thereof, peripheral blood mononuclear cells (PBMCs) being the commonest source for T cells. Interferon-γ (IFN-γ) secretion is the most commonly chosen cytokine output, but other cytokines are sometimes also measured, as is the production of granzyme B. Cytokine flow cytometry (CFC) is often used as a readout, and there is generally a good correlation between ELISpot and CFC assay results. An advantage of the CFC assay is that it can directly identify the phenotype of responding T cells, which requires depletions of cellular subsets in ELISpot assays. Assays for antigen-specific CD4+ T cells sometimes measure the up-regulation of activation-induced surface markers. However, these methods do not measure T cell avidity or test the potency of cells in viral inhibition assays (57). Here, we confine our discussion to ELISpot assays, with some exceptions.
Depending on the vaccine candidate antigen, a T cell assay can use individual peptides, mostly derived from the S protein, pooled or matrix-pooled peptides, or protein or vector-expressed antigen as a source of peptides to bind to the major histocompatibility complex molecules that are expressed on the cell surface and recognized by a specific T cell receptor. As cross-reactive T cells are known to occur, most assays will not specifically identify a response that was elicited by prior exposure to a cross-reactive pathogen (or a different vaccine). For example, an earlier infection with one of the common cold coronaviruses might lead to a secondary memory response that could skew the outcome of the SARS-CoV-2 vaccine trial analysis, unless prior infection by those other coronaviruses is an exclusion criterion (which is rarely if ever the case).
ELISpot results are usually expressed as spot-forming cells (SFCs) per 106 input PBMCs, but this is not a uniform practice. For example, some investigators use SFCs per 105 cells as their read out; we multiply their values by 10 and report them as SFCs per 106 cells. We also use the abbreviation SFC rather than SFU (spot-forming units) when the latter is used in the original paper. There are also variations in methodologies, including the length of time between blood draw and cryopreservation, the method used for thawing, the peptide concentration used, the duration of peptide incubation with the cells, the time taken to complete the assay, and whether responding T cells are separated. All of these factors can affect an ELISpot result and need to be considered when comparing different studies. A general feature of the papers we have summarized is a lack of detail on how the assays were performed. It would also be useful if images of key ELISpot plates were provided as raw data, to allow the spots to be recounted. The timing of when cell samples are collected after a vaccine prime or boost is also relevant. Thus, the time-dependent decay of circulating T cells affects the magnitude of the responses measured in vitro, to a greater extent than applies to the more stable antibody responses.
Concerns have been expressed that SARS-CoV-2 vaccines may exacerbate disease in infected animals, based on data arising in earlier animal model experiments with vaccines against other coronaviruses (34, 35, 45, 58–60). One particular potential problem is referred to as vaccine-associated enhanced respiratory disease (VAERD) (59). While it is not possible to determine whether VAERD will be a problem with SARS-CoV-2 vaccines before the outcome of efficacy trials and after licensure safety assessments, the pulmonary dysfunctions are associated with increased production of interleukin-4 (IL-4), IL-5, and IL-13, eosinophil recruitment, and impeded CD8+ T cell responses (59–61). This pattern of immune responses is indicative of T helper 2 (TH2) polarization. Accordingly, some of the NHP and human experiments include analyses of in vitro cytokine release profiles, to seek signs of unwanted, TH2-biased responses. To date, TH2 responses have rarely been seen. We briefly note the outcomes of these analyses when they were performed.
IMMUNOGENICITY OF VACCINE CANDIDATES IN NHPs
Immunogenicity studies have been performed in rhesus or cynomolgus macaques or, in one case, baboons (1–13). The immunogens were generally tested beforehand in small animals, often but not always mice, to provide initial assessments of their performance and to provide some indication of the dose or dose range to then evaluate in NHPs. Here, we focus only on the NHP studies themselves; the primary papers should be consulted for the small-animal data. In general, the NHP experiments also involved safety assessments. The outcomes were unexceptional in that no significant problems were reported in the primary papers, which should, again, be consulted for details. Key serum antibody titer values recorded in this section are summarized in Table 2 and, for data obtained at the time closest to challenge, also in Table 3. T cell response data are similarly summarized and tabulated, although these assays were not performed in several of the studies. In all cases, the vaccines were administered intramuscularly, which also applies to the human clinical studies (see below). However, small-animal studies of a chimp adenovirus vaccine and an Ad5 vaccine (not the AstraZeneca/Oxford and CanSinoBIO clinical candidates, respectively) suggest that viral vectors might be very fruitfully delivered by the intranasal route instead (50, 62).
Usually, one or more subgroups of macaques were rolled over into a SARS-CoV-2 challenge study, or the optimal regimen was tested in a de novo experiment. Some details and the outcomes of the virus challenges are summarized separately below and in Table 3.
The first macaque immunogenicity paper to appear described PiCoVacc, the Sinovac β-propiolactone–inactivated, Vero cell–produced virus vaccine (1). Note that this vaccine was renamed CoronaVac for human clinical trials (Table 1) (14). Two vaccine doses (3 and 6 μg of viral protein) with an Alum adjuvant were tested on groups of four rhesus macaques by three immunizations on days 0, 7, and 14. The 6-μg dose elicited slightly the stronger antibody responses on day 21, when the anti–S protein geometric mean (GM) ELISA end-point titers were ~12,800 and NAb GM median inhibitory dilution (ID50) values were ~50 in an RV assay (Table 2). Antibodies specific for the RBD dominated the antibody response to the inactivated virus vaccine, which is relevant to understanding the outcome of the challenge experiment (see below).
The Sinopharm/Beijing Institute of Biological Products (BIBP) inactivated virus vaccine, BBIBP-CorV, was also produced in Vero cells and inactivated with β-propiolactone. Mixed with Alum adjuvant, three different doses (2, 4, and 8 μg of viral protein) were administered to groups of 10 cynomolgus macaques on days 0, 7, and 14 (2). The resulting NAb titers, measured in an RV assay, were dose dependent, with a GM ID50 value of ~210 reported for the highest-dose group on day 21 (Table 2).
The ChAdOx1 nCoV-19 recombinant virus vector expresses a nonstabilized form of the SARS-CoV-2 S protein (3). Groups of six rhesus macaques received this vaccine (2.5 × 1010 particles) either once (day 0) or twice (days 0 and 28) in a prime-boost protocol. In the single-dose group, the anti–S protein median end-point titer on day 14 was ~600, and the median NAb ID50 value was ~20 in an RV assay. The second dose boosted these responses to ~28,000 and ~280, respectively, on day 42 (Table 2). The animals were challenged with SARS-CoV-2 on day 28 (one-dose group) and day 56 (prime-boost group), as summarized below (Table 3).
Rhesus macaques were used to identify and evaluate the optimal design of the Janssen Ad26.COV2.S vaccine candidate (4). First, antibody responses to seven different S protein variants were compared using a range of assays, leading to the selection of the optimal Ad26 S.PP design. After a single dose of this immunogen, median RBD-ELISA end-point titers at week 4 were ~4000, while the pseudo-virus (PV) and RV NAb median ID50 values were 408 and 113, respectively (Table 2). In an IFN-γ ELISpot, at week 4, the median response elicited by the S.PP vaccine was only ~80 SFCs per 106 cells. The data were insufficient to confidently assess the TH1 versus TH2 bias, as only IFN-γ and IL-4 responses were measured. T cell response data were presumably not factored into the decision to choose the S.PP construct as the clinical candidate, as this virus was the least immunogenic of the seven variants from the perspective of inducing CD4+ and CD8+ T cell immunity. The antibody responses were prioritized (4). In a larger and more complex study, various Ad26.COV2.S vaccine-dosing parameters were evaluated in adult macaques, including the number of virus particles administered (5 × 1010 versus 1 × 1011), the benefits of one dose versus two doses, and the interval between the first and second dose in a two-dose regimen (4 weeks versus 8 weeks) (5). Antibody and T cell responses were assessed using similar assays to the initial study (Table 2). The various intergroup comparisons showed that two doses of 5 × 1010 virus particles given at weeks 0 and 8 were the superior regimen for human studies (5). These findings presumably influenced the decision to begin a phase 3 trial of the two-dose regimen, to supplement the ongoing one-dose trial (see below). An additional aspect of the macaque experiment was testing in aged macaques, defined as animals 14 to 22 years old. Here, the immunogenicity of the preferred two-dose, 0- and 8-week regimen was found to be comparable to what was seen in the younger adult animals (5). One final element of the overall experiment was the use of an Alum-adjuvanted recombinant, stabilized S protein, principally for assessing TH1 versus TH2 bias compared to the Ad26 vector regimens. As expected, the responses to the Ad26 virus vectors were more TH1 polarized than those to the Alum-adjuvanted S protein (5). No virus challenges were conducted.
DNA vaccines expressing six different SARS-CoV-2 S protein variants, including the full-length S protein and the RBD, were tested, without adjuvant, in rhesus macaques (6). Median end-point anti–S protein titers at week 5 varied moderately with the immunogen but were 140 to 180 for the full-length S protein and RBD immunogen groups. Midpoint NAb titers at week 5 also varied by immunogen, with median ID50 values of ~50 to 200 and ~30 to 40 in PV and RV assays, respectively (Table 2). The full-length S protein construct elicited somewhat stronger NAb responses than its RBD counterpart. At week 5, T cell responses were detectable in ELISpot assays with pooled S peptides (~80 SFCs per 106 cells in the S group; Table 2). Intracellular staining showed IFN-γ responses both in CD4+ and CD8+ subpopulations; the responses to full-length S were stronger than to S1 and RBD. Last, IL-4 responses were barely detectable, which is compatible with a TH1 bias of the cellular immune responses (6).
INOVIO’s INO-4800 S protein–based DNA vaccine was given to five rhesus macaques in 1 mg of doses at weeks 0 and 4 by an intramuscular electroporation device that provides a mild electric shock to open membrane channels in muscle cells (7). The peak anti–S protein GM end-point titer (week 6) was ~130,000 but it dropped ~40-fold by the time of challenge at week 17. The binding antibody end-point titers against the RBD were ~5-fold lower than against the S protein at week 6. In the PV NAb assay, the peak GM ID50 titers were ~1000 but had declined to ~250 by week 12 (i.e., a four- or fivefold titer decrease over 6 weeks against the two PVs tested). Low titers of anti S protein IgG (~10) were also detected in bronchoalveaolar lavage (BAL) samples from vaccinated animals. An IFN-γ ELISpot was used to measure T cell responses triggered by five peptide pools at week 6. Signals were seen with PBMCs from four of the five animals, with a range of 0 to 518 SFCs per 106 cells and an arithmetic mean (AM) value of ~140. By week 12, the mean value had declined to only ~30 (7).
The Moderna S protein–based vaccine candidate, designated mRNA-1273, was also tested in rhesus macaques (8) The lipid-encapsulated mRNA formulation was given intramuscularly at doses of 10 or 100 μg to each of two groups of eight animals, and at weeks 0 and 4, anti–S protein ELISA data were presented only in the form of area under the curve (AUC) values, precluding direct comparison with other studies. For the high-dose group at 4 weeks after the second dose, the NAb GM ID50 values in PV and RV assays were 1862 and 3481, respectively. In a CFC assay, TH1 responses were dose-dependent, while TH2 and CD8+ T cell responses were at most minimal. Although all animals responded according to prespecified criteria, the T cell assay signals were generally weak, even at the highest vaccine dose. There were no differences in TH1- or TH2-associated cytokines or chemokines in BAL samples from the vaccine and control animals (8).
A paper describing the immunogenicity of the Pfizer/BioNTech BNT162b2 S protein–expressing mRNA vaccine in mice and rhesus macaques appeared several weeks after one that reported on the performance of the same vaccine in humans (Tables 2 to 4) (11, 25). Either 30- or 100-μg doses of the mRNA were given intramuscularly to the macaques on days 0 and 21. Serum anti–S1 antibodies were quantified by ELISA on days 21, 28, 35, 42, and 56 and presented as ELISA units per milliliter (derived from comparison with a standard curve). On day 28, these values were 30,339 and 34,668 for the 30- and 100-μg groups, respectively, but had declined ~5- to 7-fold to 4236 and 6317 by day 56. The pattern of NAb data, assessed by an RV assay, was similar; the peak NAb GM ID50 values were 962 on day 35 and 1689 on day 28 for the two dosing groups, but by day 56, they had dropped 3- to 5-fold to 285 and 310, respectively. The inferred half-life for the early-phase decline is approximately 1 week for the binding antibodies (ELISA) and 2 weeks for NAbs, although the latter titers declined more rapidly at the later time points. Antibody decay rates this high are a potential concern for the longevity of any protection seen in humans, particularly if they are also seen in trials of the other vaccines. T cell responses, measured by IFN-γ ELISpot on days 28 and 42, were ~750 SFCs per 106 PBMCs for both dosing groups, with IL-4 responses below 250 SFCs per 106 PBMCs (11).
A third S protein–based mRNA vaccine has now been described, MRT5500 from Sanofi Pasteur (13). Initial studies in mice led to the choice of a full-length S protein construct, 2P/GSAS, that contains the commonly used two proline mutations (2P) and a furin cleavage site knockout mutation (GSAS). The 2P/GSAS mRNA, formulated as lipid nanoparticles, was tested in a cynomolgus macaque dose-ranging study. Thus, 15, 45, or 135 μg of the mRNA were given on days 0 and 28, leading to NAb responses on day 35 that trended upward in a dose-dependent manner. For the 135-μg dose group, the NAb GM ID50 titers were 2871 (PV assay) and 1877 (RV assay). PBMC T cell responses, assessed using a cytokine release (ELISpot) assay, were TH1-biased (IFN-γ release but not IL-13). The magnitude of the macaque IFN-γ response was very low, around 30 to 40 SFCs per 106 PBMCs (Table 2). No virus challenge was performed (13).
The Novavax NVX-CoV3273 vaccine is an insect cell–derived S protein that is mixed with detergent to form what are described as nanoparticles (9, 10, 21, 63). That formulation is combined with the Matrix-M adjuvant. The immunogenicity study in baboons compared 1-, 5-, and 25-μg doses of the S protein in a two-dose (days 0 and 21) regimen, while a fourth group received 25 μg with no adjuvant. Antibody assays on days 21, 28, and 35 showed that the optimal dose was 5 μg with adjuvant, with the peak response reached by day 28. The highest GM anti–S protein GM median effective dilution (EC50) and NAb ID>99 values were 174,000 and 17,000, respectively (9). The same protein/adjuvant combination was then tested in cynomolgus macaques (10). The animals were immunized on days 0 and 21 at different doses, with both the protein and adjuvant amount varying (protein at 2.5-, 5-, and 25-μg doses). At the highest dose (25 μg of S protein, 50 μg of adjuvant, mirroring one of the human study groups; see below), the anti–S protein GM ED50 value on day 35 was 469,739. Note that binding antibody data were presented as ED50 values, not the more usual end-point titers, which would be substantially higher (perhaps 10- to 100-fold). In the RV NAb assay, the CPE100 (inhibition of ~100% of the cytopathic effect, approximately equivalent to ID>99) was measured, with GM values ranging from 17,920 to 23,040 in the different dosing groups. It should be noted that the neutralization titers for near complete efficacy of neutralization measured in these studies (CPE100 or ID>99) will be substantially lower than the more conventional ID50 values, although we cannot estimate by how much. In summary, the NAb titers in these papers are based on highly stringent assessments of virus neutralization, which should be borne in mind to avoid underestimating the strong antibody immunogenicity of NVX-CoV3273 (9, 10).
Another recombinant S protein vaccine has been tested in rhesus macaques, in this case, from the company, Clover Biopharmaceuticals (12). The S protein contains a C-terminal “TRIMER-Tag,” to promote trimer formation and stability, and was produced in a high-yielding, stable Chinese hamster ovary cell line. After pilot experiments in mice, rhesus macaques were given 30 μg of the S protein on days 0 and 21 in either AS03 or CpG/Alum adjuvant, or vehicle as a control (Table 2). On day 35, the strongest anti–S protein antibody responses were in the AS03 group (GM end-oint titers of ~17,497 versus ~3157 in the CpG/Alum group). At the same time, the GM NAb ID50 titers in the AS03 group were 5227 and 20,234 in a PV and an RV assay, respectively. The D614G mutation in the recombinant trimer did not affect ACE2 binding or the competition therewith by murine immune sera. The partial protection observed in a virus challenge experiment is summarized below (Table 3). A phase 1 clinical trial of the S protein with either AS03 or CpG/Alum adjuvant is now in progress in China (12).
OUTCOME AND INTERPRETATION OF MACAQUE CHALLENGE EXPERIMENTS
When interpreting the outcome of macaque challenge experiments, it should be borne in mind that SARS-CoV-2 does not cause a lethal COVID-19–like disease in these animals. The macaques do become ill, rhesus perhaps more so than cynomolgus, but the disease course is generally mild, self-limiting, and overcome within ~2 weeks (51, 64–66). In general, the various vaccines reduce the severity of this mild disease, including by reducing or even preventing the transient lung damage that can be seen in postmortem samples taken from control animals. No signs of vaccine-mediated enhancement of infection, including VAERD, were reported. The most common way in which vaccine efficacy is assessed is by determining the viral load (VL) in samples from various locales and tissues at short intervals during the week after challenge. In some experiments, both viral RNA copies per milliliter and subgenomic RNA copies per milliliter are determined, the latter avoiding problems associated with the presence of residual challenge virus and more unambiguously demonstrating viral replication in the infected animal (Table 3) (6, 51, 67). Lung pathology was also generally assessed, although the criteria chosen tend to vary among the different experiments. For a particularly detailed discussion of the variables associated with challenge experiments in macaques and how they are best interpreted, see (51).
Antibody titers in the animals on or very close to the day of challenge were reported in some of the papers and are summarized below and also in Table 3. In the other papers, the antibody data were derived at an earlier time point (Table 3). The inconsistencies in how the different studies were conducted and/or reported are another factor that blurs attempts to compare and interpret the performances of the different vaccines. Only four of the reports include data on T cell responses at any time point before challenge, which limits understanding of any role they may play (Table 2) (4–7). In a separate section, we discuss what, if any, correlates of protection (CoPs) can be inferred from some of the challenge experiments.
Most of the experiments involved SARS-CoV-2 challenges within a few weeks of the final (or only) vaccine dose, i.e., at a time when the immune response is likely to be close to its peak. The exception is the INOVIO DNA vaccine study in which the challenge was delayed by 13 weeks (7). The next longest delays are 55 days after the second dose of the BNT162b2 mRNA vaccine and 42 days after the delivery of a single dose of the Ad26.COV2.S vaccine (Table 3) (4, 11). Thus, it is not yet known whether these various vaccines would be as effective against challenges conducted many months after the immunization protocol was completed. Extrapolating to what might happen when vaccinated humans become exposed to SARS-CoV-2 over the subsequent months or years is not possible.
Another issue when considering these macaque experiments concerns the SARS-CoV-2 challenge itself. There is no generally accepted standard, and various different challenge virus stocks (in several cases, of unspecified origin) were used. The challenge dose also varies 100-fold, and the route of challenge is another variable (Table 3). In one experiment, the virus was even administered by four different routes (3). All of these protocol variations constitute yet another factor hindering cross-study comparisons (34, 51). As a general principle, it will be easier to protect against a low dose of a challenge virus than a higher one, all other things being equal. Thus, would a vaccine that protected against a relatively low challenge dose be as protective against the 100-fold higher dose used in other experiments (34, 51)? Or would its protection break down under those conditions? We return to this point at the end of this section. Challenge doses for vaccine experiments are traditionally predetermined in naïve animals, to identify an inoculum size that is neither too low to be consistently infectious nor too high to protect against. It is rarely clear from the papers whether these titrations were performed. In one report, nasal swab VL samples taken from SARS-CoV-2–infected humans and from the virus-challenged macaques soon after infection were said to be comparable (~1 × 106 RNA copies per milliliter) (8). However, the initial infection and subsequent replication efficiencies are likely to differ substantially between the two species so it is not clear that this comparison is meaningful.
In the Sinovac PiCoVacc study, groups of four rhesus macaques were immunized with either 3 or 5 μg of the inactivated virus vaccine on days 0, 7, and 14 and challenged intratracheally with the CN1 strain of SARS-CoV-2 on day 22 (1). At this time, the anti–S protein GM end-point titer was ~12,800, while the NAb GM ID50 titer was ~50 when measured 7 days earlier. All the vaccinated animals became infected after challenge, but disease severity was reduced compared to the control group (adjuvant only) as judged by lung pathology assessments. VLs (i.e., viral RNA) were frequently detected at high levels in lung samples from control animals but in none of the high-dose vaccine recipients and only sporadically at significantly lower levels in the low-dose group. Viral RNA levels in throat swabs were also lower and declined more rapidly, particularly in the higher vaccine-dose group (Table 3). The observed increases in NAb titers day 7 after infection may be consistent with an anamnestic antibody response (1).
The Sinopharm/BIBP inactivated virus vaccine experiment involved two groups of four cynomolgus macaques that were immunized with different doses (2 or 8 μg of viral protein) on days 0 and 14 (2). Binding antibodies were not measured. The NAb GM ID50 values in an RV assay were ~200 and ~230 in the low- and high-dose groups when the animals were challenged on day 24 with a SARS-CoV-2 isolate from the Chinese Center for Disease Control and Prevention, by the tracheal route (Table 3). There were no changes in body temperature in either the vaccine or placebo groups over the next 7 days, which is indicative of a mild disease course. Viral RNA in all lung lobes was analyzed postmortem, but none was detected in any lobe taken from vaccine recipients (in either dosing group). In contrast, the RNA copy number per milliliter ranged from ~30,000 to 3,000,000 in the lower lobes of the control animals. Lung pathology was also prevented or reduced in the vaccine groups. Although viral RNA in throat swabs became undetectable 7 days after challenge in the high-dose group, other evidence suggests that these animals did become infected, albeit to a much lesser extent than the control and low-dose vaccine groups. Thus, gastrointestinal virus (detected in anal swabs) remained stable in the high dose at ~100 RNA copies per milliliter from days 3 to 7, whereas the corresponding values in the two other groups fluctuated around 100,000. While this study only analyzed viral, not subgenomic, RNA, it seems highly unlikely that gastrointestinal viral RNA could simply represent residual challenge virus. Thus, the higher-dose animals were at least strongly, but apparently not completely, protected from infection, and in both dosing groups, the vaccine reduced the extent of virus replication after infection (2).
All 12 of the ChAdOx1-vaccinated macaques became infected when they were challenged 28 days after their final immunization (they received either one or two vaccine doses; see above) (3). The SARS-CoV-2 challenge strain was WA1-2020 (MN985325.1). At the time of challenge, the median binding antibody end-point titers were ~6300 and median NAb ID50 values ~60 in an RV assay (Table 3). The vaccinated animals had fewer symptoms than the control group, less lung damage, and lower VLs (measurements included subgenomic RNA) in BAL and lung samples. No virus was detected in BAL samples from the vaccinated animals on day 5, but subgenomic RNA could be detected in lung samples from some animals in both groups. No antibody or T cell data after challenge were reported, so it is unknown whether there were anamnestic responses to the infecting virus (3).
Seven different Ad26-based vectors were given once to groups of four to six rhesus macaques before challenge 6 weeks later with an unspecified isolate of SARS-CoV-2 (4). Compared to a control group of 20 animals, VLs in BAL and nasal swabs were significantly reduced in each of the seven Ad26 vector groups, by >5 logs in the case of BAL samples. The best performing vector, from this perspective, was the one designated S.PP; it was chosen to become the Ad26.COV2.S clinical vaccine candidate. Overall, the authors assessed that 17 of the 32 vaccinated macaques were protected from infection, judged by the VL data. There was no evidence for anamnestic B and T cell responses in the protected Ad26.COV2.S-vaccinated animals, although NAb titer increases were seen in other vaccine groups. The strongest, and perhaps complete, protection was seen in the S.PP group. Thus, virus (subgenomic RNA) could not be detected in BAL from 6/6 and in IN swabs (INSs) from 5/6 animals (4). More recently, hamsters immunized once with the S.PP-expressing Ad26 clinical vaccine candidate were protected from severe disease when challenged with SARS-CoV-2 nasally 4 weeks later (49).
In another study, rhesus macaques were immunized intramuscularly with S protein–expressing DNA plasmids at weeks 0 and 3 and challenged at week 6 with an unspecified SARS-CoV-2 isolate (6). All of the 10 control animals became infected, acquiring BAL and nasal swab peak subgenomic RNA copy levels in the range 104 to 107 per milliliter. However, 8 of the 25 vaccine recipients were RNA negative in BAL and nasal swab samples, while median subgenomic RNA levels in the other 17 macaques were 3 to 4 logs lower than the median values from the 10 control animals. Even when subgenomic RNA was undetectable in vaccinated animals, the observation of anamnestic antibody and T cell responses does imply that the animals were not completely protected from infection. Instead, initially replicating virus may have been suppressed by vaccine-mediated immunity (6).
The INOVIO INO-4800 DNA vaccine was given to five rhesus macaques at weeks 0 and 4 (7). The SARS-CoV-2 challenge (USA-WA1/2020 strain) was then delayed until week 17 (i.e., 13 weeks after the second immunization), a substantially longer period than applies in the other studies summarized here. Upon challenge, all the macaques became infected, judged by VLs in various samples. However, VLs in the vaccinated group were lower and declined more quickly than in five control animals, the reduction in medians being in the 10- to 300-fold range depending on the sample site and the time point assayed; the difference was significant for BAL but not INS samples. Antibody and T cell recall responses were quantified in the animals after virus challenge. Thus, by 14 days after challenge, anti–S protein antibody and NAb measurements were higher (~10- to 30-fold) in the vaccinated than control animals, while there was an ~2-fold increase in IFN-γ signals. Overall, the vaccine-mediated reduction in viremia after challenge was attributed to recall responses (i.e., T and B cell memory) (7).
Two dosing groups of eight rhesus macaques were immunized with the Moderna mRNA-1273 vaccine at weeks 0 and 4 and then challenged with the USA-WA1/2020 strain of SARS-CoV-2 at week 8 (8). Judged by VLs, most (~7 of 8) of the animals in the higher-dose group were protected, but most (~5 of 8) of the lower-dose group became infected (the exact numbers vary per time point and depend on the VL sample site). There were indications of anamnestic responses in some animals, including in BAL. Postmortem analyses of the lungs found little or no signs of inflammation in the higher-dose group, but some indications of pathology in the lower-dose animals that became infected. Neutralization titers in both PV and RV assays correlated inversely with INS VLs; virus-specific IgG and IgA levels in BAL were elevated in the high-dose group on days 2 to 7 after challenge, an anamnestic response that may perhaps have contributed to VL reduction. In the high-dose, high-protection group, the GM ID50 values from PV or RV NAb assays were >900 in 7/8 animals, whereas the corresponding values were <900 for 7/8 animals in the low-dose, low-protection group. The data pattern implied that NAbs were protective in the high-dose group (8).
In the BNT162b2 vaccine study, six rhesus macaques immunized on days 0 and 21 with 100 μg of the mRNA and three naïve controls were challenged on day 76 overall with 1.05 × 106 plaque-forming units (PFU) of the USA-WA1/2020 strain of SARS-CoV-2 by the intranasal (IN)–intratracheal (IT) routes (11). Antibody titers on the day of challenge were not reported, but measurements made on day 56 are summarized above and in Tables 2 and 3. Infection was monitored by viral RNA copies in BAL, nasal swabs, and oropharyngeal swab on days 3, 6, and 10. No viral RNA was detected at any time in the BAL samples from the immunized macaques but was present in control samples. A similar data pattern, after day 1, was seen when the nasal swabs were analyzed, and in the oropharyngeal swabs, 2/6 were positive on day 3. The GM reductions in VL measured as viral RNA copies were ~3.0 log for BAL, 1.5 log for INSs, and 2.5 log for oropharyngeal swabs (the first and last of which were statistically significant). One unusual aspect of the experiment was that the complete absence of disease symptoms in the control animals (and, of course, also in the vaccine recipients). As noted above, SARS-CoV-2 infection generally causes moderate disease in rhesus macaques, including in other vaccine experiments. Why no such symptoms were seen in the present study was not explained but could perhaps be rooted in the challenge virus stock or the origins of the macaques (11).
The adjuvanted Novavax NVX-CoV2373 recombinant protein vaccine was given to cynomolgus macaques on days 0 and 21 before the animals were challenged with the WA1 strain of SARS-CoV-2 via the nasal and tracheal routes on day 35 (10). Judged by VLs (subgenomic RNA) in BAL and nasal swabs, performed 2 and 4 days later, every animal was virus-negative except for one in the lowest-dose group that had a weakly positive BAL sample. Postmortem lung samples in the vaccine groups showed no sign of the pathologies that were visible in the control animals. To the extent that can be judged, most vaccinated animals may have been completely protected from infection. This outcome may reflect the very high GM antibody titers on the day of challenge (anti–S protein ED50 of 469,739 and NAb CPE100 of 23,040) (10).
Clover Biopharmaceuticals tested their recombinant TRIMER-Tag S protein and AS03 or CpG/Alum adjuvants in rhesus macaques (12). After two vaccine doses on days 0 and 21, the animals were challenged on day 35 with 2.6 × 106 median tissue culture infectious dose (TCID50) of SARS-CoV-2 by the IN and IT routes (Table 3). Both of the trimer/adjuvant groups of animals were partly protected as measured by body mass and temperature and VL in anal, throat, and tracheal samples but less so in nasal swab samples. However, VLs in the lungs indicated complete protection in both of the trimer groups, compared with the vehicle controls. Serum Ab responses dropped a little in the week after challenge, which the authors suggested reflected the formation of immune complexes with the incoming virus and hence Ab-mediated virus clearance (12). Whether this explanation is correct remains to be determined.
In summary, all of the vaccines tested to date have conferred a substantial degree of protection to the immunized macaques. In some macaques, there is reasonable evidence for complete protection (i.e., “sterilizing immunity”), but the more common outcome is a reduction in the severity of the already mild disease course seen in control animals. We discuss in the next section what immune factors and other variables may have influenced the outcomes of the different experiments. In respect of what the outcomes may mean for vaccine efficacy in humans, we note that it is generally easier to protect animals against mild infections than severe ones. Hence, it is hard to assess whether and how any of the present findings in macaques might translate to the subset of humans who need protection from severe and lethal COVID-19. Moreover, as noted above, it is not known whether the various vaccines would still protect macaques, and by extrapolation humans, after a substantial period (multiple months) has elapsed.
TOWARD CORRELATES AND MECHANISMS OF PROTECTION
It is notable that in the various macaque immunization studies, similar outcomes of virus challenges were associated with substantial (~2000-fold) differences in serum antibody titers to the S protein, the recombinant protein vaccines being the strongest immunogen for inducing binding antibodies (9, 10) or NAbs (9, 10, 12) (Table 2). It can also reasonably be concluded that the ChAdOx1 vaccine is not a strong inducer of antibody responses to the S protein, particularly when given only once (Table 2) (3). The same conclusion can be made about the DNA plasmid vaccines (Table 2) (6, 7). Are the serum antibody responses induced by the weaker vaccines solely responsible for any protection that was conferred? Perhaps, cellular immune responses or some other unmeasured factor, such as mucosal IgA, were contributory? The potential protective role of mucosal immunity is highlighted by the outcomes of experiments involving a ChAd virus vector in mice (50). These observations are similar to those in studies of other vaccines, such as HIV-1 Env, where only protein-based immunogens induce very strong antibody titers (68).
What protected the vaccinated animals from SARS-CoV-2 infection and/or disease? CoPs (correlates of protection) are important in vaccine development, because they can serve as robust predictors of future vaccine efficacy whether they are derived from animal experiments or clinical trials and whether the end points involve protection from infection or a reduction in disease severity. There are nuances to the identification of CoPs in population-wide studies that we cannot address here (69, 70). The present macaque challenge studies are not sufficiently powered and are not wide-ranging enough in scope to allow the identification of CoPs with high confidence. The few attempts to identify CoPs have pointed toward a predominant role for NAbs, which is not unexpected, but it is premature to conclude that NAb titers at the time of challenge (i.e., in humans, virus exposure) tell the entire story. The possible role of recall responses (i.e., T and B cell memory) in clearing a transient infection has only been addressed in the INOVIO DNA vaccine study, which involved the longest delay between immunization and challenge (Table 3). Even in that experiment, the amount of information available is quite limited (7).
Despite the limitations of the available data, we sought hints of CoPs. Thus, we analyzed the relationships between, on the one hand, binding antibody and NAb titers at the prechallenge peak or within 2 weeks before challenge and, on the other hand, VL reductions in vaccinated animals compared with controls (summed for two locales of sampling and based only on subgenomic RNA; Tables 2 and 3). We found no tangible nonparametric (Spearman) positive correlations of any significance between VL reduction and any antibody parameters, which is not unexpected given the number of variables between the different experiments. We were also unable to identify any consistent relationship between the challenge virus dose or delivery route and the degree of protection. The challenge dose was not a consistent predictor of the magnitude or duration of high VLs in the control animals, but we should bear in mind that the challenge virus stocks represent another variable, as does the subspecies (and sources) of the animals involved. Nonetheless, the wide variation in challenge dose between experiments should not be ignored. Would a vaccine that protected against a relatively low challenge dose be as protective against the 100-fold higher dose used in other experiments? Or would its protective capabilities degrade under those conditions? Experimental conditions yielding high VLs in control animals may impede complete protection while giving the potential for greater VL reductions. The smallest VL decrease in the vaccine group compared to control was seen in the INOVIO DNA vaccine experiment that involved one of the lowest challenge doses (Table 3) (7). In the report on the Ad26.COV2 vaccine, there were indications of sterilizing immunity against an intermediate challenge dose (4).
In four studies, some groups of vaccine recipients seem to be completely protected, or nearly so (4, 6, 10, 11). In one experiment, no anamnestic antibody or cellular immune responses were detected in the protected animals, which suggested that immunity was sterilizing (4). However, in another study, there were anamnestic immune responses in animals with undetectable VLs, which is more indicative of incomplete but aborted infection (6). Anamnestic responses were not analyzed in the other reports (10). In two other cases, lung lobes in the vaccine groups where protection was strongest were free of viral RNA 7 days after infection, which contrasted with the high levels found in the lower lobes of control animals (1, 2). The criteria for sterilizing immunity, or at least complete protection against persistent infection, are neither defined nor standardized in the SARS-CoV-2 animal model field, which also extends into other protection challenge systems involving small animals and both vaccines and antiviral antibodies (34, 51, 64–66).
Within individual studies there are fewer confounding factors than in a cross-study meta-analysis. Some evidence was presented that antibodies were the CoP in the Moderna mRNA vaccine study (8). In the report on the Janssen Ad26.COV2 vector vaccine, comparing various antibody and T cell responses with infection outcomes (as judged by VLs) identified NAbs as the strongest CoP, with some possible contribution from Ab effector functions such as antibody-dependent cellular phagocytosis and antibody-dependent activation of natural killer cells. In contrast, T cell responses [measured by ELISpot or intracellular cytokine staining (ICS)] did not correlate with protection (4). Similar inferences about an antibody but not a T-cell–associated CoP emerged from the experiments involving DNA-plasmid immunizations (6). However, even when significant differences were identified, the ranges of the various measurements were generally overlapping between completely and partially protected animals (4, 6). This degree of variation compromises attempts to identify the threshold response required for protection, particularly in the study of the Ad26 vector vaccine (4). Overall, the available evidence from macaque challenge experiments does point toward a protective role for antibody-based immunity (probably NAbs), but not to the extent that a protective titer can be inferred and then extrapolated to the outcome of human efficacy trials.
IMMUNOGENICITY OF VACCINE CANDIDATES IN HUMANS
Key antibody and T-cell response data summarized below for individual trials are presented in Table 4. As with the NHP studies, the primary papers and reviews should be consulted for additional details of the human trials, which are variously described as phase 1, phase 2, or combined phase 1/2a trials (14–32). Vaccine safety assessments were a key component of these trials; in all cases, reported side effects and adverse events were considered to be minor or moderate; the primary papers contain the details, which we have not attempted to summarize. A grade 3 serious adverse event (SAE) in the form of a neurological complication happened in the AstraZeneca/Oxford vaccine phase 3 trial in the United Kingdom, leading to a now-concluded pause while the case was investigated. A placebo recipient in the Brazilian arm of the phase 3 trial of this vaccine reportedly died of COVID-19. An SAE, also triggering a temporary clinical hold, occurred in the Janssen phase 3 trial, although no details have been reported.
Details of the phase 2b/3 efficacy trials for vaccines reaching that stage can be found at https://clinicaltrials.gov/ct2/results?recrs=&cond=Covid19&term=vaccine&cntry=&state=&city=&dist=. The preliminary outcomes of the Pfizer/BioNTech, Moderna, Gamalaya Center, and AstraZeneca phase 3 trials, as judged by information in press releases, are summarized in a separate section later here.
The initial human trials have predominantly involved young or middle-aged, healthy adults (see primary papers for details). Some information is becoming available on age-dependent decreases in immunogenicity. In the CanSinoBIO Ad5-nCoV vaccine, participants aged older than 55 responded with weaker antibody responses than their younger counterparts. However, that outcome could reflect either the aging process or time-dependent increases in exposure to other Ad5 viruses that compromise expression of the immunogen from by the vector (24). An ~2- to 3-fold reduction in antibody responses was seen in older adults (aged 65 to 85) compared to younger ones (aged 18 to 55) in a Pfizer/BioNTech mRNA vaccine trial (27). Moderna has now reported similar findings for their mRNA vaccine; in a small-scale (40 volunteers) extension to their original phase 1 trial, binding-antibody and NAb responses were comparable in volunteers aged 56 to 60 and over 71 and similar to what was reported for those in the 18 to 55 age range (19, 20). NAb responses were slightly lower in volunteers aged over 60 than in ones in the 18 to 59 range, during the BBIBP-CorV–inactivated phase 1 trial. The ratio was ~2-fold but varied with the time point and vaccine dose, and group sizes were small (15). The Sinovac inactivated virus vaccine trial only involved volunteers under 60, but an analysis of the 18 to 29 versus 50 to 59 age groups suggested that NAb responses were ~2-fold higher in the younger people. Overall, there was a modest trend toward weaker immunogenicity with age (14). A limited amount of preliminary data on the Janssen Ad26.COV2 vaccine also indicates that immunogenicity in volunteers aged over 65 is only modestly reduced (18). When the ChAdOx1 nCoV-19 vaccine two-dose regimen was tested in volunteers aged 18 to 55, 56 to 69, or over 70, there was little or no age-dependent reduction in immunogenicity, judged by the same suite of antibody and T cell assays as in the earlier trial (16, 17). In a macaque study, the immunogenicity of the Janssen Ad26.COV2 vaccine was comparable in adult and aged animals (5).
Together, the studies summarized above encourage the belief that the efficacy of the various vaccines in older adults are comparable to what is seen in the phase 3 trials that are being mostly conducted in younger people. There has also been an underrepresentation of minority groups in the U.S. and European trials; thus, again, information about how immunogenicity might vary in different populations is lacking. These various lacunae will need to be filled in phase 3 trials, given that COVID-19 is more severe in older people and in African-American and Latinx populations.
Inactivated virus vaccines
The Sinopharm/Wuhan Institute of Biological Products (WIBP) inactivated virus vaccine was delivered in Alum adjuvant. It was first tested in 96 volunteers in a phase 1 trial and then in 224 more people in a phase 2 study (22). The study cohorts were based on healthy individuals aged from 18 to 59. The phase 1 trial was dose-ranging (2.5, 5, and 10 μg of viral protein) and involved intramuscularly injections on days 0, 28, and 56, while in phase 2, only the 5-μg dose was tested in two substudies that involved immunizations on days 0 and 14 or on days 0 and 21. Immune responses were measured by ELISA using inactivated virus as the detecting antigen, which does not allow a comparison with other vaccines, and by an RV neutralization assay. For sera collected 14 days after the final dose, the NAb titers (GM ID50 values) in the phase 1 trial were 316, 206, and 297 in the low-, medium-, and high-dose groups respectively. Allowing for the titer ranges among participants, the three doses induced similar antibody responses. In the phase 2 trial, the corresponding NAb titer values were 121 and 247 for the 0- and 14-day groups and the 0- and 21-day groups, respectively. Antivirus ELISA end-point GM titers were also similar among the different test groups in the two trials and were 200 to 300 in phase 1 and 90 to 200 in phase 2 (Table 4). There were no T-cell data in the paper. Phase 3 trials are now in progress in South America, although the vaccine dose and delivery regimen (i.e., the number and spacing of doses) were not specified in the report on the phase 1 and phase 2 trials (22).
Sinovac’s PiCoVacc-inactivated vaccine was renamed CoronaVac before phase 1 and phase 2 human trials (Tables 1 and 4) (1, 14). The production process used to make the vaccine was stated to be changed between the phase 1 and phase 2 trials to yield a product with an ~2-fold higher S protein content, which the authors suggest improved its immunogenicity (14). However, only data from the phase 1 trial have been reported to date, and the phase 2 results are still pending. For phase 1, the Alum-adjuvanted vaccine (or a placebo) was given to 600 adults aged 18 to 59 years in a two-dose regimen on days 0 and 14 or days 0 and 28. For each of the two regimens, two vaccine doses, 3 and 6 μg, were tested in 120 volunteers, compared to a placebo group of 60. The safety profile was unexceptional. Antibody responses were measured on days 28 and 42 in an anti-RBD ELISA and NAbs in an RV assay with a CPE readout (the cutoff for neither assay was reported). Anti-RBD GM titers were ~1000 in all the four vaccine groups on day 28. The NAb GM titer values for all the groups at all the time points were generally in the 32 to 64 range (Table 4). The age dependency of the antibody responses was noted above. No T-cell data were reported (14).
The BBIBP-CorV Alum-adjuvanted inactivated virus vaccine also advanced from macaque studies into phase 1 and phase 2 human trials (15). The phase 1 trial involved 192 volunteers aged 18 to 59 and 60 or older, who received vaccine doses of 2, 4, and 8 μg on days 0 and 28. Only volunteers in the younger age-range participated in the phase 2 trial, in which 448 people received a single vaccine dose of 8 μg or two 4-μg doses given first on day 0 and then on day 14, 21, or 28. Together, the trials involved multiple small subgroups, which limit the statistical power of any comparisons. The paper should be consulted for details of how the different dosing regimens performed. The vaccine was generally safe, with only minor adverse events reported. Immunogenicity was assessed only in an RV NAb assay. The resulting NAb titers were modestly dose-dependent, were much stronger after two doses than one, and were slightly lower in the younger than older age groups. In phase 1, the 8 μg on day 0 and day 28 regimen gave a GM titer of 228.7; in phase 2, the highest NAb titers were seen in the group given an 8-μg dose on days 0 and 21, for which the GM titer was 282.7 (Table 4). No data on ELISA antivirus or anti–S protein titers, or on T-cell responses, were reported. Phase 3 trials are underway, using a two-dose regimen, but no details of the doses and scheduling chosen were provided (15).
A fourth Alum-adjuvanted, inactivated virus vaccine, from Institute of Medical Biology (IMB)/Chinese Academy of Medical Sciences (CAMS)/Peking Union Medical College (PUMC), has also entered human trials, although no preclinical data were reported (29). This vaccine virus, KMS-1, was also produced in Vero cells, but unlike the other three, it was inactivated first with formaldehyde before β-propiolactone treatment. In the phase 1 trial, the IMB/CAMS/PUMC vaccine was given twice to 192 people aged 18 to 59 on days 0 and either 14 or 28, at doses of 50, 100, or 150 EU (the stated unit of immunogen content). There were no significant adverse events, and sera from selected volunteers did not trigger antibody-dependent enhancement in vitro. Immunogenicity was assessed at several time points by an RV NAb assay, various ELISAs including anti–S protein and antivirion, and IFN-γ ELISpot. The paper should be consulted for data on the nine individual subgroups in the trial, but in general, the GM NAb titers (CPE with unspecified cutoff) were all <100 and often <50. Anti–S protein GM titers (with no cutoff specified) were 2000 to 4000 for the two highest doses of immunogen (Table 4). Only limited data were presented for the day 0 and 28 group after the second dose. Thus, on day 90, the GM NAb titer was <10, and the anti–S protein titer was ~500, which indicates a time-dependent reduction in the initial antibody levels. IFN-γ ELISpot assays using S peptides gave AM values of 30 to 250 per 106 cells for the two vaccine doses, indicative of a generally weak T-cell response (29). In a phase 2 study, 742 adults (aged 18 to 59) were given the higher dose of the inactivated vaccine on days 0 and 14 (30). The binding Ab and NAb titers reported were similar to those seen in the phase 1 trial, but T-cell responses were not analyzed (Table 4).
Adenovirus vector vaccines
In a phase 1 trial, the immunogenicity of the CanSinoBIO Ad5-nCoV vaccine candidate was found to be dose-dependent (23). Doses of 5 × 1010, 1 × 1011, or 1.5 × 1011 virus particles were given once to three different subgroups. In the highest-dose group, the anti–S protein and anti-RBD GM titers on day 28 were 596.4 and 1445.8, respectively [in (12, 13), the cutoffs for titer determinations are not specified; we refer to the values as “titers”]. The GM NAb titers were 34.0 and 45.6 in RV and PV assays, respectively, and were strongly correlated with anti-S and anti-RBD titers. A phase 2 trial was then conducted on 508 participants, of whom 126 received a placebo (24). The protocol again involved a single administration of the Ad5 virus, which was tested at doses of 1 × 1010 or 1.5 × 1010 in subgroups. The anti-RBD GM titers on day 28 were 656.5, which is ~2-fold lower than in the phase 1 trial. NAb titers in the RV and PV assays were 19.5 and 61.4, respectively, and hence similar to the phase 1 trial data. T-cell responses were measured by ELISpot on samples taken before vaccination and then on days 14 and 28. Freshly drawn PBMCs were incubated with S protein peptide pools for >12 hours, the data expressed as SFCs per 105 cells after subtraction of background values derived from unstimulated control cells. (Note that the data in Table 4 have been adjusted to SFCs per 106 cells to facilitate comparison to other datasets). There was no mention of a positive control method nor of the number of replicates. An ELISpot result was stated to be positive if the number of IFN-γ–secreting T cells responding to the S protein peptides was increased two times above baseline after vaccination. Tumor necrosis factor–α, IL-2, and IFN-γ responses to the vaccine were also assessed by CFC. T cell responses peaked at day 14 after vaccine and ranged from 200 SFCs per 106 cells in the low-dose group to 580 SFCs per 106 cells in the high-dose group. In CFC assays, both CD4+ and CD8+ T cells were found to be responsive (24).
AstraZeneca’s ChAdOx1 nCoV-19 recombinant virus vaccine (also known as AZD1222) was tested in a randomized phase 1/2 trial involving 543 people; another 544 participants were given a meningococcal control vaccine (16). The original protocol involved a single dose of 5 × 1010 virus particles, which is twice the amount per dose given to macaques (Tables 2 and 4). However, a decision was taken during the trial to give 10 participants a second dose of ChAdOx1 on day 28 in a nonrandomized boosting protocol. It is assumed that the decision was taken because of the limited immunogenicity of the single-dose regimen (a modest boosting effect of a second dose was seen in the NHP study; see above) (3). Anti–S protein binding was measured at single dilutions and converted to “ELISA units,” an approach that complicates comparisons with anti–S protein responses to other vaccine candidates in humans and that represents an unexplained change from how the macaque sera were analyzed by titration in ELISA (3). By days 14 and 28, the responses in most of the participants were in the 100 to 1000 unit range (medians, 102.7 and 157.1, respectively), with little change by day 56 in the subgroup that was assayed at that time point (median, 119). After the second vaccine dose in the prime-boost protocol, a ~5-fold increase in median anti–S protein ELISA units was measured 14 days later (median, 997.5), and the levels were largely maintained by day 56 (median, 639.2). NAbs were measured 14 days after the booster immunization using one PV and three different RV assays. NAb data from the PV assay and from the only RV assay that reported ID50 values are given in Table 4. The primary paper should be consulted for other aspects of the neutralization data generated in various assays (16). Overall, the apparently modest NAb responses to the single-dose vaccine were increased a few-fold by the day 28 boosting immunization, at least in the short term (until day 42). The median titers for the prime-boost group on day 42 were 372 to 450.9 (Table 4). ELISpot assays were performed on freshly isolated PBMCs at days 0, 7, 14, 28, and 56 and at day 35 for the participants who received two doses. Pooled peptides were used as antigens, and data were excluded if the assay background response rate was deemed to be too high. The measured responses peaked at day 14 at a value of 856 SFCs per 106 PBMCs in the prime group and 1642.3 SFCs per 106 PBMCs in the prime-boost group (i.e., after one dose in either group). The results for other time points are given in Table 4. Notably, ~10% of recipients of this vaccine appear to generate no measurable T-cell response after the first dose. Furthermore, the booster dose given to 10 trial participants did not further increase their T-cell responses (16). The initial U.K.-based phase 1/2 clinical trial was extended to encompass 20 different subgroups with variables that included the number of vaccine doses (one or two) given to volunteers in three different age groups: 18 to 55 (n = 100), 56 to 69 (n = 120), and 70+ (n = 200) (17). Control group members were given a Meningitis vaccine. Because there were 560 volunteers in total, the number of people in each individual subgroup was necessarily small, which limits the statistical power of any comparisons between the subgroups. Nonetheless, the data pattern supported the use of two doses in the subsequent phase 3 trial. The immunogenicity of the two-dose regimen was comparable among the different age groups, judged by the same suite of antibody and T-cell assays as in the earlier trial (16, 17). However, the confidence intervals on the various datasets are quite wide, which may have prevented the detection of modest differences. It was also noted that reactogenicity diminished with age (17). This vaccine has now advanced into phase 3 trials in several international locations, including Brazil and South Africa. These trials were initiated as a single-dose regimen but were later changed to incorporate the second, boosting dose. A two-dose phase 3 trial started in the United States at the beginning of September 2020.
The initial phase 3 trial of the Janssen Ad26.COV2 adenovirus vector involves a one-dose regimen. Some of the human immunogenicity data on which this scheme was reportedly based have been described in an “interim report” of a phase 1/2a trial (18). The vaccine was tested at two doses (5 × 1010 and 1 × 1011 viral particles) that were given intramuscularly either once (day 0 only) or twice (days 0 and 56) to healthy adults aged 18 to 55 (n = 402) or >65 (n = 394). Safety data were generally unexceptional, although two SAEs were reported and deemed, after investigation, to be either not vaccine-related or not problematic (a high fever that was resolved). Antibody immunogenicity was measured by S protein ELISA, with data reported as units per milliliter and an RV NAb assay with an IC50 end-point. T-cell responses to the S protein were measured by ICS, and cytokine release profiles were used to gauge TH1 versus TH2 bias. The paper should be consulted for data on the multiple individual subgroups. Here, we will summarize what was reported for the initial phase 3 trial regimen, a single dose of 5 × 1010 virus particles (Table 4). Moreover, although the two-dose groups are mentioned in the paper, no data were presented for the antibody and T-cell responses to the second dose. In addition, only a small subset of one-dose groups was included in several immunogenicity analyses. For the phase 3 regimen, the GM anti–S protein ELISA values on day 29 were 528 and 507 units for 15 of the younger and older volunteers, respectively. The corresponding NAb GM ID50 titers were 214 and 196, although some of the samples were said to need reassaying, and additional data from a PV NAb assay are reportedly pending. The weak T-cell response data show the expected TH1 bias. The ICS percentages of CD4+ and CD8+ T-cells expressing IFN-γ and IL-2 at day 15 for the phase 3 regimen were 0.08 and 0.07% for the younger adults, respectively, and 0.36 and 0.05% for the older group, respectively (with large confidence intervals). The T cell response rate varied depending on the subgroup and assay and ranged from 33 to 100%, although, given the low number of samples in many cases, the meaning of these data is not clear (18). Although the phase 3 trial of the one-dose regimen is still ongoing, Janssen has initiated a second efficacy trial, in this case involving two doses of 5 × 1010 virus particles given at weeks 0 and 8. As noted above, this regimen evoked stronger and more sustained antibody responses than a single dose when evaluated in rhesus macaques (5).
Several weeks after the Russian government approved the widespread use an adenovirus vector–based vaccine, Gam-COVID-Vac, a report appeared on how it had performed in phase 1/2 trials (28). The vaccine involves the sequential delivery of rAd5 and rAd26 vectors that each express a full-length S protein, which are given intramuscularly at 1 × 1011 particles per dose. Two subtrials, each involving 38 volunteers aged 18 to 60, compared frozen/thawed (Gam-COVID-Vac) or lyophilized/reconstituted (Gam-COVID-Vac-Lyo) vaccine formulations, which performed similarly (Gam-COVID-Vac was chosen for widespread use on convenience grounds). In the combination trial (n = 20), the first dose was rAd26 on day 0 followed by rAd5 on day 21. Smaller subgroups (n = 9) received only rAd5 or only rAd26. Phase 2 trials began a mere 5 days after phase 1 ended, based on a successful interim safety assessment. Safety studies over 42 days (maximum) revealed nothing other than the generally mild reactions reported in other Ad-vector studies. Immunogenicity assessments involved determining end-point titers in anti-RBD and anti–S1 IgG ELISAs and in an RV NAb assay, at weekly intervals. T-cell response data were derived from an IFN-γ ELISpot and CD4+ and CD8+ T cell proliferation assays. Anti-rAd5 antibodies were also measured to assess the possible influence of preexisting immunity. Anti-RBD end-point titers peaked at ~2000 by day 21 in the single-vaccine groups and were boosted to 10,000 to 15,000 in the combination vaccine groups by day 42. On day 42, the anti–S1 GM end-point titer in the phase 2 trial combination group was 53,006. NAb GM CPE67 titers at on day 28 in the single vaccine groups were in the range 5 to 10 but rose to values of 45.95 to 49.25 by day 42 in the combination groups. The authors themselves note that these titers are lower than those seen in the AstraZeneca/Oxford ChAdOx1 and mRNA vaccine trials, a difference to which the different measurements of NAb titers (ID50 versus CPE67) may contribute (see Table 4). Cell-mediated immunity was measured in a T-cell proliferation assay that is rarely used in the trials of the other vaccines reviewed here. Proliferative responses were detected in all participants but seem weak in magnitude and were not boosted by the second dose. On day 28, after the boosting immunization, median T-cell proliferation values were 2.5% versus 1.3% for CD4+ and 1.3% versus 1.1% for CD8+ cells in the groups receiving the frozen and lyophilized formulations, respectively. IFN-γ ELISpot data were presented only as fold increase from baseline values, and therefore cannot be compared with other studies. However, there was again no boosting effect of the second immunization. A phase 3 trial of the Gam-COVID-Vac combination vaccine began on 26 August and is planned to involve 40,000 volunteers of various ages and risk groups (28).
mRNA vaccines
The phase 1 trial of the Moderna mRNA-1273 vaccine involved 45 volunteers in three dosing groups who were given 25, 100, or 250 μg of the active vaccine by the intramuscular route on days 1 and 29 (19). Antibody immunogenicity was dose-dependent and much stronger after the second dose than the first. Anti–S protein GM end-point titers in the 100-μg group on day 57 (28 days after the second dose) were 782,000, while the corresponding anti-RBD end-point titers were ~30,000. Most of the NAb data were derived from a PV assay; on day 43, the GM ID50 titer for the 100-μg group was 344. An RV assay was also used on a subset of day 43 samples. The resulting ID80 titers were 654 for the 100-μg group. Note that these are not ID50 values, which would be higher numbers. No detailed data on the longevity of the antibody responses were reported, but inspection of the graphs suggests that the antibody titers are on a downward trend at the day 57 time point compared with days 36 and 43. T-cell responses were measured only by CFC, and no data on their magnitude were reported. For both vaccine dose groups, the peptide pools activated specific TH1 responses from <0.3% of the CD4+ T cells, and no TH2 responses were detectable. CD8+ T cell activity was, at most, minimal (19). In an extension of the trial that involved older adults (56 to 70 and over 71), the magnitudes of the anti–S protein, anti-RBD, and NAb responses to the two-dose regimens (25 or 100 μg) were similar to those reported for the 18 to 55 age groups (Table 4) (20). The 100-μg, two-dose regimen was chosen for the phase 3 studies that began in the United States during August 2020.
The Pfizer/BioNtech consortium has conducted three phase 1 trials of lipid nanoparticle–encapsulated mRNAs that eventually led to the selection of the clinical candidate for now ongoing phase 2/3 studies (25–27). In the first trial, the BNT162b1 mRNA expressing a soluble, trimerized version of the RBD was given at two doses (10 and 30 μg) on days 1 and 21 to groups of 12 participants and, once, at 100 μg on day 1 to a third group of 12. There were also nine placebo recipients (25). Immunogenicity was assessed by anti-RBD (25, 26) or anti-S1 (27) binding antibodies on days 7, 21, 28, and 35, although the data were reported in a nontraditional format that does not allow for cross-study comparison (25–27). All recipients in the two lower-dose groups developed anti-RBD antibodies by day 21 that were boosted 10- to 20-fold by the second immunization when measured on day 28 and unchanged by the end of the study on day 35. The 30-μg group was more immunogenic than 10 μg by ~3-fold. The pattern of the NAb data was similar, although fewer time points were studied. In all three groups, the NAb responses to the initial immunization were low but were boosted by the second dose. On day 28, the GM ID50 values in an RV assay for the 10- and 30-μg groups were 168 and 267, respectively (25).
A second phase 1 trial, conducted in Germany, also explored dosing regimens (26). Multiple doses of BNT162b1 mRNA, in the range 1 to 50 μg, were tested, as were single doses and a prime-boost protocol involving two doses on days 0 and 21. Overall, and as expected, the immunogenicity data were comparable to what was seen in the first trial. Higher immunogen doses and the prime-boost format were associated with stronger responses, as expected. The anti-RBD ELISA data were again presented in a nontraditional format. After the second dose, NAb ID50 GM titers in the higher-dose groups were 578 in an RV assay and ~3100 in a PV assay. In an additional analysis, selected sera were tested in the PV-NAb assay against RBD and S protein sequence variants (including the D614G change); no significant sensitivity differences were observed. T cell responses were measured by a modified ELISpot, in which either CD4+ or CD8+ T cells were depleted from the effector population, or by CFC. An unpublished “normalization” method was applied to enable direct comparison of spot counts/strength of response to anti-CD3 stimulation between individuals. Because PBMCs were separated into either CD4+ or CD8+ subpopulations in the ELISpot assay, no direct comparison can be made with ELISpot data on the other vaccines reviewed here because of differences in methodology. CD4+ and CD8+ T-cell responses were analyzed immediately before vaccination and then on day 29, i.e., 7 days after the booster immunization. The magnitudes of both T-cell responses were dose-dependent. At the highest dose, most participants had T-cell responses of >1500 SFCs per 106 cells. The magnitudes of the CD4+ and CD8+ responses were comparable. Approximately equal proportions of the CD4+ responders fell into groups with <500, 501 to 1500, and >1500 SFCs per 106 cells. Cytokine secretion profiles showed that CD4+ T cells producing only IL-2 were the most abundant subset, while IL-4 release was minimal. This pattern is of potential concern, as CD4+ cells secreting IL-2 can polarize CD4+ T cells toward the TH2 phenotype that may be associated with VAERD (60). The responding CD8+ T cells mostly produced IFN-γ (26).
The third Pfizer/BioNTech phase 1 trial compared the BNT162b1 RBD-based construct with BNT162b2, an mRNA expressing a full-length, membrane-anchored S protein (27). The two constructs were comparably immunogenic, but BNT162b2 was associated with lower reactogenicity levels. Accordingly, BNT162b2 at a 30-μg dose was selected to progress into phase 2/3 trials. The phase 1 trial had two principal subcomponents, involving adults aged 18 to 55 and 65 to 85. For each of the two mRNAs, three or four different doses (10, 20, 30, and, in one case, 100 μg of mRNA, together with placebo) were tested in a two-immunization protocol (days 0 and 21), so the 195 participants were split among 13 different groups in all. Here, we list the immunogenicity data only for the clinical candidate (BNT162b2, 30 μg), on day 28. NAb GM ID50 titers in an RV assay were 361 and 149 for the age groups 18 to 55 and 65 to 85, respectively, while the corresponding GM antibody end-point titers to the S1 protein in a Luminex assay were 9136 and 7985 (see also Table 4). Thus, for the clinical candidate, the NAb titers for the older group were 41% of those in their younger counterparts. Visual inspection of other antibody datasets suggests that the age-related reduction is generally 2- to 3-fold, a decline that is perhaps meaningful but not catastrophic. No longer-term antibody data and no information about T cell responses were presented (27). The BNT162b2 vaccine candidate is now in phase 3 trials in the United States and Europe, which involve a two-dose regimen.
The Curevac CVnCoV mRNA vaccine was tested in 231 German adults aged 18 to 60, who were given different vaccine doses (2 to 12 μg) twice, on days 0 and 28 (32). Moderate dose-dependent side effects were reported. Anti–S protein and anti-RBD median end-point titers on day 43 were moderately dose-dependent and highest for the 12-μg group (5463 and 1007, respectively). The median CPE50 NAb titer measured in an RV assay at this time was 113. No T-cell response data were presented. The 12-μg dose was chosen for a phase 2b/3 trial (32).
Recombinant protein vaccines
The first report on how a recombinant S protein performs in humans described the Novavax NVX-CoV2373 vaccine candidate (21). The immunogen is an insect cell–derived soluble S protein. When mixed with detergent, five or six S-protein molecules become attached noncovalently via their bases to the resulting micelles (63). This component of the immunogen was coadministered with Matrix-M adjuvant. Two formulations (5 and 25 μg of S protein) were tested in 106 people with or without adjuvant, in a two-dose regimen on days 0 and 21. In the absence of adjuvant, antibody responses were, as expected, very weak, while the 5- and 25-μg doses performed comparably when the adjuvant was present. Anti–S protein ELISA data were presented as EU, which again prevents cross-study comparison. The highest values recorded, on day 35, were 63,160. NAbs were measured in an RV assay and reported as ID>99 values. Here, the peak GM values were 3906 and 3305 for the 5- and 25-μg groups, respectively, on day 35. As noted above when discussing the corresponding macaque experiment, when NAb data are presented as ID>99 or CPE100 values, the reported numbers are likely to be several-fold lower than the more commonly used ID50 values. CFC was used to measure CD4+ T cell responses at days 0 and 28, but in only four participants per group. There were no responses in the placebo or protein with no-adjuvant recipients, but CD4+ T-cell signals could be measured in the adjuvanted protein groups at day 28, with two protein doses inducing similar but moderate responses. Both TH1 and TH2 cytokines were released although the TH1 signals were more consistent, particularly at the lower protein dose (21). A phase 3 trial of the Novavax vaccine began in the United Kingdom in September 2020, and its U.S. counterpart is scheduled to commence in December 2020.
The CoVLP vaccine from Medicago Inc. and McGill University is based on a stabilized S protein engrafted to an influenza hemagglutinin-transmembrane-cytoplasmic-tail region. These constructs, expressed in the plant cells, self-assemble into virus-like particles (VLPs) (31). In a phase 1 study, the VLPs were administered without adjuvant or in either AS03 (GSK) or CpG 1018 (Dynavax) adjuvants, on days 0 and 21, at doses of 3.75, 7.5, or 15 μg. The safety profile was unexceptional. Antibody responses were assessed by S protein ELISA and in RV and PV NAb assays. The responses in the no-adjuvant group were, as expected, weak. The three dosing groups behaved fairly similarly; the lowest-dose responses were at least as high as the others. The AS03 adjuvant consistently outperformed CpG 1018 10- to 50-fold in various groups at different times, which is a useful result with more general implications. On day 42, GM ED50 anti–S titers in the AS03 groups were ~300,000; NAb GM ID50 values were ~2200 in the PV assay and ~630 in the RV assay (responses after the first dose only were very much weaker). T cell responses were assessed by IFN-γ and IL-4 ELISpot assays and were again strongest in the AS03 groups (the differential versus CpG 1018 was greater for IFN-γ than IL-4). The highest IFN-γ signal (AS03, day 42) was ~500 SFU per million cells and ~400 for IL-4. The lowest-dose (3.75 μg) group with AS03 will proceed into additional clinical trials involving a two-immunizations regime (31).
Together, and with caveats about comparing data from different studies, two features of the binding antibody and NAb data stand out (Table 4). The seemingly strongest responses were induced by the NVX-CoV2373–adjuvanted recombinant S protein. This judgment takes into account the presentation of ID>99 values, rather than the more commonly used ID50 titers (21). The relationship between ID>99 and ID50 values depends on the shape of the titration curve, but ID>99 values are by necessity lower, often >10-fold (Fig. 3). The superior immunogenicity of the recombinant S protein mirrors its performance in macaques (Table 2) (9, 10). The second conclusion we can draw is that binding antibody and NAb responses to the single-dose adenovirus vector vaccines are quite weak, although a second dose does improve their performance, as judged by the limited dataset available (Table 4) (16–18, 23, 24). The T cell response data are too limited, and the protocols used are too variable, for us to draw any conclusions about relative immunogenicity.
VACCINE EFFICACY, AS REPORTED IN PRESS RELEASES
The press releases referred to below are all archived on the websites of the relevant institutions and should be consulted for additional details or the precise language used. The first indications of vaccine efficacy came from a Pfizer/BioNTech release issued on 9 November 2020. At that point, the companies had accumulated data from 94 COVID-19 cases during the BNT162b2 phase 3 trial in which 38,955 volunteers had been fully vaccinated (7 days after the second dose). Although there was no breakdown of the 94 cases by vaccine versus placebo, the efficacy level was stated to be “above 90%.” Two linked releases from the same group on 18 and 20 November 2020 provided additional information based on over 41,135 fully vaccinated trial participants. By then, the number of cases had reached 170 that were now broken down into 162 placebo recipients versus 8 given the vaccine, leading to an efficacy estimate of 95%. High-level efficacy was reported for all demographic groups, including adults over 65. In addition, it was announced that 10 severe COVID-19 cases had been documented, of which 9 were in the placebo group, an early although inconclusive indication that preventing severe disease might be possible.
Within about a day of the initial Pfizer/BioNTech release, one was issued by the Russian Direct Investment Fund and The Gamalaya National Center concerning their Sputnik V vaccine (11 November 2020). It reported that 16,000 trial participants had received both vaccine doses; with 20 confirmed symptomatic cases and a calculated efficacy of 92%. No vaccine versus placebo breakdown was provided. It was also stated that the number of COVID-19 cases identified in 10,000 more vaccine recipients who were not enrolled in clinical trials confirmed that vaccine efficacy was over 90%. On 24 November 2020, a second press announcement on Sputnik V reported vaccine efficacy of 91.4% based on 39 COVID-19 cases among 18,794 fully vaccinated volunteers. Of these cases, 8 occurred in 14,095 vaccinated volunteers, while 31 were in 4699 placebo recipients. The press release also mentioned that vaccine efficacy was over 95% when an analysis was performed at a later time point (21 days after the second dose, as opposed to 7 days), but no additional details were given.
Moderna issued its first press release on mRNA-1273 efficacy on 16 November 2020. In the COVE trial of over 30,000 volunteers, 95 symptomatic cases had been documented, of which 90 were in the placebo group. Vaccine efficacy was reported to be 94.5%. An additional analysis showed that all 11 cases of severe COVID-19 were in the placebo group. Consistent efficacy was seen in all demographic and age groups, although no details were reported. A further announcement on 30 November 2020 reported that 196 symptomatic cases had now accrued in the trial, 185 of them being in the placebo group. Vaccine efficacy was accordingly stated to be 94.1%. Furthermore, all 30 cases of severe COVID-19, including one death, were in the placebo group, which strengthens the evidence that this vaccine also prevents serious disease. This second press release again stated that efficacy was consistent across age, race, ethnicity, and gender demographics.
A press release was issued by AstraZeneca on 23 November 2020, reporting on the company’s AZD1222 (ChAdOx1) vaccine. The information it contained was based on two trials in the United Kingdom and Brazil and could be considered quite confusing (www.wired.com/story/the-astrazeneca-covid-vaccine-data-isnt-up-to-snuff/). Some clarifications emerged in subsequent media reporting and oral statements. The summary below reflects what is known at the time of writing. In total, 131 COVID-19 cases occurred in the trials, but their distribution was not broken down either by trial or vaccine versus placebo. One dosing regimen involving 2,741 U.K. volunteers was stated to confer 90% efficacy. It involved a half dose of vaccine followed by a full dose at least 1 month later, a regimen that was the result of an apparent error in dose calculations. In contrast, the trials involving the originally intended protocol of two full doses yielded an efficacy of 62% from 8895 vaccinated volunteers. Combining all the trials, which may or may not be appropriate for regulatory approval, led to a stated “average efficacy” of 70%. It was also reported that no hospitalizations or severe cases of COVID-19 occurred among vaccine recipients, which is presumably a reference only to the unstated number who became infected. Additional data from an ongoing phase 3 trial in the United States and from a new one that may be initiated in the United Kingdom may eventually clarify where this vaccine stands on the efficacy spectrum.
Although the press releases summarized above are sometimes confusing and generally contain less information than is desirable, together they imply that high-level protection against symptomatic SARS-CoV-2 infection can be achieved by vaccination. Moreover, there are early indications that vaccines will also be able to protect against severe COVID-19 disease.
Whether all the first-generation vaccines can protect at efficacy levels of around 90% will require more data to answer, including from phase 3 trials that are yet to report end points. It should also be noted that differences in apparent efficacy may, at least in part, depend on how symptomatic infections are documented in the different trials, which seems to vary. In some trials (e.g., Pfizer/BioNTech and Moderna), infected people are identified when they report to trial sites with symptoms and are RNA-tested, and in another (AstraZeneca), participants are RNA-tested weekly and asked about symptoms. Whether these end points are equivalent is not yet clear. It is also unknown whether the vaccine protects against asymptomatic infection, which is important if asymptomatic infected vaccine recipients are still capable of transmitting virus to other people. While this scenario cannot be dismissed, we think that it is perhaps unlikely to be common. As we discussed above, several of the vaccines provide what seems to be sterilizing immunity to at least some of the immunized macaques, which represents protection against asymptomatic infection. While extrapolation from NHPs to humans is also fraught with uncertainties, we are now seeing solid signs of protection in both species that increase the weight that can be put on the monkey models.
We await information about CoPs for a human SARS-CoV-2 vaccine, although it does seem increasingly likely that they are antibody-associated. Although serum NAb titers may well be the principal CoP, we do not yet know what a highly protective minimum NAb titer will be. In an earlier review, we suggested that NAb titers in the low hundreds might be sufficient for protection (34). In one study, when 122 Seattle seamen sailed seaward, 104 of them had become SARS-CoV-2 RNA–positive from a single source of virus by the time their boat returned to port 18 days later. However, three sailors who had NAb responses at the time of departure were not reinfected while at sea. Their serum PV ID50 NAb titers were 174, 161, and 3082 (71). Thus, while certainly far from definitive, this study further suggests that a protective serum NAb titer may lie in the low hundreds. The peak NAb responses to many, but not all, of the leading vaccines exceed that mark (Table 4). Once completed and fully analyzed, the ongoing efficacy trials should yield information about the threshold NAb titers giving certain percentages of protection from infection or disease. Again, however, the use of NAb assays with different properties and sensitivities is likely to blur comparisons of the datasets generated in the various trials (34, 51). Now that robust, reliable, and potentially high-throughput assays are available, perhaps some of these uncertainties can be resolved in centralized testing programs under national or even international coordination (72, 73).
One caveat about the press releases is that all the efficacy data so far are derived from analyses conducted within the first few weeks to months after completion of the vaccination protocol. The duration of vaccine-induced immunity is unknown. We also know little about the role of T cells in protection, and almost no information is available about mucosal immunity or immune memory. Memory B and T cells will be relevant to sustained protection by the vaccines. Significant and highly encouraging information is now emerging on the extent of immunological memory and the maintenance of NAb responses after ~6 months of SARS-CoV-2 infection (54, 55). Similar studies will need to be performed on vaccine recipients after sufficient time has elapsed. The magnitude of the initial NAb responses to the vaccines may be relevant in this context. Plasma antibody titers can be expected to diminish over time, and, at some point, they may drop below a protective threshold. The stronger the initial peak response, the longer that process will take. Time will tell. Nonetheless, it is hard to argue against the long-term benefits of a strong initial NAb response when it comes to conferring protection during the first year after vaccination, which may be particularly critical in curtailing the COVID-19 pandemic.
Next-generation immunogens now at the preclinical stage of development may play a role if some of the leading vaccines are less effective than is hoped (34, 35, 45). Combining vaccines in prime-boost formats may provide superior and longer lasting protection than single agents (34). Nonetheless, there are now highly encouraging signs for the control of the pandemic if and when multiple SARS-CoV-2 vaccines can be rolled out en masse during 2021.
Supplementary Material
Acknowledgments
We are grateful to K. Guemo for administrative support and to N. Dean for helpful comments on the press releases summarized here. Funding: This work was supported by the National Institute of Allergy and Infectious Diseases of the NIH (HIVRAD P01 AI110657 and R01 AI36082) and the Bill and Melinda Gates Foundation (OPP1132237 and INV-002022). Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/sciadv.abe8065/DC1
REFERENCES AND NOTES
- 1.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z., Gao H., Ge X., Kan B., Hu Y., Liu J., Cai F., Jiang D., Yin Y., Qin C., Li J., Gong X., Lou X., Shi W., Wu D., Zhang H., Zhu L., Deng W., Li Y., Lu J., Li C., Wang X., Yin W., Zhang Y., Qin C., Rapid development of an inactivated vaccine candidate for SARS-CoV-2. Science 369, 77–81 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., Xu W., Zhao Y., Li N., Zhang J., Liang H., Bao L., Xu Y., Ding L., Zhou W., Gao H., Liu J., Niu P., Zhao L., Zhen W., Fu H., Yu S., Zhang Z., Xu G., Li C., Lou Z., Xu M., Qin C., Wu G., Gao G. F., Tan W., Yang X., Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell 182, 713–721.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J. N., Port J. R., Avanzato V. A., Bushmaker T., Flaxman A., Ulaszewska M., Feldmann F., Allen E. R., Sharpe H., Schulz J., Holbrook M., Okumura A., Meade-White K., Pérez-Pérez L., Edwards N. J., Wright D., Bissett C., Gilbride C., Williamson B. N., Rosenke R., Long D., Ishwarbhai A., Kailath R., Rose L., Morris S., Powers C., Lovaglio J., Hanley P. W., Scott D., Saturday G., de Wit E., Gilbert S. C., Munster V. J., ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 586, 578–582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercado N. B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., Mahan K. M., Tostanoski L. H., He X., Martinez D. R., Rutten L., Bos R., van Manen D., Vellinga J., Custers J., Langedijk J. P., Kwaks T., Bakkers M. J. G., Zuijdgeest D., Rosendahl Huber S. K., Atyeo C., Fischinger S., Burke J. S., Feldman J., Hauser B. M., Caradonna T. M., Bondzie E. A., Dagotto G., Gebre M. S., Hoffman E., Jacob-Dolan C., Kirilova M., Li Z., Lin Z., Mahrokhian S. H., Maxfield L. F., Nampanya F., Nityanandam R., Nkolola J. P., Patel S., Ventura J. D., Verrington K., Wan H., Pessaint L., Van Ry A., Blade K., Strasbaugh A., Cabus M., Brown R., Cook A., Zouantchangadou S., Teow E., Andersen H., Lewis M. G., Cai Y., Chen B., Schmidt A. G., Reeves R. K., Baric R. S., Lauffenburger D. A., Alter G., Stoffels P., Mammen M., Van Hoof J., Schuitemaker H., Barouch D. H., Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 586, 583–588 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.L. Solforosi, H. Kuipers, S. K. Rosendahl Huber, J. E. M. van der Lubbe, L. Dekking, D. N. Czapska-Casey, A. Izquierdo Gil, M. R. M. Baert, J. Drijver, J. Vaneman, E. van Huizen, Y. Choi, J. Vreugdenhil, T. J. Dalebout, S. K. Myeni, M. Kikkert, E. J. Snijder, D. H. Barouch, G. Koopman, P. Mooij, W. M. J. M. Bogers, L. Muchene, J. T. B. M. Tolboom, R. Roozendaal, H. Schuitemaker, F. Wegmann, R. C. Zahn, Immunogenicity of one- and two-dose regimens of the Ad26.COV2.S COVID-19 vaccine candidate in adult and aged rhesus macaques. bioRxiv 2020.11.17.368258 [Preprint]. 17 November 2020. 10.1101/2020.11.17.368258. [DOI]
- 6.Yu J., Tostanoski L. H., Peter L., Mercado N. B., Mahan K. M., Mahrokhian S. H., Nkolola J. P., Liu J., Li Z., Chandrashekar A., Martinez D. R., Loos C., Atyeo C., Fischinger S., Burke J. S., Slein M. D., Chen Y., Zuiani A., Lelis F. J. N., Travers M., Habibi S., Pessaint L., Van Ry A., Blade K., Brown R., Cook A., Finneyfrock B., Dodson A., Teow E., Velasco J., Zahn R., Wegmann F., Bondzie E. A., Dagotto G., Gebre M. S., He X., Jacob-Dolan C., Kirilova M., Kordana N., Lin Z., Maxfield L. F., Nampanya F., Nityanandam R., Ventura J. D., Wan H., Cai Y., Chen B., Schmidt A. G., Wesemann D. R., Baric R. S., Alter G., Andersen H., Lewis M. G., Barouch D. H., DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 369, 806–811 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A. Patel, J. Walters, E. L. Reuschel, K. Schultheis, E. Parzych, E. N. Gary, I. Maricic, M. Purwar, Z. Eblimit, S. N. Walker, D. Guimet, P. Bhojnagarwala, A. Doan, Z. Xu, D. Elwood, S. M. Reeder, L. Pessaint, K. Y. Kim, A. Cook, N. Chokkalingam, B. Finneyfrock, E. Tello-Ruiz, A. Dodson, J. Choi, A. Generotti, J. Harrison, N. J. Tursi, V. M. Andrade, Y. Dia, F. T. Zaidi, H. Andersen, H. G. Lewis, K. Muthumani, J. J. Kim, D. W. Kulp, L. M. Humeau, S. Ramos, T. R. F. Smith, D. B. Weiner, K. E. Broderick, Intradermal-delivered DNA vaccine provides anamnestic protection in a rhesus macaque SARS-CoV-2 challenge model. bioRxiv 10.1101/2020.07.28.225649 [Preprint]. 29 July 2020. 10.1101/2020.07.28.225649. [DOI]
- 8.Corbett K. S., Flynn B., Foulds K. E., Francica J. R., Boyoglu-Barnum S., Werner A. P., Flach B., O’Connell S., Bock K. W., Minai M., Nagata B. M., Andersen H., Martinez D. R., Noe A. T., Douek N., Donaldson M. M., Nji N. N., Alvarado G. S., Edwards D. K., Flebbe D. R., Lamb E., Doria-Rose N. A., Lin B. C., Louder M. K., O’Dell S., Schmidt S. D., Phung E., Chang L. A., Yap C., Todd J.-P. M., Pessaint L., Van Ry A., Browne S., Greenhouse J., Putman-Taylor T., Strasbaugh A., Campbell T.-A., Cook A., Dodson A., Steingrebe K., Shi W., Zhang Y., Abiona O. M., Wang L., Pegu A., Yang E. S., Leung K., Zhou T., Teng I.-T., Widge A., Gordon I., Novik L., Gillespie R. A., Loomis R. J., Moliva J. I., Stewart-Jones G., Himansu S., Kong W.-P., Nason M. C., Morabito K. M., Ruckwardt T. J., Ledgerwood J. E., Gaudinski M. R., Kwong P. D., Mascola J. R., Carfi A., Lewis M. G., Baric R. S., Dermott A. M., Moore I. N., Sullivan N. J., Roederer M., Seder R. A., Graham B. S., Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 383, 1544–1555 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.J. H. Tian, N. Patel, R. Haupt, H. Zhou, S. Weston, H. Hammond, J. Lague, A. D. Portnoff, J. Norton, M. Guebre-Xabier, B. Zhou, K. Jacobson, S. Maciejewski, R. Khatoon, M. Wisniewska, W. Moffitt, S. Kluepfel-Stahl, B. Ekechukwu, J. Papin, S. Boddapati, C. J. Wong, P. A. Piedra, M. B. Frieman, M. J. Massare, L. Fries, K. L. Bengtsson, L. Stertman, L. Ellingsworth, G. Glenn, G. Smith, SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 elicits immunogenicity in baboons and protection in mice. bioRxiv 10.1101/2020.06.29.178509 [Preprint]. 30 June 2020. 10.1101/2020.06.29.178509. [DOI]
- 10.M. Guebre-Xabier, N. Patel, J.-H. Tian, B. Zhou, S. Maciejewski, K. Lam, A. D. Portnoff, M. J. Massare, M. B. Frieman, P. A. Piedra, L. Ellingsworth, G. Glenn, G. Smith, NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge. bioRxiv 10.1101/2020.08.18.256578 [Preprint]. 19 August 2020. 10.1101/2020.08.18.256578. [DOI] [PMC free article] [PubMed]
- 11.A. Vogel, I. Kanevsky, Y. Che, K. Swanson, A. Muik, M. Vormehr, L. Kranz, K. Walzer, S. Hein, A. Gueler, J. Loschko, M. Maddur, K. Tompkins, J. Cole, B. G. Lui, T. Ziegenhals, A. Plaschke, D. Eisel, S. Dany, S. Fesser, S. Erbar, F. Bates, D. Schneider, B. Jesionek, B. Saenger, A. K. Wallisch, Y. Feuchter, H. Junginger, S. Krumm, A. Heinen, P. Adams-Quack, J. Schlereth, C. Kroener, S. Hall-Ursone, K. Brasky, M. C. Griffor, S. Han, J. Lees, E. Mashalidis, P. Sahasrabudhe, C. Tan, D. Pavliakova, G. Singh, C. Fontes-Garfias, M. Pride, I. Scully, T. Ciolino, J. Obregon, M. Gazi, R. Carrion, K. Alfson, W. Kalina, D. Kaushal, P. Y. Shi, T. Klamp, C. Rosenbaum, A. Kuhn, O. Tuereci, P. Dormitzer, K. Jansen, U. Sahin, A prefusion SARS-CoV-2 spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates. bioRxiv 10.1101/2020.09.08.280818 [Preprint]. 8 September 2020. 10.1101/2020.09.08.280818. [DOI]
- 12.J. G. Liang, D. Su, T.-Z. Song, Y. Zeng, W. Huang, J. Wu, R. Xu, P. Luo, X. Yang, X. Zhang, S. Luo, Y. Liang, X. Li, J. Huang, Q. Wang, X. Huang, Q. Xu, M. Luo, A. Huang, D. Luo, C. Zhao, F. Yang, J.-B. Han, Y.-T. Zheng, P. Liang, S-Trimer, A COVID-19 subunit vaccine candidate, induces protective immunity in nonhuman primates. bioRxiv 10.1101/2020.09.24.311027 [Preprint]. 24 September 2020. 10.1101/2020.09.24.311027. [DOI]
- 13.K. V. Kalnin, T. Plitnik, M. Kishko, J. Zhang, D. Zhang, A. Beauvais, N. G. Anosova, T. Tibbitts, J. M. DiNapoli, P.-W. D. Huang, J. Huleatt, D. Vincent, K. Fries, S. Karve, R. Goldman, H. Gopani, A. Dias, K. Tran, M. Zacharia, X. Gu, L. Boeglin, S. Chivukula, R. Swearingen, V. Landolfi, T.-M. Fu, F. DeRosa, D. Casimiro, Immunogenicity of novel mRNA COVID-19 vaccine MRT5500 in mice and non-human primates. bioRxiv 10.1101/2020.10.14.337535 [Preprint]. 14 October 2020. 10.1101/2020.10.14.337535. [DOI]
- 14.Y.-J. Zhang, G. Zeng, H.-X. Pan, C.-G. Li, B. Kan, Y.-L. Hu, H.-Y. Mao, Q.-Q. Xin, K. Chu, W.-X. Han, Z. Chen, R. Tang, W.-D. Yin, X. Chen, X.-J. Gong, C. Qin, Y.-S. Hu, X.-Y. Liu, G.-L. Cui, C.-B. Jiang, H.-M. Zhang, J.-X. Li, M.-N. Yang, X.-J. Lian, Y. Song, J.-X. Lu, X.-X. Wang, M. Xu, Q. Gao, F.-C. Zhu, Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: Report of the randomized, double-blind, and placebo-controlled phase 2 clinical trial. medRxiv 10.1101/2020.07.31.20161216 [Preprint]. 10 August 2020. 10.1101/2020.07.31.20161216. [DOI]
- 15.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G. F., Tan W., Wu G., Xu M., Lou Z., Huang W., Xu W., Huang B., Wang H., Wang W., Zhang W., Li N., Xie Z., Ding L., You W., Zhao Y., Yang X., Liu Y., Wang Q., Huang L., Yang Y., Xu G., Luo B., Wang W., Liu P., Guo W., Yang X., Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 21, 39–51 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folegatti P. M., Ewer K. J., Aley P. K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E. A., Dold C., Faust S. N., Finn A., Flaxman A. L., Hallis B., Heath P., Jenkin D., Lazarus R., Makinson R., Minassian A. M., Pollock K. M., Ramasamy M., Robinson H., Snape M., Tarrant R., Voysey M., Green C., Douglas A. D., Hill A. V. S., Lambe T., Gilbert S. C., Pollard A. J.; Oxford COVID Vaccine Trial Group , Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396, 467–478 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramasamy M. N., Minassian A. M., Ewer K. J., Flaxman A. L., Folegatti P. M., Owens D. R., Voysey M., Aley P. K., Angus B., Babbage G., Belij-Rammerstorfer S., Berry L., Bibi S., Bittaye M., Cathie K., Chappell H., Charlton S., Cicconi P., Clutterbuck E. A., Colin-Jones R., Dold C., Emary K. R. W., Fedosyuk S., Fuskova M., Gbesemete D., Green C., Hallis B., Hou M. M., Jenkin D., Joe C. C. D., Kelly E. J., Kerridge S., Lawrie A. M., Lelliott A., Lwin M. N., Makinson R., Marchevsky N. G., Mujadidi Y., Munro A. P. S., Pacurar M., Plested E., Rand J., Rawlinson T., Rhead S., Robinson H., Ritchie A. J., Ross-Russell A. L., Saich S., Singh N., Smith C. C., Snape M. D., Song R., Tarrant R., Themistocleous Y., Thomas K. M., Villafana T. L., Warren S. C., Watson M. E. E., Douglas A. D., Hill A. V. S., Lambe T., Gilbert S. C., Faust S. N., Pollard A. J.; Oxford COVID Vaccine Trial Group , Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 396, 1979–1993 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.J. Sadoff, M. Le Gars, G. Shukarev, D. Heerwegh, C. Truyers, A. M. de Groot, J. Stoop, S. Tete, W. Van Damme, I. Leroux-Roels, P.-J. Berghmans, M. Kimmel, P. Van Damme, J. De Hoon, W. Smith, K. Stephenson, D. Barouch, S. De Rosa, K. Cohen, J. McElrath, E. Cormier, G. Scheper, J. Hendriks, F. Struyf, M. Douoguih, J. Van Hoof, H. Schuitemaker, Safety and immunogenicity of the Ad26.COV2.S COVID-19 vaccine candidate: Interim results of a phase 1/2a, double-blind, randomized, placebo-controlled trial. medRxiv 2020.09.23.20199604 [Preprint]. 25 September 2020. 10.1101/2020.09.23.20199604. [DOI]
- 19.Jackson L. A., Anderson E. J., Rouphael N. G., Roberts P. C., Makhene M., Coler R. N., McCullough M. P., Chappell J. D., Denison M. R., Stevens L. J., Pruijssers A. J., McDermott A., Flach B., Doria-Rose N. A., Corbett K. S., Morabito K. M., O’Dell S., Schmidt S. D., Swanson P. A. II, Padilla M., Mascola J. R., Neuzil K. M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J. E., Graham B. S., Beigel J. H.; mRNA-1273 Study Group , An mRNA vaccine against SARS-CoV-2 –– Preliminary report. N. Engl. J. Med. 383, 1920–1931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson E. J., Rouphael N. G., Widge A. T., Jackson L. A., Roberts P. C., Makhene M., Chappell J. D., Denison M. R., Stevens L. J., Pruijssers A. J., McDermott A. B., Flach B., Lin B. C., Doria-Rose N. A., O’Dell S., Schmidt S. D., Corbett K. S., Swanson P. A. II, Padilla M., Neuzil K. M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V. V., Floyd K., Suthar M. S., Martinez D. R., Baric R., Buchanan W., Luke C. J., Phadke V. K., Rostad C. A., Ledgerwood J. E., Graham B. S., Beigel J. H.; mRNA-1273 Study Group , Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 383, 2427–2438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., Plested J. S., Zhu M., Cloney-Clark S., Zhou H., Smith G., Patel N., Frieman M. B., Haupt R. E., Logue J., McGrath M., Weston S., Piedra P. A., Desai C., Callahan K., Lewis M., Price-Abbott P., Formica N., Shinde V., Fries L., Lickliter J. D., Griffin P., Wilkinson B., Glenn G. M., Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 383, 2320–2332 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., Li X., Peng C., Zhang Y., Zhang W., Yang Y., Chen W., Gao X., You W., Wang X., Wang Z., Shi Z., Wang Y., Yang X., Zhang L., Huang L., Wang Q., Yang J. L. Y., Guo J., Zhou W., Wan X., Wu C., Wang W., Huang S., Du J., Meng Z., Pan A., Yuan Z., Shen S., Guo W., Yang X., Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: Interim analysis of 2 randomized clinical trials. JAMA 324, 951–960 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu F. C., Li Y. H., Guan X. H., Hou L. H., Wang W. J., Li J. X., Wu S. P., Wang B. S., Wang Z., Wang L., Jia S. Y., Jiang H. D., Wang L., Jiang T., Hu Y., Gou J. B., Xu S. B., Xu J. J., Wang X. W., Wang W., Chen W., Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 395, 1845–1854 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu F. C., Guan X. H., Li Y. H., Huang J. Y., Jiang T., Hou L. H., Li J. X., Yang B. F., Wang L., Wang W. J., Wu S. P., Wang Z., Wu X. H., Xu J. J., Zhang Z., Jia S. Y., Wang B. S., Hu Y., Liu J. J., Zhang J., Qian X. A., Li Q., Pan H. X., Jiang H. D., Deng P., Gou J. B., Wang X. W., Wang X. H., Chen W., Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 396, 479–488 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulligan M., Lyke K., Kitchin N., Absalon J., Gurtman A., Lockhart S. P., Neuzil K., Raabe V., Bailey R., Swanson K. A., Li P., Koury K., Kalina W., Cooper D., Fonter-Garfias C., Shi P.-Y., Tuereci O., Tompkins K. R., Walsh E. E., Frenck R., Falsey A. R., Dormitzer P. R., Gruber W. C., Sahin U. C., Jansen K. U., Phase 1/2 study to describe the safety and immunogenicity of a COVID-19 RNA vaccine candidate (BNT162b1) in adults 18 to 55 years of age: Interim report. Nature 586, 589–593 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L. M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., Brachtendorf S., Lörks V., Sikorski J., Hilker R., Becker D., Eller A.-K., Grützner J., Boesler C., Rosenbaum C., Kühnle M.-C., Luxemburger U., Kemmer-Brück A., Langer D., Bexon M., Bolte S., Karikó K., Palanche T., Fischer B., Schultz A., Shi P.-Y., Fontes-Garfias C., Perez J. L., Swanson K. A., Loschko J., Scully I. L., Cutler M., Kalina W., Kyratsous C. A., Cooper D., Dormitzer P. R., Jansen K. U., Türeci Ö., COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586, 594–599 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Walsh E. E., Frenck R. W. Jr., Falsey A. R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M. J., Bailey R., Swanson K. A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K. R., Lyke K. E., Raabe V., Dormitzer P. R., Jansen K. U., Şahin U., Gruber W. C., Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 383, 2439–2450 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logunov D. Y., Dolzhikova I. V., Zubkova O. V., Tukhvatullin A. I., Shcheblyakov D. V., Dzharullaeva A. S., Grousova D. M., Erokhova A. S., Kovyrshina A. V., Botikov A. G., Izhaeva F. M., Popova O., Ozharovskaya T. A., Esmagambetov I. B., Favorskaya I. A., Zrelkin D. I., Voronina D. V., Shcherbinin D. N., Semikhin A. S., Simakova Y. V., Tokarskaya E. A., Lubenets N. L., Egorova D. A., Shmarov M. M., Nikitenko N. A., Morozova L. F., Smolyarchuk E. A., Kryukov E. V., Babira V. F., Borisevich S. V., Naroditsky B. S., Gintsburg A. L., Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 396, 887–897 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.J. Pu, Q. Yu, Z. Yin, Y. Zhang, X. Li, D. Li, H. Chen, R. Long, Z. Zhao, T. Mou, H. Zhao, S. Feng, Z. Xie, L. Wang, Z. He, Y. Liao, S. Fan, Q. Yin, R. Jiang, J. Wang, L. Zhang, J. Li, H. Zheng, P. Cui, G. Jiang, L. Guo, M. Xu, H. Yang, S. Lu, X. Wang, Y. Gao, X. Xu, L. Cai, J. Zhou, L. Yu, Z. Chen, C. Hong, D. Du, H. Zhao, Y. Li, K. Ma, Y. Ma, D. Liu, S. Yao, C. Li, Y. Che, L. Liu, Q. Li, An in-depth investigation of the safety and immunogenicity of an inactivated SARS-CoV-2 vaccine. medRxiv 10.1101/2020.09.27.20189548 [Preprint]. 6 October 2020. 10.1101/2020.09.27.20189548. [DOI]
- 30.Che Y., Liu X., Pu Y., Zhou M., Zhao Z., Jiang R., Yin Z., Xu M., Yin Q., Wang J., Pu J., Zhao H., Zhang Y., Wang L., Jiang Y., Lei J., Zheng Y., Liao Y., Long R., Yu L., Cui P., Yang H., Li J., Chen W., He Z., Ma K., Hong C., Li D., Jiang G., Liu D., Xu X., Fan S., Cheng C., Yang J., Li Y., Zou Y., Zhu Y., Zhou Y., Guo Y., Yang T., Chen H., Xie Z., Li C., Li Q., Randomized, double-blinded and placebo-controlled phase II trial of an inactivated SARS-CoV-2 vaccine in healthy adults. Clin. Infect. Dis. 9, ciaa1703 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.B. J. Ward, P. Gobeil, A. Séguin, J. Atkins, I. Boulay, P.-Y. Charbonneau, M. Couture, M.-A. D’Aoust, J. Dhaliwall, C. Finkle, K. Hager, A. Mahmood, A. Makarkov, M. Cheng, S. Pillet, P. Schimke, S. St-Martin, S. Trépanier, N. Landry, Phase 1 trial of a candidate recombinant virus-like particle vaccine for Covid-19 disease produced in plants. medRxiv 2020.11.04.20226282 [Preprint]. 6 November 2020. 10.1101/2020.11.04.20226282. [DOI]
- 32.P. Kremsner, P. Mann, J. Bosch, R. Fendel, J. J. Gabor, A. Kreidenweiss, A. Kroidl, I. Leroux-Roels, G. Leroux-Roels, C. Schindler, M. Schunk, T. P. Velavan, M. Fotin-Mleczek, S. Müller, G. Quintini, O. Schönborn-Kellenberger, D. Vahrenhorst, T. Verstraeten, L. Walz, O.-O. Wolz, L. Oostvogels, Phase 1 Assessment of the safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2 in human volunteers. medRxiv 2020.11.09.20228551 [Preprint]. 9 November 2020. 10.1101/2020.11.09.20228551. [DOI]
- 33.Slaoui M., Hepburn M., Developing safe and effective Covid vaccines — Operation Warp Speed’s strategy and approach. N. Engl. J. Med. 383, 1701–1703 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Moore J. P., Klasse P. J., COVID-19 vaccines: “Warp Speed” needs mind melds, not warped minds. J. Virol. 94, e01083–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krammer F., SARS-CoV-2 vaccines in development. Nature 586, 516–527 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Korber B., Fischer W. M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E. E., Bhattacharya T., Foley B., Hastie K. M., Parker M. D., Partridge D. G., Evans C. M., Freeman T. M., de Silva T. I.; Sheffield COVID-19 Genomics Group, McDanal C., Perez L. G., Tang H., Moon-Walker A., Whelan S. P., LaBranche C. C., Saphire E. O., Montefiori D. C., Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell 182, 812–827.e19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Y. J. Hou, S. Chiba, P. Halfmann, C. Ehre, M. Kuroda, K. H. Dinnon, S. R. Leist, A. Schäfer, N. Nakajima, K. Takahashi, R. E. Lee, T. M. Mascenik, C. E. Edwards, L. V. Tse, R. C. Boucher, S. H. Randell, T. Suzuki, L. E. Gralinski, Y. Kawaoka, R. S. Baric, SARS-CoV-2 D614G variant exhibits enhanced replication. bioRxiv 2020.09.28.317685 [Preprint]. 29 September 2020. 10.1101/2020.09.28.317685. [DOI]
- 38.L. Yurkovetskiy, X. Wang, K. E. Pascal, C. Tomkins-Tinch, T. Nyalile, Y. Wang, A. Baum, W. E. Diehl, A. Dauphin, C. Carbone, K. Veinotte, S. B. Egri, S. F. Schaffner, J. E. Lemieux, J. Munro, A. Rafique, A. Barve, P. C. Sabeti, C. A. Kyratsous, N. Dudkina, K. Shen, J. Luban, SARS-CoV-2 Spike protein variant D614G increases infectivity and retains sensitivity to antibodies that target the receptor binding domain. bioRxiv 2020.07.04.187757 [Preprint]. 16 July 2020. 10.1101/2020.07.04.187757. [DOI]
- 39.Hsieh C.-L., Goldsmith J. A., Schaub J. M., DiVenere A. M., Kuo H. C., Javanmardi K., Le K. C., Wrapp D., Lee A. G., Liu Y., Chou C. W., Byrne P. O., Hjorth C. K., Johnson N. V., Meyers L.-J., Nguyen A. W., Park J., Wang N., Amengor D., Maynard J. A., Finkelstein I. J., McLellan J. S., Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science 369, 1501–1505 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLellan J. S., Chen M., Joyce M. G., Sastry M., Stewart-Jones G. B., Yang Y., Zhang B., Chen L., Srivatsan S., Zheng A., Zhou T., Graepel K. W., Kumar A., Moin S., Boyington J. C., Chuang G. Y., Soto C., Baxa U., Bakker A. Q., Spits H., Beaumont T., Zheng Z., Xia N., Ko S. Y., Todd J. P., Rao S., Graham B. S., Kwong P. D., Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342, 592–598 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadoff J., De Paepe E., Haazen W., Omoruyi E., Bastian A. R., Comeaux C., Heijnen E., Strout C., Schuitemaker H., Callendret B., Safety and immunogenicity of the Ad26.RSV.preF investigational vaccine coadministered with an Influenza vaccine in older adults. J. Infect. Dis., jiaa409 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Bos R., Rutten L., van der Lubbe J. E. M., Bakkers M. J. G., Hardenberg G., Wegmann F., Zuijdgeest D., de Wilde A. H., Koornneef A., Verwilligen A., van Manen D., Kwaks T., Vogels R., Dalebout T. J., Myeni S. K., Kikkert M., Snijder E. J., Li Z., Barouch D. H., Vellinga J., Langedijk J. P. M., Zahn R. C., Custers J., Schuitemaker H., Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 5, 91 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.F. Amanat, S. Strohmeier, R. Rathnasinghe, M. Schotsaert, L. Coughlan, A. García-Sastre, F. Krammer, Introduction of two prolines and removal of the polybasic cleavage site leads to optimal efficacy of a recombinant spike based SARS-CoV-2 vaccine in the mouse model. bioRxiv 2020.09.16.300970 [Preprint]. 18 September 2020. 10.1101/2020.09.16.300970. [DOI] [PMC free article] [PubMed]
- 44.Buchbinder S., McElrath M. J., Dieffenbach C., Corey L., Use of adenovirus type-5 vectored vaccines: A cautionary tale. Lancet 395, e68–e69 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amanat F., Krammer F., SARS-CoV-2 vaccines: Status report. Immunity 52, 583–589 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith T. R. F., Patel A., Ramos S., Elwood D., Zhu X., Yan J., Gary E. N., Walker S. N., Schultheis K., Purwar M., Xu Z., Walters J., Bhojnagarwala P., Yang M., Chokkalingam N., Pezzoli P., Parzych E., Reuschel E. L., Doan A., Tursi N., Vasquez M., Choi J., Tello-Ruiz E., Maricic I., Bah M. A., Wu Y., Amante D., Park D. H., Dia Y., Ali A. R., Zaidi F. I., Generotti A., Kim K. Y., Herring T. A., Reeder S., Andrade V. M., Buttigieg K., Zhao G., Wu J. M., Li D., Bao L., Liu J., Deng W., Qin C., Brown A. S., Khoshnejad M., Wang N., Chu J., Wrapp D., McLellan J., Muthumani K., Wang B., Carroll M. W., Kim J. J., Boyer J., Kulp D. W., Humeau L., Weiner D. B., Broderick K. E., Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 11, 2601 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corbett K. S., Edwards D. K., Leist S. R., Abiona O. M., Boyoglu-Barnum S., Gillespie R. A., Himansu S., Schäfer A., Ziwawo C. T., DiPiazza A. T., Dinnon K. H., Elbashir S. M., Shaw C. A., Woods A., Fritch E. J., Martinez D. R., Bock K. W., Minai M., Nagata B. M., Hutchinson G. B., Wu K., Henry C., Bahi K., Garcia-Dominguez D., Ma L., Renzi I., Kong W. P., Schmidt S. D., Wang L., Zhang Y., Phung E., Chang L. A., Loomis R. J., Altaras N. E., Narayanan E., Metkar M., Presnyak V., Liu C., Louder M. K., Shi W., Leung K., Yang E. S., West A., Gully K. L., Stevens L. J., Wang N., Wrapp D., Doria-Rose N. A., Stewart-Jones G., Bennett H., Alvarado G. S., Nason M. C., Ruckwardt T. J., McLellan J. S., Denison M. R., Chappell J. D., Moore I. N., Morabito K. M., Mascola J. R., Baric R. S., Carfi A., Graham B. S., SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586, 567–571 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham S. P., McLean R. K., Spencer A. J., Belij-Rammerstorfer S., Wright D., Ulaszewska M., Edwards J. C., Hayes J. W. P., Martini V., Thakur N., Conceicao C., Dietrich I., Shelton H., Waters R., Ludi A., Wilsden G., Browning C., Bialy D., Bhat S., Stevenson-Leggett P., Hollinghurst P., Gilbride C., Pulido D., Moffat K., Sharpe H., Allen E., Mioulet V., Chiu C., Newman J., Asfor A. S., Burman S., Crossley S., Huo J., Owens R. J., Carroll M., Hammond J. A., Tchilian E., Bailey D., Charleston B., Gilbert S. C., Tuthill T. J., Lambe T., Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. NPJ Vaccines 5, 69 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tostanoski L. H., Wegmann F., Martinot A. J., Loos C., McMahan K., Mercado N. B., Yu J., Chan C. N., Bondoc S., Starke C. E., Nekorchuk M., Busman-Sahay K., Piedra-Mora C., Wrijil L. M., Ducat S., Custers J., Atyeo C., Fischinger S., Burke J. S., Feldman J., Hauser B. M., Caradonna T. M., Bondzie E. A., Dagotto G., Gebre M. S., Jacob-Dolan C., Lin Z., Mahrokhian S. H., Nampanya F., Nityanandam R., Pessaint L., Porto M., Ali V., Benetiene D., Tevi K., Andersen H., Lewis M. G., Schmidt A. G., Lauffenburger D. A., Alter G., Estes J. D., Schuitemaker H., Zahn R., Barouch D. H., Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters. Nat. Med. 26, 1694–1700 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hassan A. O., Kafai N. M., Dmitriev I. P., Fox J. M., Smith B. K., Harvey I. B., Chen R. E., Winkler E. S., Wessel A. W., Case J. B., Kashentseva E., McCune B. T., Bailey A. L., Zhao H., VanBlargan L. A., Dai Y.-A., Ma M., Adams L. J., Shrihari S., Danis J. E., Gralinski L. E., Hou Y. J., Schäfer A., Kim A. S., Keeler S. P., Weiskopf D., Baric R. S., Holtzman M. J., Fremont D. H., Curiel D. T., Diamond M. S., A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 183, 169–184.e13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khoury D. S., Wheatley A. K., Ramuta M. D., Reynaldi A., Cromer D., Subbarao K., O’Connor D. H., Kent S. J., Davenport M. P., Measuring immunity to SARS-CoV-2 infection: Comparing assays and animal models. Nat. Rev. Immunol. 20, 727–738 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klasse P. J., Neutralization of virus infectivity by antibodies: Old problems in new perspectives. Adv. Biol., 157895 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K. J. A., Hemmings O., O’Byrne A., Kouphou N., Galao R. P., Betancor G., Wilson H. D., Signell A. W., Winstone H., Kerridge C., Huettner I., Jimenez-Guardeño J. M., Lista M. J., Temperton N., Snell L. B., Bisnauthsing K., Moore A., Green A., Martinez L., Stokes B., Honey J., Izquierdo-Barras A., Arbane G., Patel A., Tan M. K. I., O’Connell L., O’Hara G., MacMahon E., Douthwaite S., Nebbia G., Batra R., Martinez-Nunez R., Shankar-Hari M., Edgeworth J. D., Neil S. J. D., Malim M. H., Doores K. J., Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 5, 1598–1607 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.C. Gaebler, Z. Wang, J. C. C. Lorenzi, F. Muecksch, S. Finkin, M. Tokuyama, M. Ladinsky, A. Cho, M. Jankovic, D. Schaefer-Babajew, T. Y. Oliveira, M. Cipolla, C. Viant, C. O. Barnes, A. Hurley, M. Turroja, K. Gordon, K. G. Millard, V. Ramos, F. Schmidt, Y. Weisblum, D. Jha, M. Tankelevich, J. Yee, I. Shimeliovich, D. F. Robbiani, Z. Zhao, A. Gazumyan, T. Hatziioannou, P. J. Bjorkman, S. Mehandru, P. D. Bieniasz, M. Caskey, M. C. Nussenzweig, Evolution of antibody immunity to SARS-CoV-2. bioRxiv 2020.11.03.367391 [Preprint]. 5 November 2020. 10.1101/2020.11.03.367391. [DOI]
- 55.J. M. Dan, J. Mateus, Y. Kato, K. M. Hastie, C. E. Faliti, S. I. Ramirez, A. Frazier, E. D. Yu, A. Grifoni, S. A. Rawlings, B. Peters, F. Krammer, V. Simon, E. O. Saphire, D. M. Smith, D. Weiskopf, A. Sette, S. Crotty, Immunological memory to SARS-CoV-2 assessed for greater than six months after infection. bioRxiv 2020.11.15.383323 [Preprint]. 16 November 2020. 10.1101/2020.11.15.383323. [DOI]
- 56.Regoes R. R., Magnus C., The role of chance in primate lentiviral infectivity: From protomer to host organism. Prog. Mol. Biol. Transl. Sci. 129, 327–351 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Makedonas G., Betts M. R., Living in a house of cards: Re-evaluating CD8+ T-cell immune correlates against HIV. Immunol. Rev. 239, 109–124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burton D. R., Walker L. M., Rational vaccine design in the time of COVID-19. Cell Host Microbe 27, 695–698 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graham B. S., Henderson G. S., Tang Y. W., Lu X., Neuzil K. M., Colley D. G., Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J. Immunol. 151, 2032–2040 (1993). [PubMed] [Google Scholar]
- 60.Graham B. S., Rapid COVID-19 vaccine development. Science 368, 945–946 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Cote-Sierra J., Foucras G., Guo L., Chiodetti L., Young H. A., Hu-Li J., Zhu J., Paul W. E., Interleukin 2 plays a central role in Th2 differentiation. Proc. Natl. Acad. Sci. U.S.A. 101, 3880–3885 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu S., Zhong G., Zhang J., Shuai L., Zhang Z., Wen Z., Wang B., Zhao Z., Song X., Chen Y., Liu R., Fu L., Zhang J., Guo Q., Wang C., Yang Y., Fang T., Lv P., Wang J., Xu J., Li J., Yu C., Hou L., Bu Z., Chen W., A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 11, 4081 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bangaru S., Ozorowski G., Turner H. L., Antanasijevic A., Huang D., Wang X., Torres J. L., Diedrich J. K., Tian J. H., Portnoff A. D., Patel N., Massare M. J., Yates III J. R., Nemazee D., Paulson J., Glenn G., Smith G., Ward A., Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science 370, 1089–1094 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cleary S. J., Pitchford S. C., Amison R. T., Carrington R., Robaina Cabrera C. L., Magnen M., Looney M. R., Gray E., Page C. P., Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology. Br. J. Pharmacol. 177, 4851–4865 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Munster V. J., Feldmann F., Williamson B. N., van Doremalen N., Pérez-Pérez L., Schulz J., Meade-White K., Okumura A., Callison J., Brumbaugh B., Avanzato V. A., Rosenke R., Hanley P. W., Saturday G., Scott D., Fischer E. R., de Wit E., Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585, 268–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rockx B., Kuiken T., Herfst S., Bestebroer T., Lamers M. M., Oude Munnink B. B., de Meulder D., van Amerongen G., van den Brand J., Okba N. M. A., Schipper D., van Run P., Leijten L., Sikkema R., Verschoor E., Verstrepen B., Bogers W., Langermans J., Drosten C., Fentener van Vlissingen M., Fouchier R., de Swart R., Koopmans M., Haagmans B. L., Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368, 1012–1015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wölfel R., Corman V. M., Guggemos W., Seilmaier M., Zange S., Müller M. A., Niemeyer D., Jones T. C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C., Virological assessment of hospitalized patients with COVID-2019. Nature 581, 465–469 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Klasse P. J., Ozorowski G., Sanders R. W., Moore J. P., Env exceptionalism: Why are HIV-1 Env glycoproteins atypical immunogens? Cell Host Microbe 27, 507–518 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plotkin S. A., Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17, 1055–1065 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klasse P. J., Moore J. P., Good CoP, bad CoP? Interrogating the immune responses to primate lentiviral vaccines. Retrovirology 9, 80 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Addetia A., Crawford K. H. D., Dingens A., Zhu H., Roychoudhury P., Huang M., Jerome K. R., Bloom J. D., Greninger A., Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with high attack rate. J. Clin. Microbiol. 58, e02107-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidt F., Weisblum Y., Muecksch F., Hoffmann H. H., Michailidis E., Lorenzi J. C. C., Mendoza P., Rutkowska M., Bednarski E., Gaebler C., Agudelo M., Cho A., Wang Z., Gazumyan A., Cipolla M., Caskey M., Robbiani D. F., Nussenzweig M. C., Rice C. M., Hatziioannou T., Bieniasz P. D., Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 217, e20201181 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.K. Y. Oguntuyo, C. S. Stevens, C. T. Hung, S. Ikegame, J. A. Acklin, S. S. Kowdle, J. C. Carmichael, H. P. Chiu, K. D. Azarm, G. D. Haas, F. Amanat, J. Klingler, I. Baine, S. Arinsburg, J. C. Bandres, M. N. Siddiquey, R. M. Schilke, M. D. Woolard, H. Zhang, A. J. Duty, T. A. Kraus, T. M. Moran, D. Tortorella, J. K. Lim, A. V. Gamarnik, C. E. Hioe, S. Zolla-Pazner, S. S. Ivanov, J. P. Kamil, F. Krammer, B. Lee, Quantifying absolute neutralization titers against SARS-CoV-2 by a standardized virus neutralization assay allows for cross-cohort comparisons of COVID-19 sera. medRxiv 10.1101/2020.08.13.20157222 [Preprint]. 27 August 2020. 10.1101/2020.08.13.20157222. [DOI] [PMC free article] [PubMed]
- 74.Polack F. P., Thomas S. J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J. L., Pérez Marc G., Moreira E. D., Zerbini C., Bailey R., Swanson K. A., Roychoudhury S., Koury K., Li P., Kalina W. V., Cooper D., Frenck R. W., Hammitt L. L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D. B., Mather S., Dormitzer P. R., Şahin U., Jansen K. U., Gruber W. C.; C4591001 Clinical Trial Group , Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Widge T., Rouphael N. G., Jackson L. A., Anderson E. J., Roberts P. C., Makhene M., Chappell J. D., Denison M. R., Stevens L. J., Pruijssers A. J., McDermott A. B., Flach B., Lin B. C., Doria-Rose N. A., O’Dell S., Schmidt S. D., Neuzil K. M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V. V., Floyd K., Suthar M. S., Buchanan W., Luke C. J., Ledgerwood J. E., Mascola J. R., Graham B. S., Beigel J. H.; mRNA-1273 Study Group , Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 384, 80–82 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barrett J. R., Belij-Rammerstorfer S., Dold C., Ewer K. J., Folegatti P. M., Gilbride C., Halkerston R., Hill J., Jenkin D., Stockdale L., Verheul M. K., Aley P. K., Angus B., Bellamy D., Berrie E., Bibi S., Bittaye M., Carroll M. W., Cavell B., Clutterbuck E. A., Edwards N., Flaxman A., Fuskova M., Gorringe A., Hallis B., Kerridge S., Lawrie A. M., Linder A., Liu X., Madhavan M., Makinson R., Mellors J., Minassian A., Moore M., Mujadidi Y., Plested E., Poulton I., Ramasamy M. N., Robinson H., Rollier C. S., Song R., Snape M. D., Tarrant R., Taylor S., Thomas K. M., Voysey M., Watson M. E. E., Wright D., Douglas A. D., Green C. M., Hill A. V. S., Lambe T., Gilbert S., Pollard A. J.; Oxford COVID Vaccine Trial Group , Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med., (2020). [DOI] [PubMed] [Google Scholar]
- 77.Voysey M., Clemens S. A. C., Madhi S. A., Weckx L. Y., Folegatti P. M., Aley P. K., Angus B., Baillie V. L., Barnabas S. L., Bhorat Q. E., Bibi S., Briner C., Cicconi P., Collins A. M., Colin-Jones R., Cutland C. L., Darton T. C., Dheda K., Duncan C. J. A., Emary K. R. W., Ewer K. J., Fairlie L., Faust S. N., Feng S., Ferreira D. M., Finn A., Goodman A. L., Green C. M., Green C. A., Heath P. T., Hill C., Hill H., Hirsch I., Hodgson S. H. C., Izu A., Jackson S., Jenkin D., Joe C. C. D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A. M., Lelliott A., Libri V., Lillie P. J., Mallory R., Mendes A. V. A., Milan E. P., Minassian A. M., McGregor A., Morrison H., Mujadidi Y. F., Nana A., O’Reilly P. J., Padayachee S. D., Pittella A., Plested E., Pollock K. M., Ramasamy M. N., Rhead S., Schwarzbold A. V., Singh N., Smith A., Song R., Snape M. D., Sprinz E., Sutherland R. K., Tarrant R., Thomson E. C., Török M. E., Toshner M., Turner D. P. J., Vekemans J., Villafana T. L., Watson M. E. E., Williams C. J., Douglas A. D., Hill A. V. S., Lambe T., Gilbert S. C., Pollard A. J.; Oxford COVID Vaccine Trial Group , Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397, 99–111 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.E. Gabitzsch, J. T. Safrit, M. Verma, A. Rice, P. Sieling, L. Zakin, A. Shin, B. Morimoto, H. Adisetiyo, R. Wong, A. Bezawada, K. Dinkins, J. Balint, V. Peykov, H. Garban, P. Liu, A. Bacon, J. Drew, P. Spilman, L. Sender, S. Rabizadeh, K. Niazi, P. Soon-Shiong, Complete protection of nasal and lung airways against SARS-CoV-2 challenge by antibody plus Th1 dominant N- and S-specific T-cell responses to subcutaneous prime and thermally-stable oral boost bivalent hAd5 vaccination in an NHP study. bioRxiv 2020.12.08.416297 [Preprint]. 9 December 2020. 10.1101/2020.12.08.416297. [DOI]
- 79.Zhang X. Y., Guo J., Wan X., Zhou J. G., Jin W. P., Lu J., Wang W. H., Yang A. N., Liu D. X., Shi Z. L., Yuan Z. M., Li X. G., Meng S. L., Duan K., Wang Z. J., Yang X. M., Shen S., Biochemical and antigenic characterization of the structural proteins and their post-translational modifications in purified SARS-CoV-2 virions of an inactivated vaccine candidate. Emerg. Microbes Infect. 9, 2653–2662 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z. J., Zhang H. J., Lu J., Xu K. W., Peng C., Guo J., Gao X. X., Wan X., Wang W. H., Shan C., Zhang S. C., Wu J., Yang A. N., Zhu Y., Xiao A., Zhang L., Fu L., Si H. R., Cai Q., Yang X. L., You L., Zhou Y. P., Liu J., Pang D. Q., Jin W. P., Zhang X. Y., Meng S. L., Sun Y. X., Desselberger U., Wang J. Z., Li X. G., Duan K., Li C. G., Xu M., Shi Z. L., Yuan Z. M., Yang X. M., Shen S., Low toxicity and high immunogenicity of an inactivated vaccine candidate against COVID-19 in different animal models. Emerg. Microbes Infect. 9, 2606–2618 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.R. Ella, K. M. Vadrevu, H. Jogdand, S. Prasad, S. Reddy, V. Sarangi, B. Ganneru, G. Sapkal, P. Yadav, P. Abraham, S. Panda, N. Gupta, P. Reddy, S. Verma, S. K. Rai, C. Singh, S. V. Redkar, C. S. Gillurkar, J. S. Kushwaha, S. Mohapatra, V. Rao, R. Guleria, K. Ella, B. Bhargava, A phase 1: Safety and immunogenicity trial of an inactivated SARS-CoV-2 vaccine-BBV152. medRxiv 2020.12.11.20210419 [Preprint]. 15 December 2020. 10.1101/2020.12.11.20210419. [DOI]
- 82.R. Ella, S. Reddy, H. Jogdand, V. Sarangi, B. Ganneru, S. Prasad, D. Das, D. Raju, U. Praturi, G. Sapkal, P. Yadav, P. Reddy, S. Verma, C. Singh, S. V. Redkar, C. S. Gillurkar, J. S. Kushwaha, S. Mohapatra, A. Bhate, S. Rai, S. Panda, P. Abraham, N. Gupta, K. Ella, B. Bhargava, K. M. Vadrevu, Safety and immunogenicity clinical trial of an inactivated SARS-CoV-2 vaccine, BBV152 (a phase 2, double-blind, randomised controlled trial) and the persistence of immune responses from a phase 1 follow-up report. medRxiv 2020.12.21.20248643 [Preprint]. 22 December 2020. 10.1101/2020.12.21.20248643. [DOI]
- 83.S. Yang, Y. Li, L. Dai, J. Wang, P. He, C. Li, X. Fang, C. Wang, X. Zhao, E. Huang, C. Wu, Z. Zhong, F. Wang, X. Duan, S. Tian, L. Wu, Y. Liu, Y. Luo, Z. Chen, F. Li, J. Li, X. Yu, H. Ren, L. Liu, S. Meng, J. Yan, Z. Hu, L. Gao, G. F. Gao, Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD protein vaccine against COVID-19 in adults: Pooled analysis of two randomized, double-blind, placebo-controlled, phase 1 and 2 trials. medRxiv 2020.12.20.20248602 [Preprint]. 22 December 2020. 10.1101/2020.12.20.20248602. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/sciadv.abe8065/DC1


